TO THE EDITOR:
Posttransplant lymphoproliferative disorder (PTLD) includes a broad spectrum of disorders, ranging from nonmalignant lymphoproliferation to lymphoma.1-5 The clinical presentation of PTLD is variable, depending on the location and pathologic features.6 The majority of pediatric PTLD cases are Epstein-Barr virus (EBV)–positive, CD20+ B-cell lymphoma, whereas T- and natural killer (NK)–cell PTLDs (T/NK-PTLDs) are rare, representing <15% of PTLD cases, which usually occur at a median posttransplant interval of 4 to 6 years, and are associated with variable responses to treatment and overall poor outcome.7-11
Peripheral T-cell lymphoma, not otherwise specified (PTCL, NOS) is the most common subtype of T/NK-PTLD, followed by anaplastic large cell lymphoma, hepatosplenic T-cell lymphoma, and cutaneous T-cell lymphoma.9,11 In contrast to B-cell PTLD, only 30% to 40% of T/NK-PTLD is EBV- related.5,11 EBV− T/NK-PTLD after solid organ transplantation (SOT) appear to have inferior survival than EBV+ cases.7,9,11,12,14,15 T/NK-PTLD is extremely rare in children; hence, the pathological and clinical features of PTLD-PTCL in pediatric patients are largely unknown. Here, we report a multi-institutional case series of PTLD-PTCL and review the clinical, genetic, and pathological features of its presentation in a pediatric cohort.
Cases of childhood (aged ≤18 years) PTCL, NOS after SOT from 2013 to 2023 in different institutions were reviewed. The diagnostic criteria of PTCL, NOS was based on the 2017 fourth World Health Organization classification of tumors of hematopoietic and lymphoid tissues.1 Related literature was reviewed, and similar data were collected, including a report of a 21-year-old patient.16 Childhood posttransplant PTCL, NOS published before 2008 and cutaneous T-cell lymphoma such as anaplastic lymphoma kinase (ALK+) anaplastic large cell lymphoma, hepatosplenic T-cell lymphoma, or other distinct diagnostic entities were excluded in our study (patients reported before 2008 were previously summarized by Swerdlow et al11 and Yang et al9). The available clinical characteristics including age, sex, disease site(s), clinical stage, treatment strategies, outcomes, pathological features (morphology and immunophenotype), and genetics were obtained and summarized. This study was approved by the relevant institutional review boards. This study was conducted in accordance with the Declaration of Helsinki.
We identified 8 PTCL, NOS in children with a history of SOT. A literature search identified 8 more such patients (Table 1). These included 9 females and 7 males, with a median age at presentation of 5 years (range, 1-21). All patients had received SOT (kidney [n = 1]; liver [n = 8]; heart [n = 5]; liver, intestine, and pancreas [n = 1], and lung [n = 1]) and presented at a median of 4 years (range, 2 months to 11 years) after transplantation. The immunosuppressive regimens included tacrolimus, azathioprine, mycophenolate mofetil, and/or prednisone.
Characteristics, therapy, and outcome of pediatric PTCL after SOT
| Patient number . | Age/sex . | Graft . | Time to PTLD (y) . | Location . | Tumor cell morphology . | EBV . | Immunophenotype . | Molecular results . | Reported diagnosis . | Treatment . | Outcome . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient 116 | 21-y male | Liver | 5.0 | Lymphadenopathy | N/A | Positive | CD3+, CD4–, CD8+, CD30 subset+ | N/A | PTCL, NOS | CHOP | In remission 9 mo later |
| Patient 225 | 7-y male | Liver | 7.0 | Gastrointestinal erosions | N/A | Negative | CD3+, CD4+, CD8–, CD5+, CD7+, CD103+, CD56– | Clonal TCR | PTCL, NOS | Reduced immunosuppressive | In remission |
| Patient 39 | 3-y female | Lung | 3.0 | Mesenteric and retroperitoneal mass | N/A | N/A | CD3 dim+, CD4+, CD8–, CD7 partial loss | Clonal TCR | PTCL, NOS | ALL-like (vincristine, daunorubicin, asparaginase, and prednisone) | In remission 20 mo later |
| Patient 49 | 9-y female | Liver | 5.0 | Hepatosplenomegaly and lymphadenopathy | N/A | N/A | CD3+, CD4+, CD8–, CD7 partial loss | Clonal TCR | PTCL, NOS | ALL-like | In remission 10 mo later |
| Patient 510 | 2-y male | Liver | 1.5 | Lymph node and graft | small to medium tumor cells | N/A | CD3+, CD4+, CD8– | N/A | PTCL, NOS | N/A | Died of disease in 1 mo |
| Patient 610 | 1-y male | Liver | 0.2 | Lymph nodes and pleural fluid | N/A | N/A | N/A | N/A | PTCL, NOS | N/A | Alive 48 mo follow-up |
| Patient 726 | 4-y female | Liver | 3.5 | Abdominal mass and bowel perforation | N/A | Negative | CD3 dim+, CD4+, CD8–, CD5 dim+, CD7– | Clonal TCR | PTCL, NOS | EPOCH and ICE × 2 cycles, HSCT | In remission 3.5 y follow-up |
| Patient 813 | 6-y female | Heart | 5.4 | Lung, small bowel and lymph node | N/A | N/A | N/A | N/A | PTCL, NOS | ALL-like | Alive at 22 mo follow-up |
| Patient 915 | 4.7-y female | Heart | 1.4 | Retroperitoneal lymph nodes and bowel | N/A | Negative | CD4+ | Clonal TCR | PTCL, NOS | Chemotherapy | In remission at 4 mo follow-up; died of heart rejection 3 y later |
| Patient 10 | 12-y female | Heart | 11 | Lymphadenopathy | Medium to large tumor cells | Negative | CD3+, CD4+, CD8–, CD7–, CD5+, CD2+, CD30+;Ki67 20%;CD34–;TCR delta– | Clonal TCR; KRAS and DICER1 mutations | PTCL, NOS | Chemotherapy (GDP-BV) | In remission; relapsed 8 mo later |
| Patient 11 | 3-y female | Liver, intestine, and pancreas | 2.0 | Stomach and lung mass | Small to medium tumor cells | Negative | CD3+, CD4+, CD8–, CD5+, CD7+; TP53–, MYC–, TdT–, CD34–; Ki67 10% | Clonal TCR | PTCL, NOS | Chemotherapy | Died of disease in 5 mo |
| Patient 12 | 15-y female | Kidney | 11 | Ovary and pelvis mass | Small to medium tumor cells | Positive (by PCR) | CD3+, CD4+, CD8–, CD5+, TdT–, CD34– | Clonal TCR | PTCL, NOS | Chemotherapy | Died of disease in 1 y |
| Patient 13 | 5-y female | Liver | 4.0 | Lymphadenopathy | Small to medium tumor cells | N/A | CD3+, CD4+, CD8–, CD5 dim, CD2 dim+, CD7 dim+ | Clonal TCR; Chr18q aberrancy | PTCL, NOS | Chemotherapy | Died of disease in 1 y |
| Patient 14 | 4-y male | Liver | 4.0 | Chest wall and pleural effusion | Small to medium tumor cells | Positive | CD3+, CD4+, CD5 dim, CD7 loss, CD30–, Ki67 50%-60% | Clonal TCR | PTCL, NOS | Chemotherapy (CHOP) | Died of disease in 3 mo |
| Patient 15 | 4-y male | Heart | 3.0 | Duodenum | Small to medium tumor cells | Negative | CD3+, CD4+ (>>CD8), CD5 and CD7 partial loss, CD30 patchy+, BF1+ | Clonal TCR | PTCL, NOS | Chemotherapy (BV-CHP) | Died of disease in 13 mo |
| Patient 16 | 12-y male | Heart | 11 | Bone marrow, splenomegaly, and mediastinal/subcarinal adenopathy | Small to medium tumor cells | Positive | CD3+, CD2+, CD8+, CD4–, CD5 +, CD7– ; BF1–; CD56 partial+; CD34– | Clonal TCR | PTCL, NOS | Chemotherapy (anakinra, dexamethasone; CHOP) | Died of disease in 3 mo |
| Patient number . | Age/sex . | Graft . | Time to PTLD (y) . | Location . | Tumor cell morphology . | EBV . | Immunophenotype . | Molecular results . | Reported diagnosis . | Treatment . | Outcome . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient 116 | 21-y male | Liver | 5.0 | Lymphadenopathy | N/A | Positive | CD3+, CD4–, CD8+, CD30 subset+ | N/A | PTCL, NOS | CHOP | In remission 9 mo later |
| Patient 225 | 7-y male | Liver | 7.0 | Gastrointestinal erosions | N/A | Negative | CD3+, CD4+, CD8–, CD5+, CD7+, CD103+, CD56– | Clonal TCR | PTCL, NOS | Reduced immunosuppressive | In remission |
| Patient 39 | 3-y female | Lung | 3.0 | Mesenteric and retroperitoneal mass | N/A | N/A | CD3 dim+, CD4+, CD8–, CD7 partial loss | Clonal TCR | PTCL, NOS | ALL-like (vincristine, daunorubicin, asparaginase, and prednisone) | In remission 20 mo later |
| Patient 49 | 9-y female | Liver | 5.0 | Hepatosplenomegaly and lymphadenopathy | N/A | N/A | CD3+, CD4+, CD8–, CD7 partial loss | Clonal TCR | PTCL, NOS | ALL-like | In remission 10 mo later |
| Patient 510 | 2-y male | Liver | 1.5 | Lymph node and graft | small to medium tumor cells | N/A | CD3+, CD4+, CD8– | N/A | PTCL, NOS | N/A | Died of disease in 1 mo |
| Patient 610 | 1-y male | Liver | 0.2 | Lymph nodes and pleural fluid | N/A | N/A | N/A | N/A | PTCL, NOS | N/A | Alive 48 mo follow-up |
| Patient 726 | 4-y female | Liver | 3.5 | Abdominal mass and bowel perforation | N/A | Negative | CD3 dim+, CD4+, CD8–, CD5 dim+, CD7– | Clonal TCR | PTCL, NOS | EPOCH and ICE × 2 cycles, HSCT | In remission 3.5 y follow-up |
| Patient 813 | 6-y female | Heart | 5.4 | Lung, small bowel and lymph node | N/A | N/A | N/A | N/A | PTCL, NOS | ALL-like | Alive at 22 mo follow-up |
| Patient 915 | 4.7-y female | Heart | 1.4 | Retroperitoneal lymph nodes and bowel | N/A | Negative | CD4+ | Clonal TCR | PTCL, NOS | Chemotherapy | In remission at 4 mo follow-up; died of heart rejection 3 y later |
| Patient 10 | 12-y female | Heart | 11 | Lymphadenopathy | Medium to large tumor cells | Negative | CD3+, CD4+, CD8–, CD7–, CD5+, CD2+, CD30+;Ki67 20%;CD34–;TCR delta– | Clonal TCR; KRAS and DICER1 mutations | PTCL, NOS | Chemotherapy (GDP-BV) | In remission; relapsed 8 mo later |
| Patient 11 | 3-y female | Liver, intestine, and pancreas | 2.0 | Stomach and lung mass | Small to medium tumor cells | Negative | CD3+, CD4+, CD8–, CD5+, CD7+; TP53–, MYC–, TdT–, CD34–; Ki67 10% | Clonal TCR | PTCL, NOS | Chemotherapy | Died of disease in 5 mo |
| Patient 12 | 15-y female | Kidney | 11 | Ovary and pelvis mass | Small to medium tumor cells | Positive (by PCR) | CD3+, CD4+, CD8–, CD5+, TdT–, CD34– | Clonal TCR | PTCL, NOS | Chemotherapy | Died of disease in 1 y |
| Patient 13 | 5-y female | Liver | 4.0 | Lymphadenopathy | Small to medium tumor cells | N/A | CD3+, CD4+, CD8–, CD5 dim, CD2 dim+, CD7 dim+ | Clonal TCR; Chr18q aberrancy | PTCL, NOS | Chemotherapy | Died of disease in 1 y |
| Patient 14 | 4-y male | Liver | 4.0 | Chest wall and pleural effusion | Small to medium tumor cells | Positive | CD3+, CD4+, CD5 dim, CD7 loss, CD30–, Ki67 50%-60% | Clonal TCR | PTCL, NOS | Chemotherapy (CHOP) | Died of disease in 3 mo |
| Patient 15 | 4-y male | Heart | 3.0 | Duodenum | Small to medium tumor cells | Negative | CD3+, CD4+ (>>CD8), CD5 and CD7 partial loss, CD30 patchy+, BF1+ | Clonal TCR | PTCL, NOS | Chemotherapy (BV-CHP) | Died of disease in 13 mo |
| Patient 16 | 12-y male | Heart | 11 | Bone marrow, splenomegaly, and mediastinal/subcarinal adenopathy | Small to medium tumor cells | Positive | CD3+, CD2+, CD8+, CD4–, CD5 +, CD7– ; BF1–; CD56 partial+; CD34– | Clonal TCR | PTCL, NOS | Chemotherapy (anakinra, dexamethasone; CHOP) | Died of disease in 3 mo |
ALL, acute lymphoblastic leukemia; BV-CHP, brentuximab vedotin, cyclophosphamide, doxorubicin, prednisone; CHOP, cyclophosphamide, doxorubicin hydrochloride, vincristine sulfate, and the steroid hormone prednisone; EPOCH, etoposide, vincristine, doxorubicin, cyclophosphamide, and prednison; GDP-BV, gemcitabine, dexamethasone, cisplatin, and brentuximab vedotin; HSCT, hematopoietic stem cell transplantation; ICE, ifosfamide, carboplatin, and etoposide phosphate; N/A, not applicable; PCR, polymerase chain reaction; PTLD, posttransplant lymphoproliferative disorder.
In this combined case series, 8 patients had nodal disease; 8 had disease in the gastrointestinal tract and abdominal/retroperitoneal mass; 2 presented with a lung mass; 1 had pelvic and ovarian mass; 1 had subcutaneous mass; and 1 had bone marrow involvement and hemophagocytic lymphohistiocytosis. Interestingly, there were 2 patients who presented with pleural effusions. Both nodal and extranodal cases revealed prominent infiltration of small- to medium-sized lymphoid cells with dense chromatin (87.5% cases), as well as some (12.5%) with medium- to large-sized tumor cells. CD4 was positive in 86% of cases; CD8 was positive in 14% (also EBV+). T-cell antigen loss (eg, CD5 and CD7) was frequently identified in all cases. CD30 was not routinely tested in most of these cases but was reported positive in 3 (75%). Ki67 proliferation rate ranged from 10% to 60%. Epstein–Barr virus–encoded small RNA (EBER) in-situ hybridization was negative in 6 (60%) and positive in 4 (40%) of 10 cases. T-cell gene rearrangements (TCRs) were clonal in all tested cases.
Next-generation sequencing (NGS) panel was performed and reported in 2 patients: 1 patient revealed DICER1 c.4049_4050+9 dup (35.6% variant allele fraction); and KRAS c.38G>A (36.2% variant allele fraction). No pathogenic somatic mutations were identified in the other patient.
All patients received systemic chemotherapy, except patient 2 who had a good response to reduced immunosuppression (RIS) only. At the end of follow-up, 8 patients (50%) were deceased, with an overall median survival time of 12 months. Interestingly, 8 patients in our series achieved remission after therapy, including 3 responding to acute lymphoblastic leukemia–like chemotherapy regimen.17 Two patients (cases 11 and 15) received chemotherapy combined with anti-CD30 brentuximab vedotin; patient 11 achieved remission but relapsed 8 months later, whereas patient 15 succumbed to disease 13 months after diagnosis. Patient 2 of our series was reported to be PTCL, NOS but showed an indolent T-cell lymphoproliferative disorder of the gastrointestinal tract in a child after SOT, which responded to RIS only.
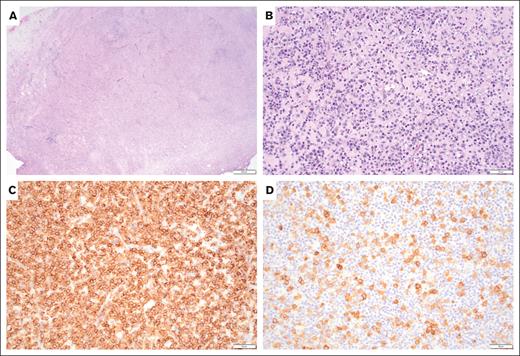
To the best of our knowledge, this is the largest series of childhood posttransplant PTCL (PT-PTCL). Similar to prior studies, pediatric PT-PTCL can be EBV+ or EBV− with heterogeneous clinical presentation and pathological features.9,11 Our study reveals that PT-PTCL often show small to medium cell size, CD4 positivity, frequent loss of T-cell antigen expression, and clonal TCR, involving both lymph nodes and extranodal organs. We only report 1 case in which the tumor cells are large, with anaplastic morphology and CD8 positivity. The pathology and clinical presentation of patient 16 overlaps with systemic EBV+ T-cell lymphoma of childhood, which is often complicated by hemophagocytic lymphohistiocytosis and fatal outcome.1-3 Moreover, compared with patient 2 of an indolent T-cell lymphoproliferative disorder of the gastrointestinal tract with response to RIS only,2,3 our patient 15 also presented with a duodenum mass (Figure 1), with tissue architecture effacement by atypical small- to medium-sized T lymphocytes with aberrant immunophenotype and clonal TCR, which failed to respond to chemotherapy; the patient succumbed to disease 13 months after diagnosis. Similar to adult patients, PT-PTCL often presents with extranodal disease. Morphology, immunophenotype, and EBV positivity are variables (supplemental Table 1).
A biopsy of duodenum mass shows an effacement of duodenum architecture. (A) Hematoxylin and eosin stain, low power, (original magnification ×40), the infiltrates are composed of small- to medium-sized atypical lymphocytes. (B) Hematoxylin and eosin stain, high power, (original magnification ×200), diffusely positive for CD3, (C) Immunostain (original magnification ×200) and patchy CD30. (D) Immunostain (original magnification ×200).
A biopsy of duodenum mass shows an effacement of duodenum architecture. (A) Hematoxylin and eosin stain, low power, (original magnification ×40), the infiltrates are composed of small- to medium-sized atypical lymphocytes. (B) Hematoxylin and eosin stain, high power, (original magnification ×200), diffusely positive for CD3, (C) Immunostain (original magnification ×200) and patchy CD30. (D) Immunostain (original magnification ×200).
Recent genomic studies of PTCL, NOS reveals distinct molecular abnormalities.18 Targeted NGS in adult T/NK-PTLDs revealed mutations of epigenetic modifiers, JAK/STAT signaling cascade, TP53, and additional recurrent genomic alterations described in PTCL of immunocompetent individuals19; however, genomic studies focusing on childhood PT-PTCL are lacking.19 Patient 10 carried somatic mutations in DICER and KRAS genes.19 A limited NGS study was negative on patient 15. These findings indicate the potentially different genetic landscapes between adult and pediatric PT-PTCL, although additional molecular study of pediatric cases is needed.
Treatment of PT-PTCL is challenging.20,21 The use of regimens developed for immunocompetent individuals is often complicated by reduced chemotherapy tolerance of transplant recipients.4 Nevertheless, our study demonstrates that these tumors can be treatment responsive to acute lymphoblastic leukemia–like chemotherapy regimens,9 although the follow-up on reported 3 patients was short.9 Moreover, CD30 is a member of the tumor necrosis factor receptor superfamily, member 8 with normal expression on activated T and B cells. Examination of CD30 expression in pediatric tumors revealed that CD30 expression is much broader than anticipated.22 Although only 2 patients from our series received anti-CD30 targeted therapy and with variable clinical outcomes, there is enough evidence to support the benefit of brentuximab vedotin on CD30+ lymphomas, in both clinical trials and real-world settings.23,24
In summary, we report the clinical and pathological features of a large series of pediatric PT-PTCL, which show distinct pathology and potentially different genetic features compared with adult PT-PTCL. These cases demonstrate the importance of accurate pathologic diagnosis and classification, which can guide clinical treatment and predict clinical outcome.1-3 It is important to recognize this rare subtype of monomorphic PTLD, and future studies are required to understand the pathobiology of this disease.
Contribution: J.C. designed the study, collected and analyzed the data, and wrote the manuscript; and R.M., J.N.P., M.d.l.C.B., P.N., M.O., W.S., L.W., O.A., and S.G. collected and analyzed data and reviewed the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jinjun Cheng, Pathology and Laboratory Medicine Division, Children’s National Hospital, Room 1629, 111 Michigan Ave NW, Washington, DC 20010-2970; email: jcheng2@childrensnational.org.
References
Author notes
All materials, data sets, and protocols are available to other investigators without unreasonable restrictions. Please email the corresponding author, Jinjun Cheng (jcheng2@childrensnational.org).
The full-text version of this article contains a data supplement.