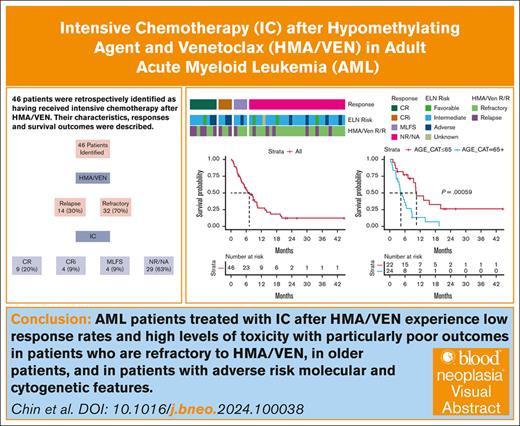
Key Points
After HMA/VEN treatment, patients with AML treated with IC had a CR/CRi rate of 28% and mOS of 7.2 months.
Patients aged >65 years performed poorly with IC after HMA/VEN, achieving a mOS of 4.3 months (vs 10.6 months for younger patients).
Visual Abstract
The combination of a hypomethylating agent (HMA) and venetoclax (VEN) is approved for adults aged >75 years with newly diagnosed acute myeloid leukemia (AML) as well as those ineligible for intensive chemotherapy (IC). HMA/VEN is increasingly substituted for IC in adults with AML aged <75 years, particularly in those with adverse cytogenetic and molecular features. When patients fail to respond or relapse after HMA/VEN, the utility of salvage IC is largely unknown. We performed a retrospective single-institution study and identified 46 patients who received IC after HMA/VEN, including 24 patients who received HMA/VEN as their first treatment for AML. This population had complete remission (CR)/CR with incomplete count recovery (CRi)/morphologic leukemia-free state rate of 37%, CR/CRi rate of 28%, and a median overall survival (mOS) of 7.2 months (95% confidence interval, 5.0-10.3). Patients who relapsed after an initial response to HMA/VEN and subsequently received IC were more likely to achieve a CR/CRi than those refractory to HMA/VEN (50% vs 19%; P = .04), although there was no statistically significant difference in survival (mOS, 8.8 vs 5.4 months; P = .64). Age >65 years predicted poorer survival (mOS, 4.3 vs 10.6 months; P < .001). IC after HMA/VEN should be further studied and chosen with caution.
Introduction
The hypomethylating agents (HMAs) azacitidine (AZA) and decitabine in combination with the B-cell lymphoma 2 (BCL2) inhibitor venetoclax (VEN) are approved for patients with newly diagnosed acute myeloid leukemia (AML) aged >75 years or who have comorbidities that preclude the use of intensive chemotherapy (IC) for induction.1 This approval is largely based on the results of the VIALE-A trial, a randomized, placebo-controlled, multicenter study, in which patients who received AZA/VEN had improved overall survival (OS) over patients who received AZA and placebo.2 Increasingly, HMA/VEN is being applied in other contexts. First, HMA/VEN is frequently used as a salvage regimen for relapsed or refractory (R/R) AML after IC, with variable response rates reported in retrospective studies featuring heterogeneous populations.3-5 Additionally, patients with poor risk cytogenetics and/or TP53 mutations are often treated with HMA/VEN instead of IC in the upfront setting, based on the results of single-arm studies demonstrating complete remission (CR)/CR with incomplete count recovery (CRi) rates of 40% to 70%.6,7 There are also emerging retrospective data supporting the use of HMA/VEN as an alternative frontline therapy to IC in subtypes of AML thought to be similarly responsive to HMA/VEN, such as secondary AML.8 The prospect of comparable or improved OS with HMA/VEN has led to randomized trials directly comparing the 2 approaches in newly diagnosed fit adults.9 In addition, some centers are already choosing HMA/VEN as frontline therapy for patients as young as 60 years.10
There is currently no standard of care after HMA/VEN failure in the frontline or R/R setting. Because HMA/VEN is increasingly used in the frontline setting for patients fit for IC, the best salvage approach after such failures will become an increasingly important question. Several retrospective studies have documented overall poor outcomes for patients who do not achieve CR/CRi with HMA/VEN. In a series of 71 patients with AML with R/R disease after frontline HMA/VEN described by Gangat et al, only 11 patients (15%) received a salvage regimen (primarily targeted therapies) with a CR rate of 27% and median OS (mOS) of <6 months from the time of HMA/VEN initiation.11 In a different series of 41 patients with R/R AML after frontline HMA/VEN from Maiti et al, 58% received some form of salvage therapy (IC, targeted therapies, and experimental agents), but the OS was only 2.4 months from the time of HMA/VEN failure.12
One unanswered question is whether there is utility in administering IC after HMA/VEN failure in patients who are medically fit. This question will become commonplace as HMA/VEN is increasingly used in young and fit patients with AML. Gangat et al11 only reported a single patient who received IC after HMA/VEN, and Maiti et al12 reported a CR/CRi rate of 25% based on 8 patients receiving this sequence of treatment. Given the limited data available, we performed a single-center retrospective cohort study to identify the characteristics and outcomes of patients who received IC after HMA/VEN for R/R AML.
Methods
Patients
We performed a retrospective cohort study among patients with AML who received IC after receipt of HMA/VEN at Memorial Sloan Kettering from 2017 to 2023. The IC regimens used in our cohort were as follows: 7 + 3 (daunorubicin + cytarabine), FLAG (fludarabine + high-dose cytarabine + granulocyte colony-stimulating factor [G-CSF]), FLAG-IDA (FLAG + idarubicin), GCLAC (G-CSF, clofarabine, and cytarabine), HiDAC (high-dose cytarabine), and MEC (mitoxantrone, etoposide, and cytarabine). Patients were included regardless of which line of therapy HMA/VEN had been and regardless of the time elapsed between HMA/VEN and IC. Once patients were identified, relevant patient and disease characteristics as well as treatment histories and outcomes were extracted from the medical record through detailed chart review. Prior therapies for antecedent diagnoses to AML such as myelodysplastic syndrome (MDS) or myeloproliferative neoplasm (MPN) were identified and reported.
Definitions
AML diagnosis, subtype, risk classification, and response were defined based on European LeukemiaNet (ELN) 2022 criteria regardless of the year of diagnosis.13 Composite CR was defined as the combination of CR and CRi. Overall response rate (ORR) was defined as the combination of CR, CRi, and morphologic leukemia-free state (MLFS). Measurable residual disease (MRD) was determined based on multiparameter flow cytometry at a cutoff of 10–4 according to leukemia-associated immunophenotype and/or different from normal aberrant immunophenotype approaches. Consistent with ELN 2022, patients with AML-defining recurrent genetic abnormalities or other specific genetic abnormalities without 20% blasts were included as AML or MDS/AML provided they also had received HMA/VEN as treatment. The time of OS was defined starting from the date of initiation of IC to the date of last follow-up or event, with censoring as appropriate.
Statistical analysis
Baseline demographic and clinical characteristics are reported descriptively. Fisher exact tests and Student t tests were used for significance testing of categorical and continuous data, respectively. Kaplan-Meier methodology was incorporated to estimate survival statistics. P values were 2-sided, and a significance level of .05 was used. All analyses were performed using R 4.2.3 (CRAN Project; www.cran.r-project.org).
Ethical considerations
This project was approved by the Institutional Review Board of Memorial Sloan Kettering Cancer Center. This study was conducted in accordance with the Declaration of Helsinki.
Results
Characteristics of patients who received IC after HMA/VEN
We identified 46 patients who received IC after prior HMA/VEN, including patients for whom HMA/VEN was given as the first treatment for AML (1L) as well as subsequent lines of therapy (2L+; Table 1; supplemental Figure 1). The median age of this group was 65 years, with an interquartile range of 50 to 68 years. Thirty-seven patients (80%) had AML, and 9 patients (20%) had MDS/AML by 2022 ELN classification at the time of initial therapy. Most patients (67%) were classified as adverse risk based on 2022 ELN classification by cytogenetic or molecular profile. HMA/VEN was the first line of AML treatment for 24 patients (52%), whereas 22 patients (48%) had received other treatments for AML before HMA/VEN. Including treatments for antecedent diagnoses such as MDS, MPN, or MDS/MPN overlap; 33% of patients had previously received an allogeneic stem cell transplant (alloSCT), and 20% of patients had received HMA monotherapy before HMA/VEN. Almost all patients who had received AML therapy before HMA/VEN received IC (n = 21 [95%]; supplemental Table 1). Of those 21 patients, 20 received 7 + 3 or Vyxeos, and 1 received FLAG. Three of them received midostaurin with 7 + 3, and 1 received ivosidenib with 7 + 3. Eighteen (86%) were able to achieve an initial CR.
Characteristics of patients with AML who received IC after HMA and VEN
| . | All (N=46) . |
|---|---|
| Sex, n (%) | |
| Female | 20 (43) |
| Male | 26 (57) |
| Age, median (interquartile range), y | 65 (50-68) |
| Diagnosis, n (%) | |
| AML | 37 (80) |
| MDS/AML | 9 (20) |
| AML classification, n (%) | |
| Recurrent genetic abnormality | 13 (28) |
| TP53 (mutated or lost) | 8 (17) |
| Myelodysplasia-related gene mutation | 14 (30) |
| Myelodysplasia-related cytogenetic abnormality | 5 (11) |
| Not otherwise specified | 6 (13) |
| AML qualifier, n (%) | |
| Therapy-related | 7 (15) |
| Prior MDS or MDS/MPN | 19 (41) |
| Germ line predisposition | 1 (2) |
| None | 19 (41) |
| ELN 2022 risk classification, n (%) | |
| Favorable | 4 (9) |
| Intermediate | 10 (22) |
| Adverse | 31 (67) |
| Unknown | 1 (2) |
| VEN combination, n (%) | |
| AZA | 37 (80) |
| Decitabine | 9 (20) |
| HMA/VEN line of treatment, n (%) | |
| 1L | 24 (52) |
| 2L+ | 22 (48) |
| Prior HMA, n (%) | 9 (20) |
| Prior IC, n (%) | 21 (46) |
| Prior transplant, n (%) | 15 (33) |
| HMA/VEN cycles, median (interquartile range) | 2 (2-4) |
| HMA/VEN response, n (%) | |
| CR | 6 (13) |
| CRi | 5 (11) |
| MLFS | 3 (7) |
| PR | 1 (2) |
| NR | 31 (67) |
| . | All (N=46) . |
|---|---|
| Sex, n (%) | |
| Female | 20 (43) |
| Male | 26 (57) |
| Age, median (interquartile range), y | 65 (50-68) |
| Diagnosis, n (%) | |
| AML | 37 (80) |
| MDS/AML | 9 (20) |
| AML classification, n (%) | |
| Recurrent genetic abnormality | 13 (28) |
| TP53 (mutated or lost) | 8 (17) |
| Myelodysplasia-related gene mutation | 14 (30) |
| Myelodysplasia-related cytogenetic abnormality | 5 (11) |
| Not otherwise specified | 6 (13) |
| AML qualifier, n (%) | |
| Therapy-related | 7 (15) |
| Prior MDS or MDS/MPN | 19 (41) |
| Germ line predisposition | 1 (2) |
| None | 19 (41) |
| ELN 2022 risk classification, n (%) | |
| Favorable | 4 (9) |
| Intermediate | 10 (22) |
| Adverse | 31 (67) |
| Unknown | 1 (2) |
| VEN combination, n (%) | |
| AZA | 37 (80) |
| Decitabine | 9 (20) |
| HMA/VEN line of treatment, n (%) | |
| 1L | 24 (52) |
| 2L+ | 22 (48) |
| Prior HMA, n (%) | 9 (20) |
| Prior IC, n (%) | 21 (46) |
| Prior transplant, n (%) | 15 (33) |
| HMA/VEN cycles, median (interquartile range) | 2 (2-4) |
| HMA/VEN response, n (%) | |
| CR | 6 (13) |
| CRi | 5 (11) |
| MLFS | 3 (7) |
| PR | 1 (2) |
| NR | 31 (67) |
PR, partial remission; NR, no response.
Responses to HMA/VEN before IC
Patients were then divided into 2 groups based on their prior response to HMA/VEN before IC treatment. Of the 46 patients, 32 patients (70%) were refractory to HMA/VEN, defined as never achieving CR/CRi/MLFS with treatment (Table 2). The other 14 patients (30%) achieved CR/CRi/MLFS initially but subsequently relapsed, necessitating further treatment. Patients who responded and then subsequently relapsed received a median of 4.5 cycles, compared with 2 cycles among patients refractory to HMA/VEN (P = .01). There were no statistically significant differences in the underlying AML classification, including ELN 2022 risk classification, between the 2 groups.
Characteristics of patients with AML who received IC after HMA and VEN stratified by prior response to HMA/VEN
| . | HMA/VEN response . | P . | |
|---|---|---|---|
| Refractory . | Relapse∗ . | ||
| No. of patients, n (%) | 32 (70) | 14 (30) | |
| Sex, n (%) | |||
| Female | 12 (38) | 8 (57) | .33 |
| Male | 20 (63) | 6 (43) | |
| Age, median (interquartile range) | 65 (53-68) | 64 (41-68) | .41 |
| Diagnosis, n (%) | |||
| AML | 26 (81) | 11 (79) | 1 |
| MDS/AML | 6 (19) | 3 (21) | |
| AML classification, n (%) | |||
| Recurrent genetic abnormality | 7 (22) | 6 (43) | .63 |
| TP53 (mutated or lost) | 6 (19) | 2 (14) | |
| Myelodysplasia-related gene mutation | 11 (34) | 3 (21) | |
| Myelodysplasia-related cytogenetic abnormality | 3 (9) | 2 (14) | |
| Not otherwise specified | 5 (16) | 1 (7) | |
| AML qualifier, n (%) | |||
| Therapy related | 4 (13) | 3 (21) | .83 |
| Prior MDS or MDS/MPN | 14 (44) | 5 (36) | |
| Germ line predisposition | 1 (3) | 0 (0) | |
| None | 13 (41) | 6 (43) | |
| ELN 2022 risk classification, n (%) | |||
| Favorable | 1 (3) | 3 (21) | .20 |
| Intermediate | 7 (22) | 3 (21) | |
| Adverse | 23 (72) | 8 (57) | |
| Unknown | 1 (3) | 0 (0) | |
| VEN combination, n (%) | |||
| AZA | 24 (75) | 13 (93) | .24 |
| Decitabine | 8 (25) | 1 (7) | |
| HMA/VEN line of treatment, n (%) | |||
| 1 | 19 (59) | 5 (36) | .20 |
| 2+ | 13 (41) | 9 (64) | |
| Prior HMA, n (%) | 7 (22) | 2 (14) | .7 |
| Prior IC, n (%) | 13 (41) | 8 (57) | .35 |
| Prior Transplant, n (%) | 4 (29) | 11 (34) | 1 |
| HMA/VEN cycles, median (interquartile range) | 2 (1-2) | 4.5 (2-6) | .01 |
| HMA/VEN response, n (%) | |||
| CR | 0 (0) | 6 (43) | N/A |
| CRi | 0 (0) | 5 (36) | |
| MLFS | 0 (0) | 3 (21) | |
| PR | 1 (3) | 0 (0) | |
| NR | 31 (97) | 0 (0) | |
| . | HMA/VEN response . | P . | |
|---|---|---|---|
| Refractory . | Relapse∗ . | ||
| No. of patients, n (%) | 32 (70) | 14 (30) | |
| Sex, n (%) | |||
| Female | 12 (38) | 8 (57) | .33 |
| Male | 20 (63) | 6 (43) | |
| Age, median (interquartile range) | 65 (53-68) | 64 (41-68) | .41 |
| Diagnosis, n (%) | |||
| AML | 26 (81) | 11 (79) | 1 |
| MDS/AML | 6 (19) | 3 (21) | |
| AML classification, n (%) | |||
| Recurrent genetic abnormality | 7 (22) | 6 (43) | .63 |
| TP53 (mutated or lost) | 6 (19) | 2 (14) | |
| Myelodysplasia-related gene mutation | 11 (34) | 3 (21) | |
| Myelodysplasia-related cytogenetic abnormality | 3 (9) | 2 (14) | |
| Not otherwise specified | 5 (16) | 1 (7) | |
| AML qualifier, n (%) | |||
| Therapy related | 4 (13) | 3 (21) | .83 |
| Prior MDS or MDS/MPN | 14 (44) | 5 (36) | |
| Germ line predisposition | 1 (3) | 0 (0) | |
| None | 13 (41) | 6 (43) | |
| ELN 2022 risk classification, n (%) | |||
| Favorable | 1 (3) | 3 (21) | .20 |
| Intermediate | 7 (22) | 3 (21) | |
| Adverse | 23 (72) | 8 (57) | |
| Unknown | 1 (3) | 0 (0) | |
| VEN combination, n (%) | |||
| AZA | 24 (75) | 13 (93) | .24 |
| Decitabine | 8 (25) | 1 (7) | |
| HMA/VEN line of treatment, n (%) | |||
| 1 | 19 (59) | 5 (36) | .20 |
| 2+ | 13 (41) | 9 (64) | |
| Prior HMA, n (%) | 7 (22) | 2 (14) | .7 |
| Prior IC, n (%) | 13 (41) | 8 (57) | .35 |
| Prior Transplant, n (%) | 4 (29) | 11 (34) | 1 |
| HMA/VEN cycles, median (interquartile range) | 2 (1-2) | 4.5 (2-6) | .01 |
| HMA/VEN response, n (%) | |||
| CR | 0 (0) | 6 (43) | N/A |
| CRi | 0 (0) | 5 (36) | |
| MLFS | 0 (0) | 3 (21) | |
| PR | 1 (3) | 0 (0) | |
| NR | 31 (97) | 0 (0) | |
Relapsed AML after previously documented CR/CRi/MLFS with HMA/VEN for AML.
Choice of IC regimens and responses to IC
Among the entire cohort of 46 patients, IC regimens varied widely after HMA/VEN and depended on previous therapy (Table 3; supplemental Table 2). The most common regimens were FLAG-IDA in 14 patients (30%), MEC in 10 patients (22%), and 7 + 3 in 9 patients (20%). The distribution of IC regimens was not statistically different between those who were refractory to HMA/VEN vs those who relapsed after achieving composite CR (P = .31). Two patients received IC in combination with a targeted therapy (1 patient received 7 + 3 + midostaurin, and 1 patient received MEC + crenolanib).
IC regimens and responses after HMA and VEN by HMA/VEN response
| . | All . | HMA/VEN response . | P . | |
|---|---|---|---|---|
| Refractory . | Relapse . | |||
| All | 46 (100) | 32 (70) | 14 (30) | |
| IC regimen∗ , n (%) | ||||
| 7 + 3 | 9 (20) | 8 (25) | 1 (7) | .31 |
| Liposomal daunorubicin/cytarabine | 7 (15) | 5 (16) | 2 (14) | |
| FLAG | 2 (4) | 2 (6) | 0 (0) | |
| FLAG-IDA | 14 (30) | 9 (28) | 5 (36) | |
| GCLAC | 2 (4) | 0 (0) | 2 (14) | |
| HiDAC | 2 (4) | 1 (3) | 1 (7) | |
| MEC | 10 (22) | 7 (22) | 3 (21) | |
| Response, n (%) | ||||
| CR | 9 (20) | 4 (13) | 5 (36) | 0.25 |
| CRi | 4 (9) | 2 (6) | 2 (14) | |
| MLFS | 4 (9) | 3 (9) | 1 (7) | |
| NR | 26 (57) | 20 (63) | 6 (43) | |
| Not assessed | 3 (7) | 3 (9) | 0 (0) | |
| CR/CRi/MLFS, n (%) | 17 (37) | 9 (28) | 8 (57) | .10 |
| CR/CRi, n (%) | 13 (28) | 6 (19) | 7 (50) | .04 |
| CR/CRi MRD negative, n (%) | 6 (13) | 3 (9) | 3 (21) | .35 |
| OS, median (95% CI), mo | 7.2 (5-10.3) | 5.4 (3.2-14.1) | 8.8 (5.8 to NA) | .64 |
| . | All . | HMA/VEN response . | P . | |
|---|---|---|---|---|
| Refractory . | Relapse . | |||
| All | 46 (100) | 32 (70) | 14 (30) | |
| IC regimen∗ , n (%) | ||||
| 7 + 3 | 9 (20) | 8 (25) | 1 (7) | .31 |
| Liposomal daunorubicin/cytarabine | 7 (15) | 5 (16) | 2 (14) | |
| FLAG | 2 (4) | 2 (6) | 0 (0) | |
| FLAG-IDA | 14 (30) | 9 (28) | 5 (36) | |
| GCLAC | 2 (4) | 0 (0) | 2 (14) | |
| HiDAC | 2 (4) | 1 (3) | 1 (7) | |
| MEC | 10 (22) | 7 (22) | 3 (21) | |
| Response, n (%) | ||||
| CR | 9 (20) | 4 (13) | 5 (36) | 0.25 |
| CRi | 4 (9) | 2 (6) | 2 (14) | |
| MLFS | 4 (9) | 3 (9) | 1 (7) | |
| NR | 26 (57) | 20 (63) | 6 (43) | |
| Not assessed | 3 (7) | 3 (9) | 0 (0) | |
| CR/CRi/MLFS, n (%) | 17 (37) | 9 (28) | 8 (57) | .10 |
| CR/CRi, n (%) | 13 (28) | 6 (19) | 7 (50) | .04 |
| CR/CRi MRD negative, n (%) | 6 (13) | 3 (9) | 3 (21) | .35 |
| OS, median (95% CI), mo | 7.2 (5-10.3) | 5.4 (3.2-14.1) | 8.8 (5.8 to NA) | .64 |
95% CI, 95% confidence interval.
7 + 3, daunorubicin + cytarabine; FLAG, fludarabine + high-dose cytarabine + granulocyte colony-stimulating factor [G-CSF]; FLAG-IDA, FLAG + idarubicin; GCLAC, G-CSF, clofarabine, and cytarabine; HiDAC, high-dose cytarabine; MEC, mitoxantrone, etoposide, cytarabine.
The response to IC after HMA/VEN was distributed as follows: 20% of patients achieved a CR, 9% CRi, 9% MLFS, 57% had no response, and 6% (n = 3) were not evaluable for response due to early death (Table 3). The ORR was 37%. Responses were determined after the first cycle of IC. Four patients with no response to IC did receive alternative IC as their next therapy, with 1 patient achieving a brief MRD-positive CR before relapse (supplemental Figure 1). ORR was numerically higher for those who relapsed after HMA/VEN (as opposed to those refractory to HMA/VEN), but this was not statistically significantly (57% vs 28%; P = .10). The CR/CRi rate for the cohort was 28%; this was significantly higher among those who relapsed after HMA/VEN (50%) than those who were refractory to HMA/VEN (19%; P = .04). Thirteen percent of patients achieved MRD-negative CR/CRi, including 21% of relapsed and 9% of refractory patients (P = .35). Response rates were not statistically different for the various IC regimens (supplemental Table 3). The 7 + 3–based regimens (7 + 3 and Vyxeos) had a CR/CRi rate of 25% and ORR of 31%, whereas HiDAC-based regimens had a CR/CRi rate of 30% and ORR of 40% (P = 1 and .75, respectively).
Survival after IC and predictors of survival
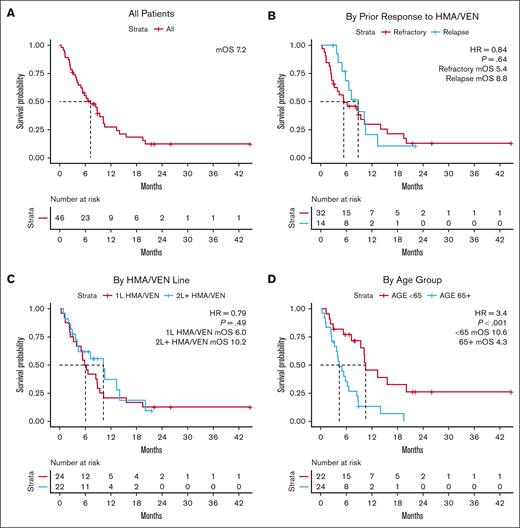
The OS for the entire cohort was 7.2 months from the start of IC (95% confidence interval, 5-10.3) at a median follow-up of 22.2 months (Figure 1A; Table 3). This was not statistically different among patients who were refractory to HMA/VEN and those who relapsed after achieving response with HMA/VEN, although the mOS numerically favored the relapsed group (Figure 1B). The line of therapy in which HMA/VEN was applied also was not associated with statistically significant differences when it came to OS, even though 95% of patients receiving HMA/VEN in 2L+ had previously received IC (Figure 1C). Notably, younger patients aged <65 years had superior mOS of 10.6 months compared with that of patients aged >65 years (4.3 months; P < .001; Figure 1D).
Survival outcomes associated with IC after HMA and VEN. (A) OS measured relative to the start of IC for all patients receiving IC after HMA/VEN. (B) OS relative to the start of IC stratified based on prior response to HMA/VEN. (C) OS relative to the start of IC stratified by the line of therapy in which HMA/VEN was used. (D) OS relative to the start of IC stratified based on age over or under 65 years. HR, hazard ratio.
Survival outcomes associated with IC after HMA and VEN. (A) OS measured relative to the start of IC for all patients receiving IC after HMA/VEN. (B) OS relative to the start of IC stratified based on prior response to HMA/VEN. (C) OS relative to the start of IC stratified by the line of therapy in which HMA/VEN was used. (D) OS relative to the start of IC stratified based on age over or under 65 years. HR, hazard ratio.
Outcomes associated with age group and transplant
In the group with patients aged 65+ (n = 24), 7 patients (29%) achieved CR/CRi/MLFS; and of those 7, 4 underwent alloSCT, whereas the other 3 relapsed before transplant (supplemental Table 4). Nine patients (38%) did not receive any further therapy due to death, including 2 who had 30-day mortality events and 4 who had 60-day mortality events. Specifically, 6 of the 9 had evidence of active leukemia from peripheral or bone marrow assessments before death, and 3 of them were pancytopenic without marrow assessment before death. Those in the group with age 65+ years who were able to proceed to transplant had a mOS of 10.4 months compared with 4 months for those who did not (P = .14; supplemental Figure 2A).
In those aged <65 years, 10 patients (45%) achieved CR/CRi/MLFS, and 5 of them proceeded to alloSCT. The 5 patients who did not proceed to alloSCT either saw rapid relapse (n = 3) or had already received multiple alloSCTs (n = 2). Of note, 3 patients who did not achieve CR/CRi/MLFS proceeded to alloSCT with active disease, and all 3 relapsed within 1 year. Four patients (18%) did not receive any further therapy after IC due to death, and of those 4, 1 had achieved MLFS briefly, whereas the other 3 did not achieve a response. None of the patients in the <65 years group had 30-day mortality events, and 1 had a 60-day mortality event. Those in the <65 years group who were able to proceed to transplant had a mOS of not reached compared with 9.4 months for those who did not (P = .0011; supplemental Figure 2B).
Patients receiving 1L HMA/VEN
Patients were then divided into those who received HMA/VEN in 1L (n = 24 [52%]) and those who received it in 2L+ (n = 22 [48%]; supplemental Table 1). Patients who received 1L HMA/VEN were generally older (median age, 66 vs 55 years; P = .02) and more likely to have AML classified to be myelodysplasia related (50% vs 32%; P = .02) or TP53 mutated (29% vs 5%; P = .02). The majority of 1L HMA/VEN patients had prior MDS or MDS/MPN overlap (58% vs 23%; P = .03), and they largely had adverse-risk disease (83% vs 50%; P = .02). The HMA of choice was similar between the 2 groups, and prior exposure to HMA and transplant was not statistically different between the 2 groups. By definition, patients receiving 1L HMA/VEN were all IC naïve. All 2L+ HMA/VEN patients received IC before HMA/VEN, except for 1 patient who received decitabine monotherapy before HMA/VEN. The number of HMA/VEN cycles and HMA/VEN response were not statistically different depending on the line it was given.
ORR (33% vs 41%), CR/CRi (25% vs 32%), and CR/CRi/MRD-negative (13% vs 14%) rates were not statistically different between 1L HMA/VEN and 2L+ HMA/VEN patients (supplemental Table 2). Nineteen 1L HMA/VEN patients (79%) were refractory (“primary refractory”) to HMA/VEN, and 5 (21%) saw response followed by relapse (Figure 2; supplemental Table 5). Both ORR (26% vs 60%, P = .29) and CR/CRi rate (21% vs 40%, P = .57) were numerically lower for the primary refractory group, although the difference was not statistically significant. Survival outcomes were similar for 1L and 2L+ HMA/VEN patients, with a mOS of 5.4 months for the refractory group and 6.6 months for the relapse group (P = .61; supplemental Table 3).
Molecular and cytogenetic characteristics of 1L HMA/VEN patients at the start of IC. Mut, mutated; NR, no response; Unkwn, unknown.
Molecular and cytogenetic characteristics of 1L HMA/VEN patients at the start of IC. Mut, mutated; NR, no response; Unkwn, unknown.
Molecular and cytogenetic predictors of response
The AML classification and molecular and cytogenetic profiles of the patients in our cohort at the time of IC treatment is shown in the Oncoprints in Figure 2 (1L HMA/VEN) and supplemental Figure 3 (all patients). Among 1L HMA/VEN patients, RUNX1 (n = 11 [46%]), TP53 (n = 7 [29%]), TET2 (n = 5 [21%]), and ASXL1 (n = 5 [21%]) were the most frequently mutated genes. No patients with these common mutations saw CR/CRi rates >20% when treated with IC. Patients with 3 less-common mutations showed CR/CRi rate >20% (NRAS, 50%; IDH2, 33%; DNMT3A, 50%). When mutations were grouped by pathway, CR/CRi rates were modest to poor in all of the groups we examined, including 22% for receptor tyrosine kinase mutations, despite 2 of 4 NRAS patients achieving CR, and 0% for splicing factor mutations (supplemental Table 6).
Looking at cytogenetic features, 8 patients (50%) had a complex karyotype, and 2 patients (8%) had chromosome 17 abnormalities. Response rates for the complex karyotype group included a CR/CRi rate of 13% and ORR of 13%, both numerically lower than the overall 1L cohort (CR/CRi of 25% and ORR of 33%). Both patients with chromosome 17 abnormalities also had TP53 mutations, and neither responded to IC.
When patients who received HMA/VEN in any line followed by IC were considered, CR/CRi rates for RUNX1, TP53, TET2, and ASXL1 remained low (22% or lower). Only 13% of all patients with receptor tyrosine kinase or splicing factor mutations achieved CR/CRi. Twenty-eight percent of patients with complex karyotype achieved CR/CRi.
Discussion
In this retrospective cohort study, we describe, to our knowledge, the largest published cohort of patients with AML who received IC after HMA/VEN failure. This is currently a small subset of patients, because HMA/VEN is usually given to newly diagnosed patients unfit for IC or after IC failure. However, given the safety and efficacy of HMA/VEN, we anticipate more patients will receive HMA/VEN early in their treatment course. Therefore, the role of IC after HMA/VEN will be increasingly debated, especially with large, randomized studies comparing IC and HMA/VEN as first-line treatment in medically fit adults with AML.9
Overall, we found that 28% of patients who receive IC after HMA/VEN failure achieved CR/CRi, including 25% of patients who received IC after 1L HMA/VEN and 21% of patients who exhibited primary refractory disease to 1L HMA/VEN. There are very limited published data available looking at responses and outcomes in this setting, but when compared with the report by Maiti et al,12 our findings are reasonably concordant, because they reported response in 2 of 8 patients receiving IC after HMA/VEN. Patients who had initially received IC followed by HMA/VEN that were rechallenged with IC did at times achieve responses, with CR/CRi rate of 32% and ORR of 41%. The majority of such patients previously responded to IC.
We attempted to identify significant predictors of response and improved survival with IC after HMA/VEN in our cohort. We hypothesized that patients who never achieved a response to HMA/VEN (“refractory”) would have more resistant disease and therefore lower response rates than those who relapsed after achieving a response. We see some evidence supporting this, with a numerically lower CR/CRi/MLFS rate (28% vs 57%; P = .10) and significantly lower CR/CRi rate (19% vs 50%; P = .04). However, possibly due to sample size limitations, there was a numeric but not statistically significant difference in survival between the 2 groups (mOS, 5.4 vs 8.8 months; P = .64). Because HMA/VEN and IC exert their antileukemic effects via distinct mechanisms, resistance to HMA/VEN does not necessarily entail resistance against IC. Nevertheless, we argue there may be some overlap, and the use of IC after HMA/VEN failure, especially primary HMA/VEN failure, must be considered carefully against available alternative therapies such as targeted therapies and clinical trials.
Another key consideration is age. Although potent therapies such as stem cell transplantation and cellular therapy are increasingly offered to older individuals with hematologic malignancies, IC for AML remains a therapy with potential toxicity for the older population.14-16 In our study, despite perceived fitness and eligibility, patients aged ≥65 years had poor outcomes, with a mOS of 4.3 months after IC, significantly worse than those aged <65 years (Figure 1C; P < .001). In our overall cohort, 13 patients (28%) died after receiving IC, with 9 of them having documented active leukemia, 1 of them achieving MLFS briefly, and the other 3 transitioning to comfort measures while being pancytopenic without marrow reassessment (supplemental Figure 1). Nine of the 13 patients who died were aged ≥65 years, and those 9 made up 38% of all patients aged ≥65 years (supplemental Table 4). Only 6 patients aged ≥65 years achieved CR/CRi/MLFS, and 4 proceeded to transplant, achieving a mOS of 10.4 months. This was not statistically different from those who did not proceed to transplant (mOS, 4.1; P = .14), albeit with a limited sample size. As such, we argue that aggressive treatments such as IC and alloSCT should be considered with particular caution after prior treatment with HMA/VEN in older individuals. In younger patients, the case for IC after HMA/VEN is somewhat stronger, with higher ORR (45%), transplant rate (36%), and survival, with the caveat that 3 of the 5 transplants occurred with active disease and saw early relapse.
Finally, we looked at molecular and cytogenetic predictors of response. In general, the most common mutations present in our cohort, notably RUNX1, TP53, TET2, and ASXL1, were all associated with CR/CRi rates of ≤20%. A small signal of favorable response was noted for NRAS mutations (50% CR after failure of 1L HMA/VEN). Complex cytogenetics were also common and associated with a 13% CR/CRi and 13% ORR after 1L HMA/VEN. No patients with chromosome 17 abnormalities responded. This is consistent with our hypothesis that patients currently being selected for 1L HMA/VEN have characteristics that are known predictors of poor outcomes with IC, and they remain poor candidates for IC after HMA/VEN failure.
This study is limited by its retrospective, single-centered design. Patients who received IC after HMA/VEN in this time frame are patients with specific characteristics that led to this sequence of treatments, presenting a risk for confounding by indication. We cannot precisely attribute the rationale for IC after HMA/VEN in this cohort in retrospect and acknowledge that patients who receive IC after HMA/VEN in the future may not have the same characteristics. However, by analyzing a multitude of underlying patient and disease characteristics, we saw that overall IC response rates are low and comparable to historical rates reported in R/R AML.17 Although this study does not contain sufficient data to fully determine effectiveness of IC after frontline HMA/VEN in young and fit individuals, it does raise concerns about the effectiveness of IC after HMA/VEN, especially in the older patients. Our findings discourage the use of IC after HMA/VEN in patients with canonically adverse-risk and cytogenetic features such as TP53 mutations, complex karyotype, and chromosome 17 abnormalities. Although ELN risk groups were developed based on IC-treated patients and is not intended to apply to HMA/VEN, it may still be informative for predicting responses to IC after HMA/VEN. Due to the relatively small sample size, this study is not powered to further detect small but potentially clinically important differences in outcomes between more rare subgroups. Larger retrospective studies and multicenter prospective studies would be beneficial, and studies examining frontline use of HMA/VEN should rigorously report response rates to subsequent therapies. There is abundant retrospective evidence supporting the use of HMA/VEN after IC, and it is important that such an evidence base for IC after HMA/VEN is developed if the alternative is to become widely considered.18
In summary, IC is an important tool in the treatment of AML. However, in the R/R setting after prior HMA/VEN treatment, we saw relatively low response rates and high levels of toxicity, with particularly poor outcomes in patients who are refractory to HMA/VEN, in older patients, and in patients with adverse-risk molecular and cytogenetic features. In such cases, the selection of IC should be made with caution. Careful exploration of alternative salvage therapies, including investigational approaches, should be performed at the time of R/R disease after HMA/VEN.
Acknowledgments
This work was supported by grant funding from the National Institutes of Health, namely the Cancer Center Support Grant/Core Grant (P30 CA008748) (Memorial Sloan Kettering Cancer Center [MSKCC]).
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Authorship
Contribution: K.K.C. designed the study, collected the data, analyzed and interpreted data, performed statistical analyses, and wrote the manuscript; Y.V. designed the study, analyzed and interpreted data, and wrote the manuscript; A.D., M.Y.S., L.B., J.L.G., and A.D.G. designed the study and analyzed and interpreted data; J.C. collected the data; E.M.S. designed and supervised the study and analyzed and interpreted data; and all authors reviewed and substantially edited the manuscript.
Conflict-of-interest disclosure: M.B.G.: Novartis: Consultancy; Sanofi: Consultancy, Research Funding; Actinium Pharmaceuticals, Inc: Research Funding; Amgen: Research Funding. A.D.G.: Genentech: Consultancy; DAVA Oncology: Honoraria; Trillium: Research Funding; AROG: Research Funding; Pfizer: Research Funding; Aptose: Research Funding; Aprea: Research Funding; ADC Therapeutics: Research Funding; Celularity: Research Funding; Prelude: Research Funding; Daiichi Sankyo: Consultancy, Research Funding; Astellas Pharma: Consultancy; Abbvie: Consultancy, Research Funding. J.H.P.: BeiGene: Consultancy; Genentech, Inc.: Research Funding; Autolus Therapeutics: Research Funding; Amgen: Consultancy; Be Biopharma: Consultancy; Fate Therapeutics: Research Funding; Allogene: Consultancy, Membership on an entity’s Board of Directors or advisory committees; Servier: Consultancy, Research Funding; Intella: Consultancy; Takeda: Consultancy, Research Funding; Sobi: Consultancy, Research Funding; Pfizer: Consultancy; Minerva Bio: Consultancy; Affyimmune: Consultancy; Artiva Biotherapeutics: Consultancy, Current holder of stock options in a privately held company, Membership on an entity’s Board of Directors or advisory committees; Bright Pharmaceuticals: Consultancy; Curocell: Consultancy; Kite: Consultancy; GC Cell: Membership on an entity’s Board of Directors or advisory committees; Incyte: Research Funding. Roeker: DAVA: Other: CME speaker; PeerView: Other: CME speaker; Curio: Other: CME speaker; Medscape: Other: CME speaker; AbbVie: Consultancy, Research Funding; Ascentage: Consultancy; AstraZeneca: Consultancy, Research Funding; Beigene: Consultancy; Janssen: Consultancy; Loxo Oncology: Consultancy, Other: travel support, Research Funding; Pharmacyclics: Consultancy; Pfizer: Consultancy, Research Funding; TG Therapeutics: Consultancy; Abbott Laboratories: Current equity holder in publicly traded company; Adaptive Biotechnologies: Research Funding; Genentech: Research Funding; Aptose Biosciences: Research Funding; Dren Bio: Research Funding; Qilu Puget Sound Biotherapeutics: Research Funding. M.S.T.: Innate Pharmaceuticals: Consultancy; Foghorn: Membership on an entity’s Board of Directors or advisory committees; Orsenix: Research Funding; UpToDate: Patents & Royalties; AbbVie: Research Funding; BioSight: Research Funding; Glycomimetics: Research Funding; Amgen: Research Funding; Rafael Pharmaceuticals: Research Funding; Novartis: Consultancy; Kura: Consultancy; Ipsen Biopharmaceuticalas: Consultancy; HOVON: Membership on an entity’s Board of Directors or advisory committees; American Society of Hematology: Honoraria, Membership on an entity’s Board of Directors or advisory committees. E.M.S.: Bristol Myers Squib: Consultancy, Research Funding; Gilead: Consultancy; Calithera: Consultancy; Syros: Consultancy; Daiichi: Consultancy; Menarini: Consultancy; OnCusp: Consultancy; Syndax: Consultancy; Aptose: Consultancy; Servier: Consultancy; Foghorn: Consultancy; CTI Biopharma: Consultancy; Neoleukin: Consultancy; Astellas: Consultancy; Ono Pharma: Consultancy; Blueprint: Consultancy; PinotBio: Consultancy; Novartis: Consultancy; Janssen: Consultancy; Agios: Consultancy; Jazz: Consultancy; Genentech: Consultancy; Genesis: Consultancy; Abbvie: Consultancy; Eisai: Research Funding. The remaining authors declare no competing financial interests.
Correspondence: Eytan M. Stein, 545 East 73rd St, New York, NY 10021; email: steine@mskcc.org.
References
Author notes
K.-K.C. and Y.V. contributed equally to this study.
Original deidentified data are available on request from the corresponding author, Eytan M. Stein (steine@mskcc.org). In general, individual participant data will not be shared but may be available depending on the request.
The full-text version of this article contains a data supplement.