Key Points
CD38-CAR NK and T cells showed enhanced killing toward CD38-expressing hematologic malignancies.
ATRA upregulates CD38, enhancing the activity of CD38-CAR NK cells against resistant CD38-low tumor cells.
Visual Abstract
CD38 is a metabolically active enzyme broadly expressed on the surface of normal and malignant hematologic cells. It has been targeted clinically with anti-CD38 monoclonal antibodies (mAbs), for which efficacy may be limited by natural killer (NK)–cell fratricide. Isatuximab is an anti-CD38 mAb that uniquely inhibits CD38 metabolic activity. Here, we used CRISPR/adeno-associated virus (AAV) to generate fratricide–resistant and metabolically–enhanced CD38KO/CD38-chimeric antigen receptor (CAR) NK cells using 2 isatuximab-based CD38 single-chain variable fragments (scFvs; reversing heavy and light chain orientation) on the same CD8α/4-1BB/CD3ζ base, and we demonstrate their activity against a range of CD38+ hematologic malignancies (acute myeloid leukemia, multiple myeloma, Burkitt lymphoma, and T-cell leukemia/lymphoma). The cytotoxicity of the CAR NK cells was enhanced by upregulating CD38 expression on the malignant targets with all-trans retinoic acid (ATRA). By generating CD38KO/CD38-CAR T cells using the same engineering approach, we show that the CAR NK cells had higher cytotoxicity than CAR T cells against all hematologic tumor targets. Additionally, AAVS1KO/CD38-CAR NK cells were capable of targeting CD38 without experiencing fratricide and have a similar enhanced metabolic activity via the inhibitory activity of the cis-acting isatuximab-based scFv. Finally, we report fratricide-resistant CD38-CAR NK cells with enhanced metabolism and cytotoxicity toward CD38+ hematologic malignancies, further increased by combination treatment with ATRA.
Introduction
Cellular therapy has shown tremendous efficacy against hematologic malignancies.1 Despite the strong antitumor efficacy of chimeric antigen receptor (CAR) T-cell therapies, these therapies are limited by associated toxicities and their inability to be accessible for off-the-shelf treatments.1-4 Natural killer (NK) cells are immune cells with an innate killing potential toward malignant and viral-infected cells that downregulate major histocompatibility complex (MHC) class I.5 Thus, NK-cell–based immunotherapies offer a unique, off-the-shelf therapeutic option with innate cytotoxicity toward cancerous cells that also can be further enhanced by genetic engineering to express CARs.6 Malignant cells can evade NK-cell killing by suppressing the expression of activating ligands, preserving MHC expression to inhibit activation, and inhibiting immune cell function through suppressive cytokines.7 By genetically engineering them to express a CAR, NK cells can directly target tumor-specific antigens and kill independently of MHC recognition or expression levels. Thus, CAR NK cells are a promising therapeutic for hematologic malignancies because they offer target-specific cellular cytotoxicity and retain their innate killing potential.
CD38 is a transmembrane glycoprotein highly expressed on the surface of plasma cells and other lymphoid and myeloid cell populations.8,9 CD38 is expressed in high levels on various normal hematologic tissues and hematologic malignancies such as multiple myeloma (MM), Burkitt lymphoma (BL), acute myeloid leukemia (AML), and T-cell leukemias.10 Although these malignancies are diverse in their origin, their high CD38 expression makes them all strong potential targets for anti-CD38 CAR NK-cell therapy. Similarly, CD38 is well studied to play a role in tumor progression and the tumor microenvironment by metabolizing NAD+ in hypoxic conditions and assisting in angiogenesis.11 CD38 targeting cellular therapies are relevant because of their broad application toward a diverse range of CD38-expressing hematologic malignancies.
Currently, CD38 has been targeted in patients with MM using the US Food and Drug Administration–approved anti-CD38 monoclonal antibodies (mAbs), isatuximab and daratumumab.11,12 Isatuximab-based combination therapies display efficacy and a tolerable safety level in patients.13 NK cells play an important role in the recognition and killing of malignant cells in the presence of anti-CD38 mAbs through antibody-dependent cellular cytotoxicity (ADCC).14 However, because NK cells also highly express CD38, anti-CD38 mAbs cause NK cells to target and kill one another (also known as fratricide), limiting their efficacy.15 The limited efficacy of CD38 mAb therapies suggests that novel approaches are needed to reduce fratricide and improve NK-cell cytotoxicity toward CD38 malignancies.10
Materials and methods
Cell lines and primary cells
CSTX002 feeder cells, derived from K562 and transduced to express membrane-bound interleukin-21 (IL-21) and 4-1BBL (referred to hereafter as FC21), were cultured in RMPI 1640 media containing 10% fetal bovine serum (FBS) at 37°C, 5% CO2. FC21 were treated with 100-Gy X-ray irradiation (Faxitron, Tucson, AZ) before coculture with NK cells.16-19 Human AML cell lines MV4-11, MM cell lines MM1S and H929, and BL cell lines Daudi and Raji were purchased from American type culture collection. Primary human AML cells (AML10) were obtained from a pediatric patient with relapsed M5 AML and passaged in immunodeficient (NOD-SCID) mice. Primary human AML, pre–T-cell acute lymphoblastic leukemia, T-cell prolymphocytic leukemia (T-PLL), MM, and hepatosplenic T-cell lymphoma were obtained from The Ohio State University (OSU) Comprehensive Cancer Center Leukemia Tissue Bank. Tumor cell lines were cultured in RPMI 1640 media plus Glutamax and 10% FBS and routinely tested for mycoplasma contamination. Anonymized human buffy coats were obtained from the American Red Cross of Ohio (Columbus, OH) as healthy donor lymphocyte source material, designated not human subjects research by the institutional review board. Primary cancer cells were obtained from the OSU leukemia tissue bank from patients undergoing therapeutic apheresis under an institutional review board–approved protocol and with patient's informed consent, consistent with the Declaration of Helsinki.
NK-cell purification and in vitro expansion
Human primary NK cells were isolated from buffy coats by negative depletion using RosetteSep Human NK-cell Enrichment Cocktail (STEMCELL Technologies) and expanded as previously described.16,18 Briefly, isolated NK cells were stimulated and expanded using FC21 cells. On day 7, NK cells were electroporated and transduced as described below and then rested in NK-cell media for 2 days. The genetically modified NK cells were then further expanded by restimulation with FC21 cells (1:1) every 7 days under the culture conditions described above.
T-cell purification and in vitro expansion
Human primary T cells were isolated from donor buffy coats using RosetteSep Human T-cell Enrichment Cocktail (STEMCELL Technologies, Vancouver, BC, Canada) as described for NK cells. Isolated T cells were stimulated on day 0 with human T-Activator CD3/CD28 (Thermo Fisher Scientific). Stimulated T cells were cultured in RPMI 1640 media plus Glutamax, 10% FBS at a concentration of 1.0e6 cells per mL, and supplemented with 50 IU/mL human recombinant IL-7 and IL-15 (BioLegend, San Diego, CA) every 2 to 3 days.
Two days after cytokine activation the T cells were electroporated and transduced as described below.19 After transduction, the cells were expanded in RPMI 1640 media plus Glutamax and 10% FBS at 1.0e6 cells per mL, with IL-7 and IL-15 supplemented at 50 IU/mL every 2 to 3 days for up to 10 days.
Generation of genome-edited NK and T cells
CD38KO/CD38-CAR T cells or NK cells were generated by electroporation of HiFi CRISPR-associated protein 9 (Cas9) Nuclease V3 (Integrated DNA Technologies, Coralville, IA) and guide RNA targeting exon 1 of the CD38 gene (5'-CTGAACTCGCAGTTGGCCAT-3'), as previously described.15,20 Knockout cells were then returned to culture media and transduced within 30 minutes with 75 000 vg per cell of adeno-associated virus type 6 (AAV6; Andelyn Biosciences) vector containing the CAR expression cassette with CD38 homology arms, as detailed.15 Similarly, AAVS1KO/CD38-CAR NK cells were generated using gRNA targeting the AAVS1 safe harbor gene (5'-GGGGCCACTAGGGACAGGAT-3') and AAV6 vectors with homology arms for the AAVS1 locus.19
Flow cytometry
Cells were stained in phosphate-buffered saline containing 2% FBS using anti-CD38/PE (Miltenyi Biotec; clone REA572) and CD38 Fc chimera protein (R&D Systems; rhCD38), followed by secondary staining with Alexa Fluor 647 AffiniPure goat anti-human immunoglobulin G (Jackson ImmunoResearch) and Tonbo Ghost Dye 450 (Tonbo Biosciences, San Diego, CA). See representative gating strategy in supplemental Figure 1.
Cytotoxicity assay
Four-hour cytotoxicity assays were performed using calcein AM (Fisher Scientific, Hampton, NH). Briefly, target tumor cells were stained with 2 μg/mL calcein AM and cocultured with effector cells at the indicated effector-to-target (E:T) ratios. After 4 hours, culture supernatant was assessed by fluorimetry, and percent specific lysis was calculated using Triton X-100 2% for maximum and untreated for spontaneous controls. Cytotoxicity is plotted as percent specific lysis.18 Statistical analysis was performed using 2-way analysis of variance main column effect.
Metabolic assay
The oxygen consumption rate and extracellular acidification rate of wild-type (WT), CD38KO/CD38-CAR, and AAVS1KO/CD38-CAR NK cells were measured using the Agilent Extracellular Flux Assay kit (Agilent Technologies, Santa Clara, CA) on the Seahorse XFe24 analyzer (Agilent Technologies, Santa Clara, CA) according to the manufacturer's instructions as previously described.15
Bio-Plex cytokine assay
Cytokine secretion was assessed in WT and CD38KO/CD38-CAR NK cells after coculture with 3 separate tumor targets (MM1S, MV4-11, and Raji). Cells were cocultured for 6 hours at a 5:1 E:T ratio before the supernatant was collected. The assay was performed according to manufacturer instructions (Bio-Plex Pro Human Cytokine 8-plex assay, Bio-Rad) on the Bio-Plex 200 system.
Mass cytometry lysis assay
A primary cell sample from a patient with AML (OSU leukemia tissue bank) was cultured in a 6-well plate (precoated with rat tail collagen [Corning]) in serum-free media supplemented with a cytokine cocktail (IL-6, IL-3, FLT3, GMCSF, GCSF, and TPO at 6.66 ng/mL, EPO at 3.33 ng/mL, and stem cell factor (SCF) at 20 ng/mL; STEMCELL Technologies). The primary AML samples were cultured for 24 hours with or without 10-nM all-trans retinoic acid (ATRA). After 24 hours, WT or CD38KO/CD38 CAR NK cells were added at a ratio of 1:1 and cocultured for 24 hours. Control wells were cultured in parallel. Cells were stained and events were acquired on the Helios CyTOF system (Fluidigm, Toronto, Canada) as previously described.20 Cells were identified using markers and analyzed using SPADE.19
CD38 qPCR
Statistical analysis
Statistical analyses were performed using Prism 9.3.0 (GraphPad Software, Inc) as described in the figure legends. Student t test was used to compare 2 independent groups. Three or more groups were compared with 2-way analysis of variance analysis. P values <.05 were considered significant.
Results
Generation of highly efficient CD38KO/CD38CAR NK cells using Cas9/RNP and AAV
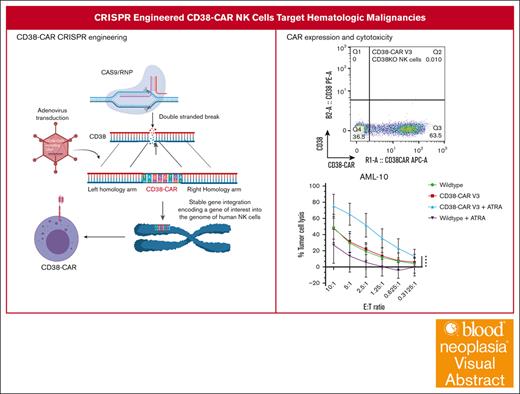
We generated 2 single-chain variable fragments (scFvs) based on publicly available variable heavy (VH) and light (VL) chains of isatuximab to generate a second-generation CAR with CD3ζ and 41BB costimulatory domains and a CD8α transmembrane/hinge (Figure 1B). CAR construct V3 expressed the scFv in VH-VL orientation and construct V4 expressed the scFv in VL-VH orientation. After the initial 7-day expansion, activated NK cells were electroporated with Cas9/RNP targeting CD38 and were transduced with AAV vectors providing the CAR constructs with homology arms for the targeting site at the CD38 locus (Figure 1A). Complete CD38 knockouts and CAR expression were measured by flow cytometry 7 days after transduction in the CAR NK cells, indicating a successful generation of the CD38KO/CD38-CAR NK cells (Figure 1B). The median adenovirus transduction efficiency for V3 was 60.7% and for V4 was 61.9% (Figure 1C).
Generation of CRISPR-engineered CD38KO/CD38-CAR human primary NK cells using Cas9/RNP and AAV. (A) Schemata of steps for CRISPR/RNP knockout of the CD38 gene and directed insertion of a CD38-CAR encoding DNA delivered by AAV6 vector with homology arms for CD38 targeting site. (B) CD38 (PE) and CAR (APC) expression levels measured by flow cytometry for binding of CD38 antigen, 7 days after stimulation. Constructs contain a 41BB signaling domain, a CD8α transmembrane domain/hinge, a CD3ζ stimulatory domain, and reversed orderings of light and heavy chain orientations. (C) Relative percentage and intensity of CD38-CAR expression (n = 10; mean ± standard deviation [SD]). (D) Fold expansion of WT and CD38-CAR NK cells over 12 days after activation with irradiated, modified mbIL21-K562 cells and IL-2 show no significant change from WT human NK cells (n = 10; mean ± SD). P values were calculated using a 2-way analysis of variance (ANOVA); ∗P = .0332; ∗∗P = .0021; ∗∗∗P = .0002; ∗∗∗∗P < .0001. (E) Cytotoxicity observed for V3 and V4 CD38KO/CD38-CAR NK cells against high CD38-expressing MM (H929), BL (Raji), and AML (MV-11) (n = 5; mean ± SD). P values were calculated using a 2-way ANOVA; ∗P < .05; ∗∗P < .01; ∗∗∗P = .001; ∗∗∗∗P < .0001.
Generation of CRISPR-engineered CD38KO/CD38-CAR human primary NK cells using Cas9/RNP and AAV. (A) Schemata of steps for CRISPR/RNP knockout of the CD38 gene and directed insertion of a CD38-CAR encoding DNA delivered by AAV6 vector with homology arms for CD38 targeting site. (B) CD38 (PE) and CAR (APC) expression levels measured by flow cytometry for binding of CD38 antigen, 7 days after stimulation. Constructs contain a 41BB signaling domain, a CD8α transmembrane domain/hinge, a CD3ζ stimulatory domain, and reversed orderings of light and heavy chain orientations. (C) Relative percentage and intensity of CD38-CAR expression (n = 10; mean ± standard deviation [SD]). (D) Fold expansion of WT and CD38-CAR NK cells over 12 days after activation with irradiated, modified mbIL21-K562 cells and IL-2 show no significant change from WT human NK cells (n = 10; mean ± SD). P values were calculated using a 2-way analysis of variance (ANOVA); ∗P = .0332; ∗∗P = .0021; ∗∗∗P = .0002; ∗∗∗∗P < .0001. (E) Cytotoxicity observed for V3 and V4 CD38KO/CD38-CAR NK cells against high CD38-expressing MM (H929), BL (Raji), and AML (MV-11) (n = 5; mean ± SD). P values were calculated using a 2-way ANOVA; ∗P < .05; ∗∗P < .01; ∗∗∗P = .001; ∗∗∗∗P < .0001.
CD38KO/CD38-CAR NK cells avoid fratricide and show enhanced cytotoxicity toward cancer cell lines
NK cells mediate the antileukemic effect of anti-CD38 mAbs via antibody-dependent cellular cytotoxicity (ADCC), but their efficacy is also limited by ADCC against each other, known as fratricide.21,22 We hypothesized that we could overcome fratricide in NK cells expressing a CD38 CAR by developing a simultaneous CD38 knockout (KO) and CD38-CAR knock-in (KI). There was no significant difference between the posttransduction expansion (Figure 1D) of the CAR NK cells compared with WT NK cells, suggesting that the CD38KO/CD38-CAR NK cells avoided fratricide during expansion.
We then performed killing assays to assess the activity of V3 and V4 CARs against CD38-expressing malignancies and observed a significant increase in cytotoxicity for the CAR NK cells against all tumor targets (MM [H929], BL [Raji], and AML [MV4-11]) for 5 NK-cell donors (Figure 1E). No significant difference was seen between NK cells expressing the V3 and V4 CAR constructs, suggesting heavy and light chain order did not affect function, so the remaining experiments were performed with V3.
CD38-CAR NK cells have enhanced killing toward patient tumor samples
To further validate the clinical potential of CD38-CAR NK cells, we tested their cytotoxicity against CD38-expressing (supplemental Figure 2) primary patient tumor samples. One to 3 NK-cell donors were tested against each tumor sample based on the availability of viable primary tumor cells after thawing. CD38-CAR NK cells showed higher killing than WT NK cells against AML (AML-1 [n = 3 NK-cell donors]; Figure 2A), MM (MM-1 and MM-2 [n = 1 NK-cell donor]; Figure 2B), lymphoma (T-PLL-1 and T-PLL-2 [n = 1 NK-cell donor]), and hepatosplenic T-cell lymphoma (n = 1 NK-cell donor; Figure 2C).
CD38-CAR NK cells exhibit enhanced cytotoxic function and cytokine secretion. CD38-CAR NK cells were tested against CD38-expressing AML, MM, and T-cell malignancies collected from patients at baseline. (A) NK and CD38-CAR NK killing of AML-1 (n = 3; mean ± SD). (B) NK and CD38-CAR NK killing of samples from patients with MM (MM-1 and MM-2; n = 1; mean ± SD). (C) NK and CD38-CAR NK killing of T-cell malignancies (hepatosplenic T-cell lymphoma and T-PLL [T-PLL-1 and T-PLL-2]; n = 1; mean ± SD). All cytotoxicity P values were calculated using a 2-way ANOVA; ∗P < .05; ∗∗P < .01; ∗∗∗P = .001; ∗∗∗∗P < .0001. (D) Bio-Plex Pro Human Cytokine assay was performed on the supernatant of WT and CD38-CAR NK cells cocultured with CD38+ malignancies (n = 9; mean ± SD). P values were calculated using a paired Student t test; ∗P = .05; ∗∗P = .01; ∗∗∗P = .001. GM-CSF, IFN-γ, MCP-1, MIP-1α, TNF-α.
CD38-CAR NK cells exhibit enhanced cytotoxic function and cytokine secretion. CD38-CAR NK cells were tested against CD38-expressing AML, MM, and T-cell malignancies collected from patients at baseline. (A) NK and CD38-CAR NK killing of AML-1 (n = 3; mean ± SD). (B) NK and CD38-CAR NK killing of samples from patients with MM (MM-1 and MM-2; n = 1; mean ± SD). (C) NK and CD38-CAR NK killing of T-cell malignancies (hepatosplenic T-cell lymphoma and T-PLL [T-PLL-1 and T-PLL-2]; n = 1; mean ± SD). All cytotoxicity P values were calculated using a 2-way ANOVA; ∗P < .05; ∗∗P < .01; ∗∗∗P = .001; ∗∗∗∗P < .0001. (D) Bio-Plex Pro Human Cytokine assay was performed on the supernatant of WT and CD38-CAR NK cells cocultured with CD38+ malignancies (n = 9; mean ± SD). P values were calculated using a paired Student t test; ∗P = .05; ∗∗P = .01; ∗∗∗P = .001. GM-CSF, IFN-γ, MCP-1, MIP-1α, TNF-α.
CD38-CAR NK cells have higher production of activating cytokines
To further evaluate the enhanced activation in the CAR NK cells, we measured the supernatant for cytokines after CAR NK cells from 3 different donors were cocultured with 3 tumor targets (MM1S, Raji, and MV4-11). The CAR NK cells showed significantly higher production of interferon gamma (IFN-γ), tumor necrosis factor alpha (TNF-α), granulocyte-macrophage colony-stimulating factor (GM-CSF), monocyte chemoattractant protein-1 (MCP-1), macrophage inflammatory protein-1 alpha (MIP-1α), and RANTES (Figure 2D), important mediators of NK-cell effector function and antitumor activity. They also showed a notable increase in the NK-cell activating cytokine IL-1RA and IL-9 (supplemental Figure 3) but no significant difference in other observed cytokines.
ATRA increases CD38 expression and improves CAR killing in certain cancer cell lines
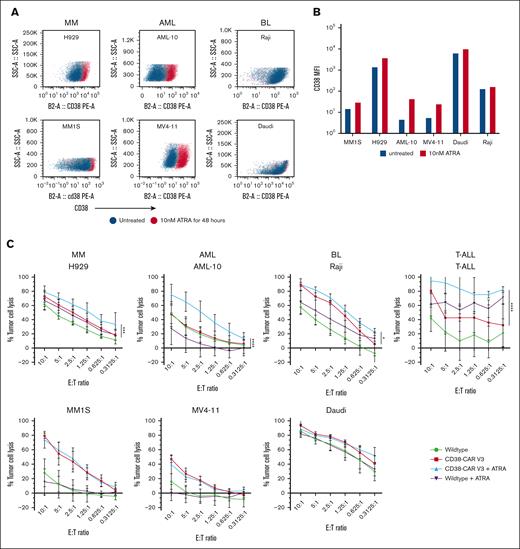
To further augment the antitumor activity of the CD38-CAR NK cells, we explored combination therapy with ATRA. ATRA is a differentiation agent that upregulates CD38 expression in many cell types.23 Across cell lines of AML, MM, and BL, we observed variable responses in CD38 expression after treatment with 10-nM ATRA for 48 hours (Figure 3A). The 2 AML cell lines with the lowest baseline CD38 expression showed the greatest proportional increase in CD38 after ATRA treatment (Figure 4B). Conversely, intermediate to high CD38-expressing MM cell lines saw a minor increase in expression, whereas high CD38-expressing BL showed no observable increase (Figure 3B). A significant increase in tumor cell killing between the CAR NK cells alone and ATRA plus CAR NK cells was observed for H929, AML-10, Raji, and T-cell acute lymphoblastic leukemia (T-ALL; Figure 3C). No increase in killing by the CAR NK cells with the addition of ATRA was seen for MM1S, MV4-11, or Daudi. Thus, ATRA appears to have a cell line–dependent effect on the increase in CD38 surface density and sensitivity to CAR NK-cell killing (Figure 3A,C). We previously reported that ATRA did not have any adverse effect on the function or viability of CD38KO NK cells.15
ATRA upregulates CD38 expression on tumor cells and can enhance antitumor activity. (A) CD38 cell surface expression as measured by flow cytometry across the hematologic malignancies MM, AML, BL, and T-ALL after treatment with 10 nM of ATRA for 48 hours. (B) Mean fluorescence intensity (MFI) of CD38 expression on cell lines with and without ATRA treatment. (C) Cytotoxicity assays performed by coculturing WT and CD38-CAR NK cells against AML, MM, BL, and T-cell malignancies with and without 48-hour, 10-nM ATRA pretreatments. MM1S (n = 4), H929 (n = 4), AML-10 (n = 4), MV4-11 (n = 4), Raji (n = 4), Daudi (n = 4), and primary cells from patients with T-ALL (n = 3; mean ± SD). P values were calculated using a 2-way ANOVA; ∗P < .05; ∗∗ P <.01; ∗∗∗P = .001; ∗∗∗∗P < .0001.
ATRA upregulates CD38 expression on tumor cells and can enhance antitumor activity. (A) CD38 cell surface expression as measured by flow cytometry across the hematologic malignancies MM, AML, BL, and T-ALL after treatment with 10 nM of ATRA for 48 hours. (B) Mean fluorescence intensity (MFI) of CD38 expression on cell lines with and without ATRA treatment. (C) Cytotoxicity assays performed by coculturing WT and CD38-CAR NK cells against AML, MM, BL, and T-cell malignancies with and without 48-hour, 10-nM ATRA pretreatments. MM1S (n = 4), H929 (n = 4), AML-10 (n = 4), MV4-11 (n = 4), Raji (n = 4), Daudi (n = 4), and primary cells from patients with T-ALL (n = 3; mean ± SD). P values were calculated using a 2-way ANOVA; ∗P < .05; ∗∗ P <.01; ∗∗∗P = .001; ∗∗∗∗P < .0001.
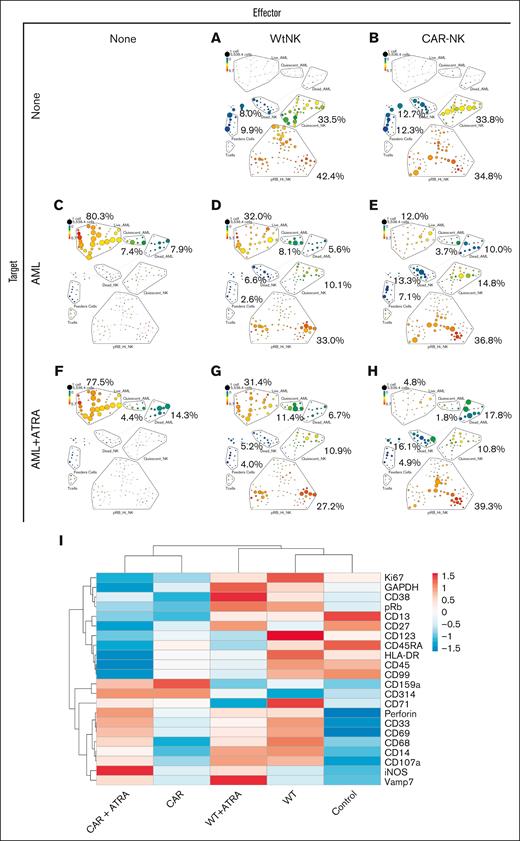
Mass cytometry analysis shows combination ATRA and CD38-CAR NK-cell treatment decreases live AML population. Mass cytometry analysis was performed 24 hours after the coculture of a primary AML cell line (AML-1) with WT or CD38-CAR NK cells with and without 48-hour, 10-nM ATRA pretreatment. Eight distinct conditions were analyzed: WT NK cells alone (A), CD38-CAR NK cells alone (B), AML cells alone (C), AML plus WT NK cells (D), AML plus CD38-CAR NK cells (E), AML cells with ATRA (F), AML plus ATRA plus WT NK cells (G), and AML plus ATRA plus CD38-CAR NK cells (H). (I) Heat map of surface marker appearing on the live AML cells after treatment analyzed by mass cytometry. Original values are ln(x)-transformed. Rows are centered; unit variance scaling is applied to rows. Both rows and columns are clustered using correlation distance and average linkage using ClustVis.
Mass cytometry analysis shows combination ATRA and CD38-CAR NK-cell treatment decreases live AML population. Mass cytometry analysis was performed 24 hours after the coculture of a primary AML cell line (AML-1) with WT or CD38-CAR NK cells with and without 48-hour, 10-nM ATRA pretreatment. Eight distinct conditions were analyzed: WT NK cells alone (A), CD38-CAR NK cells alone (B), AML cells alone (C), AML plus WT NK cells (D), AML plus CD38-CAR NK cells (E), AML cells with ATRA (F), AML plus ATRA plus WT NK cells (G), and AML plus ATRA plus CD38-CAR NK cells (H). (I) Heat map of surface marker appearing on the live AML cells after treatment analyzed by mass cytometry. Original values are ln(x)-transformed. Rows are centered; unit variance scaling is applied to rows. Both rows and columns are clustered using correlation distance and average linkage using ClustVis.
ATRA and CD38-CAR NK-cell combination treatment reduced live primary AML cell populations
Mass cytometry analysis was performed to further evaluate the efficacy of the combination of ATRA and CD38-CAR NK-cell treatment. Primary AML cells with and without ATRA before coculture with NK cells had live cell populations of 77.5% and 80.3%, respectively (Figure 4C,F). After coculture with WT NK cells with and without ATRA, AML live cell populations decreased to 31.4% and 32.0%, respectively (Figure 4D,G). A further reduction was observed when AML cells were treated with CD38-CAR NK cells, showing only 12% remaining live AML without ATRA and 4.8% with ATRA (Figure 4E,H). The greatest reduction in live AML cells and increase in AML dead population (17.8%) seen with the ATRA and CD38-CAR NK cells combination is a result of ATRA-mediated upregulation of CD38 (Figure 4I). Notably, there was an increase in live proliferating (pRb+) CD38-CAR NK cells after coculture with AML (Figure 4E) or AML and ATRA (Figure 4H), but the WT population decreased after coculture with AML (Figure 4D) or AML and ATRA (Figure 4G), suggesting that the CAR might also deliver a survival or proliferative signal to the NK cells.
The live AML cell populations (Figure 4C-E,G-H) identified by marker CD34 and cleavage product poly-ADP-ribose polymerase (cPARP) were further analyzed for surface expression of cell markers and clustered accordingly. CD38 behaves as anticipated, with increased expression from ATRA and depletion by the CAR (Figure 4I). Clusters reveal differential expression of surface markers between AML cells that survived treatment with WT vs CAR and ATRA or no ATRA (Figure 4I).
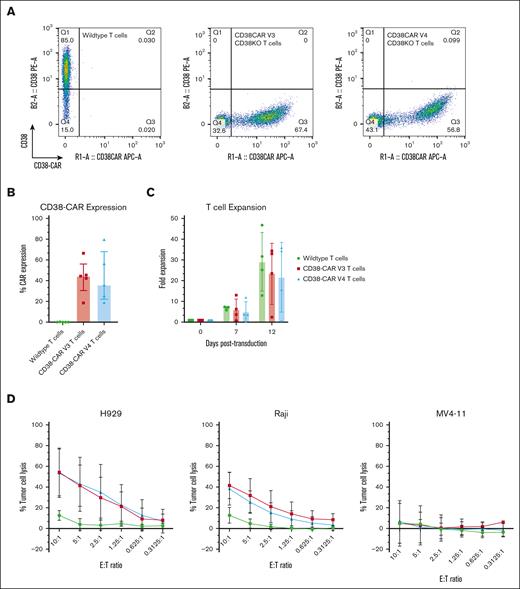
CD38KO/CD38-CAR is reproducible and functional in T cells
CAR T cells are effective treatments for a variety of hematologic malignancies as an alternative to chemotherapy.24 Anti-CD38 CAR T cells have recently shown efficacy against MM, T-ALL, and AML in xenografted mouse models.20 To understand how the cytotoxic function of CAR NK cells compares with CAR T cells, we also generated CD38KO/CD38-CAR T cells using the same CRISPR/Cas9 and adenovirus transduction method. We feel this provides the most direct comparison of the same CAR in 2 different cell types, and to our knowledge, this is the first comparison of CAR NK and CAR T cells in donor-matched pairs using the same site-directed CAR integration method. CD38-CAR expression and complete CD38 knockouts were observed in CAR T cells 7 days after transduction (Figure 5A). The median adenovirus transduction efficiency for V3 was 44.4% and for V4 was 35.9% (Figure 5B). Using the same transduction conditions of 75 000 vg per cell of AAV6, this transduction efficiency in T cells was lower than in NK cells (60.7% vs 61.9% for V3 and V4, respectively [Figure 1C]). The CAR T cells expanded the same as WT T cells (Figure 5C), which indicates that the cells avoid fratricide as was observed with CAR NK cells. CAR T cells had enhanced cytotoxicity toward high antigen density tumor cell lines H929 and Raji, but they showed no significant increase in killing of CD38low MV4-11 (Figure 5D). In this single time point assay, CAR T cells had lower percent tumor cell lysis than CAR NK cells across all cell lines and E:T ratios, potentially due to lower CAR expression in the T cells or additional innate cytolytic activity of the NK cells. Maximum killing at the 10:1 E:T ratio was 54.1% for H929 and 41.5% for Raji with CAR T cells but was 73.7% for H929 and 84.9% for Raji with the CAR NK cells (Figures 1E and 5D).
CD38KO/CD38-CAR T cells generated by Cas9/RNP and AAV6 show antitumor activity. (A) CD38 (PE) and CAR (APC) expression levels measured by flow cytometry. (B) Relative percentage of CD38-CAR expression (n = 5; mean ± SD). (C) Ten-day fold expansion of generated CD38-CAR T cells with matched donors (n = 4; mean ± SD). P values were calculated using a 2-way ANOVA; ∗P = .0332; ∗∗P = .0021; ∗∗∗P = .0002; ∗∗∗∗P < .0001. (D) Cytotoxicity of CD38KO/CD38-CAR T cells toward H929, Raji, and MV4-11 cell lines (n = 4; mean ± SD). P values were calculated using a 2-way ANOVA; ∗P < .05; ∗∗P < .01; ∗∗∗P = .001; ∗∗∗∗P < .0001.
CD38KO/CD38-CAR T cells generated by Cas9/RNP and AAV6 show antitumor activity. (A) CD38 (PE) and CAR (APC) expression levels measured by flow cytometry. (B) Relative percentage of CD38-CAR expression (n = 5; mean ± SD). (C) Ten-day fold expansion of generated CD38-CAR T cells with matched donors (n = 4; mean ± SD). P values were calculated using a 2-way ANOVA; ∗P = .0332; ∗∗P = .0021; ∗∗∗P = .0002; ∗∗∗∗P < .0001. (D) Cytotoxicity of CD38KO/CD38-CAR T cells toward H929, Raji, and MV4-11 cell lines (n = 4; mean ± SD). P values were calculated using a 2-way ANOVA; ∗P < .05; ∗∗P < .01; ∗∗∗P = .001; ∗∗∗∗P < .0001.
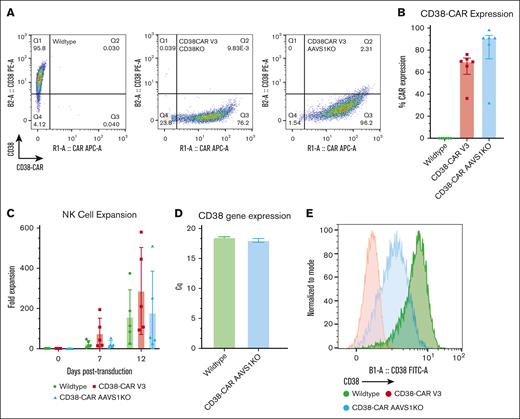
CD38-CAR NK cells avoid fratricide without CD38KO
To establish whether CD38 deletion is necessary to avoid fratricide among the CAR NK cells, we generated CD38-CAR NK cells using the same CAR constructs but with AAVS1 as the knockout/gene insertion site, leaving the CD38 gene intact. Because the CAR NK cells still express CD38, we hypothesized that they would experience fratricide, similar to that seen for WT NK cells in the presence of daratumumab. Instead, the AAVS1KO/CD38-CAR NK cells were 90% CAR-positive 7 days after transduction, and CD38 was not detectable by flow cytometry (Figure 6A). The median transduction efficiency for the CD38KO/CD38-CAR NK cells was 69.6%, and for the AAVS1KO/CD38-CAR NK cells, it was 91.2% (Figure 6B). AAVS1KO/CD38-CAR NK cells expanded similarly to WT NK cells, implying that no significant fratricide was occurring and that CAR NK cells were not dying (Figure 6C). Because the CD38 gene was left intact for the AAVS1KO/CD38-CAR NK cells but CD38 was undetectable initially, we used reverse transcriptase qPCR to assess CD38 gene expression. Surprisingly, the AAVS1KO CAR NK cells showed equivalent CD38 gene expression to WT NK cells (Figure 6D). To further confirm expression at the protein level, we reassessed by flow cytometry using a polyclonal anti-CD38 antibody and observed CD38 surface expression (Figure 6E). The lack of expression using the mAb is likely explained by steric hindrance through cis interactions of CD38 with the CAR. The reduced detection by the polyclonal antibody can also be explained by steric inhibition of some, but not all, epitopes recognized by the antibody. The qPCR results show that the CAR had not selected for cells with reduced transcription of CD38, but it is still possible that the CAR selected for cells with some as-yet-unknown epigenetic mechanism that results in reduced protein translation or surface translocation.
AAVS1KO/CD38-CAR NK cells avoid fratricide. (A) CD38 (PE) and CAR (APC) expression levels measured by flow cytometry for the CD38-CAR inserted into different loci. All CAR NK cells were generated with matched donors. (B) Fold expansion of WT and CD38-CAR NK cells over 12 days (n = 6; mean ± SD). P values were calculated using a 2-way ANOVA; ∗P < .05; ∗∗P < .01; ∗∗∗P = .001; ∗∗∗∗P < .0001. (C) Relative percentage of CD38-CAR expression (n = 6; mean ± SD). (D) Reverse-transcription qPCR was performed using CD38 primer probes to detect transcription of the CD38 gene in the WT and AAVS1KO/CD38-CAR NK cells. (E) CD38 expression measured by flow cytometry using a polyclonal anti-CD38 antibody.
AAVS1KO/CD38-CAR NK cells avoid fratricide. (A) CD38 (PE) and CAR (APC) expression levels measured by flow cytometry for the CD38-CAR inserted into different loci. All CAR NK cells were generated with matched donors. (B) Fold expansion of WT and CD38-CAR NK cells over 12 days (n = 6; mean ± SD). P values were calculated using a 2-way ANOVA; ∗P < .05; ∗∗P < .01; ∗∗∗P = .001; ∗∗∗∗P < .0001. (C) Relative percentage of CD38-CAR expression (n = 6; mean ± SD). (D) Reverse-transcription qPCR was performed using CD38 primer probes to detect transcription of the CD38 gene in the WT and AAVS1KO/CD38-CAR NK cells. (E) CD38 expression measured by flow cytometry using a polyclonal anti-CD38 antibody.
CD38KO in CD38-CAR NK cells is not necessary for increased cytotoxicity and metabolism
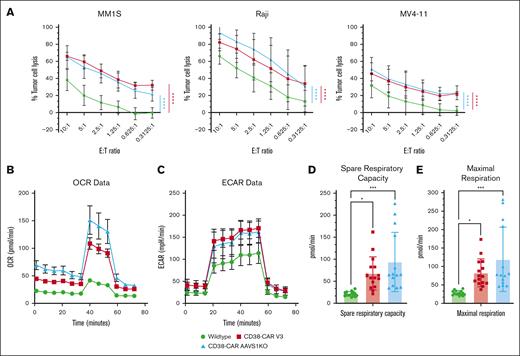
The function of CAR NK cells in both insertion sites (CD38KO and the AAVS1KO) was analyzed by examining cytotoxicity and metabolism in these cells compared with WT NK cells. Cytotoxicity assays were performed against MM1S, Raji, and MV4-11. Regardless of the insertion site, both CAR NK cells had significantly higher killing than WT NK cells against CD38+ MM, BL, and AML cell lines (Figure 7A). In addition, both CAR NK cells had significantly higher rates of oxidative phosphorylation than WT NK cells (Figure 7B). The AAVS1KO/CD38-CAR NK cells had a higher basal respiration rate and maximal respiration capacity than the CD38KO/CD38-CAR NK cells (Figure 7B; supplemental Figure 5). Extracellular acidification rates for both CARs, regardless of the insertion site, were higher than those of WT NK cells, which indicated that the CAR NK cells perform glycolysis at a faster rate and have a higher overall glycolytic capacity (Figure 7C). In addition, CD38-CAR NK cells, regardless of the insertion site, had significantly higher spare respiratory capacity than WT NK cells (Figure 7D). Both CAR conditions also displayed significantly higher maximal respiration than WT NK cells (Figure 7E). Overall, these data suggest that CD38 inhibition is present in both CD38-CAR NK products, resulting in increased metabolic fitness and flexibility.
AAVS1KO/CD38-CAR NK cells display enhanced cytotoxicity and metabolism. CD38-CAR NK cells were tested against CD38-expressing AML, MM, and BL. (A) CD38-CAR NK-cell killing of CD38-expressing cell lines (n = 3; mean ± SD). P values were calculated using a 2-way ANOVA; ∗P < .05; ∗∗P < .01; ∗∗∗P = .001; ∗∗∗∗P < .0001. (B) Oxygen consumption rate (OCR) for CD38-CAR NK cells. (C) Glycolytic capacity, measured by the extracellular acidification rate (ECAR), observed in CD38-CAR NK cells. (D) Spare respiratory capacity, a measure of the cell's ability to produce adenosine triphosphate (ATP) in response to stress, measured in CAR NK cells. (E) CAR NK-cell maximal respiration rates compared with WT.
AAVS1KO/CD38-CAR NK cells display enhanced cytotoxicity and metabolism. CD38-CAR NK cells were tested against CD38-expressing AML, MM, and BL. (A) CD38-CAR NK-cell killing of CD38-expressing cell lines (n = 3; mean ± SD). P values were calculated using a 2-way ANOVA; ∗P < .05; ∗∗P < .01; ∗∗∗P = .001; ∗∗∗∗P < .0001. (B) Oxygen consumption rate (OCR) for CD38-CAR NK cells. (C) Glycolytic capacity, measured by the extracellular acidification rate (ECAR), observed in CD38-CAR NK cells. (D) Spare respiratory capacity, a measure of the cell's ability to produce adenosine triphosphate (ATP) in response to stress, measured in CAR NK cells. (E) CAR NK-cell maximal respiration rates compared with WT.
Discussion
Our study reports the generation and in vitro evaluation of CD38-CAR NK cells as potential immunotherapeutics against a broad range of hematologic malignancies. We developed two unique CD38-CAR constructs with isatuximab-based scFv, CD3ζ and 41BB costimulatory domains, and a CD8α transmembrane, having reversed heavy and light chain orientations. Both constructs were evaluated for CAR expression, proliferation, and cytolytic activity. After determining scFv constructs were comparable, we probed the cytolytic capacity, cytokine production, and metabolism of the V3 CD38KO/CD38-CAR NK cells. We additionally explored ATRA for enhancing the expression of CD38 density on tumor cells and thereby improving CD38-CAR NK-cell killing.
CD38-targeting mAbs (eg, isatuximab and daratumumab) are effective and well-tolerated therapies for MM.25 There is, however, an unmet need for novel treatments for relapsed/refractory T-cell lymphomas, BL, and AML,26-28 which also express CD38. We explored the feasibility of engineering NK cells with an anti-CD38 CAR construct to improve specificity and augment their innate killing potential toward these hematologic malignancies.27,29 CD38 mAbs induce fratricide among NK cells, but previous work by our laboratory and others showed that CD38KO NK cells avoided CD38 mAb-mediated fratricide.15,30 Here, we inserted the isatuximab-based CAR into the CD38 locus in NK cells, creating a simultaneous knockout/knockin. The CD38KO/CD38-CAR NK cells are fratricide resistant, proliferate comparable with WT NK cells, and show enhanced killing toward cell lines and primary patient tumor cells of a wide range of hematologic malignancies. CD38-CAR NK cells displayed increased production of proinflammatory cytokines (interferon gamma and tumor necrosis factor α), granulocyte-macrophage colony-stimulating factor, and proinflammatory chemokines (monocyte chemoattractant protein 1, macrophage inflammatory protein 1α, and RANTES), which are released by activated NK cells to promote immune cell recruitment and maturation.22,31,32
ATRA has previously been reported to reverse anti-CD38 resistance to daratumumab by upregulating CD38 expression on tumor cells.33 We used ATRA to enhance CD38 expression on AML, MM, BL, and T-ALL to improve the sensitivity of tumor cells to CD38-CAR–mediated lysis in an isatuximab-based therapy. ATRA induced the greatest increases in surface CD38 expression in immature, CD38-low tumor cell lines, including AML-10, MV4-11, and AML-1. Additionally, primary patient AML cells (AML-10 and AML-1) with lower CD38 surface density displayed a significant increase in cell lysis when cotreated with ATRA and CD38-CAR NK cells. Mass cytometry revealed combination treatments of ATRA and CD38-CAR NK cells against primary patient AML displayed the greatest decreases in live AML populations. The combination therapy also showed a significant decrease in the population of quiescent AML cells that are responsible for the progression and maintenance of the leukemia clone, a population involved in residual disease and relapse.34 The therapeutic potential of an ATRA and CD38-CAR NK-cell combination therapy may be most important in targeting CD38-low disease.
Previously published findings revealed the efficacy of CD38-targeted CAR T-cell therapies in AML, MM, and T-ALL, establishing their broad impact.21 To provide a benchmark for the relative potency of CAR NK cells compared with the better-understood CAR T-cell population, we performed the same CRISPR/Cas9 AAV gene-engineering approach on T cells from matched donors. CD38-CAR T cells displayed lower cytotoxicity toward the tumor cell lines AML, MM, and BL across all E:T ratios than CAR NK cells. CD38-CAR T cells also displayed no cytotoxic function against the CD38-low expressing AML cell line MV4-11.
Although CD38 knockout is necessary to avoid fratricide in NK cells among CD38 mAbs,15 CD38-CAR NK cells displayed fratricide resistance regardless of surface CD38 expression. AAVS1KO/CD38-CAR NK cells had intact CD38 gene expression and surface expression measured with a polyclonal antibody but undetected with a mAb, which could be explained by steric hindrance through cis interactions masking CD38 epitopes. Sterically blocking the detection of CD38 from surrounding cells would allow the CAR NK cells to avoid fratricide without a CD38KO. Both CD38KO and AAVS1KO CD38-CAR NK cells showed similar cytotoxicity toward CD38+ malignancies. Additionally, both CD38-CAR NK cells, regardless of CD38KO or AAVS1KO, had increased metabolic function compared with conventional NK cells, suggesting that CD38, even if expressed, is not functional. Unlike daratumumab, isatuximab’s binding site mediates inhibition of CD38 metabolic activity,23 which may also be the mechanism for metabolic enhancement of this isatuximab-derived CAR. In summary, CD38 deletion may not be crucial for the generation of metabolically enhanced, fratricide-resistant CD38-CAR NK-cell therapy. Further studies will be needed to confirm the mechanism by which CD38-CAR NK cells avoid fratricide while still expressing CD38.
Acknowledgments
This work was supported by grants from the Nationwide Children’s Hospital Research Institute (grants AWD00001677 and 45306-0015-1222) and Cancer Free Kids (grant 810753-1222-00).
Authorship
Contribution: E.T., Y.S., M.S.F.P., J.C., and J.L. performed the research; G.B., D.A.L., and M.N.K. provided vital reagents; and E.T., M.N.K., and D.A.L. designed the research, analyzed the data, and wrote the manuscript.
Conflict-of-interest disclosure: E.T., D.A.L., M.N.K., and Y.S. have a provisional patent for the described technology. D.A.L., M.N.K., and M.S.F.P. have received royalties from Sanofi/Kiadis. The remaining authors declare no competing financial interests.
Correspondence: Meisam Naeimi Kararoudi, Center for Childhood Cancer and Blood Diseases, Abigail Wexner Research Institute at Nationwide Children’s Hospital, 700 Children's Dr, Columbus, OH 43205; email: meisam.naeimikararoudi@nationwidechildrens.org.
References
Author notes
D.A.L. and M.N.K. contributed equally as principal investigators in this study.
Presented as an oral presentation at the American Society of Hematology (ASH) Summit on Immunotherapies for Hematologic Disease, Washington, DC, 3 March 2023 and presented in abstract form at the 65th annual meeting of ASH, San Diego, CA, 10 December 2023.
Original data are available on request from the corresponding author, Meisam Naeimi Kararoudi (meisam.naeimikararoudi@nationwidechildrens.org).
The full-text version of this article contains a data supplement.

![Generation of CRISPR-engineered CD38KO/CD38-CAR human primary NK cells using Cas9/RNP and AAV. (A) Schemata of steps for CRISPR/RNP knockout of the CD38 gene and directed insertion of a CD38-CAR encoding DNA delivered by AAV6 vector with homology arms for CD38 targeting site. (B) CD38 (PE) and CAR (APC) expression levels measured by flow cytometry for binding of CD38 antigen, 7 days after stimulation. Constructs contain a 41BB signaling domain, a CD8α transmembrane domain/hinge, a CD3ζ stimulatory domain, and reversed orderings of light and heavy chain orientations. (C) Relative percentage and intensity of CD38-CAR expression (n = 10; mean ± standard deviation [SD]). (D) Fold expansion of WT and CD38-CAR NK cells over 12 days after activation with irradiated, modified mbIL21-K562 cells and IL-2 show no significant change from WT human NK cells (n = 10; mean ± SD). P values were calculated using a 2-way analysis of variance (ANOVA); ∗P = .0332; ∗∗P = .0021; ∗∗∗P = .0002; ∗∗∗∗P < .0001. (E) Cytotoxicity observed for V3 and V4 CD38KO/CD38-CAR NK cells against high CD38-expressing MM (H929), BL (Raji), and AML (MV-11) (n = 5; mean ± SD). P values were calculated using a 2-way ANOVA; ∗P < .05; ∗∗P < .01; ∗∗∗P = .001; ∗∗∗∗P < .0001.](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodneoplasia/1/4/10.1016_j.bneo.2024.100032/2/m_bneo_neo-2024-000186-gr1.jpeg?Expires=1769087900&Signature=RlpDLFakZTFPXGcBchqhlJ8h7oWv92jW1bXcSCJWOO~SQBO9VssAmL6qK70Y~zisguUqFHpRDl6SBHbVZLMcfBsxeKLRooLXv0Bzk9HD6TbK5hSms03DXLAozlAOFjWkhC6~5NDYO-j91xDbKq8oZi4NYALWR~vHmRhGpmB1eQcgtEwLAZiiLHYNO6L7FLkoYrcaDJhlqIQtcR3p-waKPhB-vZbNziBWTyjp7fArLf6HuOWX7Kl1n58kTKBwBtPAaCHVOs2nJBth4fu5WWbmAeo-jyKJx2eL7gs1~b5YqG1LVrmNFkpt8mN5bcOoTG~Qt3NsYLgxbg3s~UK7XCZCBw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![CD38-CAR NK cells exhibit enhanced cytotoxic function and cytokine secretion. CD38-CAR NK cells were tested against CD38-expressing AML, MM, and T-cell malignancies collected from patients at baseline. (A) NK and CD38-CAR NK killing of AML-1 (n = 3; mean ± SD). (B) NK and CD38-CAR NK killing of samples from patients with MM (MM-1 and MM-2; n = 1; mean ± SD). (C) NK and CD38-CAR NK killing of T-cell malignancies (hepatosplenic T-cell lymphoma and T-PLL [T-PLL-1 and T-PLL-2]; n = 1; mean ± SD). All cytotoxicity P values were calculated using a 2-way ANOVA; ∗P < .05; ∗∗P < .01; ∗∗∗P = .001; ∗∗∗∗P < .0001. (D) Bio-Plex Pro Human Cytokine assay was performed on the supernatant of WT and CD38-CAR NK cells cocultured with CD38+ malignancies (n = 9; mean ± SD). P values were calculated using a paired Student t test; ∗P = .05; ∗∗P = .01; ∗∗∗P = .001. GM-CSF, IFN-γ, MCP-1, MIP-1α, TNF-α.](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodneoplasia/1/4/10.1016_j.bneo.2024.100032/2/m_bneo_neo-2024-000186-gr2.jpeg?Expires=1769087900&Signature=Vq5DSh9ZTLCprl2G0MsYpHpPfknMuEjBXoI09JfGug-7oDglwehssMwr~esVo0vGV8~XBCGjfW8h1MJjyS~PsPdKt04l3ummFM7RzBy-aY-wxaNF-NNLyFF9BoT1mZAVkzN~aL81ojVnW8~56MqZVE~5uKYDd6l4YHMcH6JWCC3mT9wxY9Pnoc9qULgMBUupFNpuDy3IghphQ5FyXYLP7YutkEdf1LcTK8-ONc4PPVZc9X4M6z~0-mQasMVDuWKTvOEo1vRYJAs3HiOKyz79MiTVYMCJXTjaO48uj39fj1viBJcIuXYkrZp5MSi-DRO8s1wtyGkifhDkQhpf-f81~g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)