Key Points
DNMT3A mutations confer enhanced survival to T-ALL cells through increased JAK/STAT signaling.
BIRC5 represents a specific genetic dependency and therapeutic vulnerability of T-ALL cells with DNMT3A mutations.
Visual Abstract
T-cell acute lymphoblastic leukemia (T-ALL) is an aggressive hematopoietic neoplasm. Although the prognosis of pediatric T-ALL has improved with intensified chemotherapy regimens, this benefit has largely not translated to the adult T-ALL population. Development of new treatments requires understanding the mechanisms driven by specific mutations. DNMT3A mutations are identified in ∼10% to 18% of adult patients with T-ALL and are associated with poor clinical outcomes. Here, using primary human specimens, we show that cells from patients with T-ALL with DNMT3A mutations are resistant to apoptosis and certain chemotherapies. Elevated JAK/STAT signaling drove prosurvival programs in patients with mutant DNMT3A, and JAK/STAT inhibition restored sensitivity to chemotherapy. The prosurvival gene BIRC5 was upregulated in patients with DNMT3A-mutant T-ALL, and these cells were specifically sensitive to the Baculoviral IAP Repeat Containing 5 (BIRC5) inhibitor YM155. Genetic inhibition of BIRC5 in vivo lead to rapid depletion of DNMT3A-mutant T-ALL cells in patient-derived xenografts, positioning BIRC5 as a precision medicine target for these patients.
Introduction
T-cell acute lymphoblastic leukemia (T-ALL) is an aggressive neoplasm of T-cell progenitors that accounts for ∼15% and 25% of pediatric and adult ALL cases, respectively.1 Although in pediatric T-ALL the introduction of high-dose, multiagent chemotherapy regimens has resulted in long-term event-free survival (EFS) rates of up to 90%,2 this survival benefit has not carried over to the adult T-ALL population, which have 5-year EFS rates of 40% to 50%. Treatment approaches for adult patients with relapsed or primary refractory T-ALL may include nelarabine or cytarabine-based salvage therapy coupled with bone marrow (BM) transplantation,3,4 but outcomes remain dismal with a median survival of 5 months.4 The differences in treatment outcomes are likely related to underlying genetic differences between children and adults. The search for molecular drug targets and the design of tumor-specific therapies is necessary to improve outcomes and reduce toxicity for high-risk adult patients with T-ALL.
In T-ALL, the accumulation of genomic abnormalities leads to the aberrant expression of select transcription factors and activation of signaling pathways that result in increased proliferation, cell survival, and impaired differentiation of T-cell progenitors, which give rise to this disease.5,6 The NOTCH signaling pathway is essential for T-lymphopoiesis and T-lineage differentiation from thymic precursors.7,8 Moreover, NOTCH1 gain-of-function mutations are the major genetic driver of T-ALL in adults (>60% of cases)9 and mouse models have demonstrated the potency for these mutations in T-ALL development.10,11 Recent genomic studies have also identified recurrent mutations in epigenetic regulators in patients with T-ALL,12,13 including the de novo DNA methyltransferase enzyme DNMT3A. Although DNMT3A mutations are rare in pediatric and adolescent T-ALL,14 they occur in 10% to 18% of adult cases and are associated with lower remission rates, higher incidence of relapse, and markedly poorer EFS and overall survival.15-18 The spectrum of DNMT3A mutations in T-ALL is distinct from that seen in myeloid neoplasms (eg, less prevalence of DNMT3AR882 dominant negative mutations), implicating divergent mechanisms of leukemogenesis. Although DNMT3A mutations in acute myeloid leukemia (AML) and myelodysplastic syndromes are almost exclusively heterozygous,19,20 patients with T-ALL can harbor homozygous or compound heterozygous DNMT3A mutations.15,17,21
DNMT3A loss-of-function mutations frequently co-occur with NOTCH1 gain-of-function mutations in patients with T-ALL.17 We previously used a conditional knockout mouse model to uncover a tumor suppressor function for Dnmt3a in T-cell transformation.22 Transplantation of control and Dnmt3a-deficient BM progenitor cells transduced with a retrovirus expressing the NOTCH1 intracellular domain (NICD)10 revealed a much shorter latency to T-ALL in a Dnmt3a-deficient background.22 Mice deficient for Dnmt3a in hematopoiesis also showed an accumulation of T-cell progenitors in the thymus as a possible mechanism of premalignant disposition.22 This developmental block of Dnmt3a-null T-cell progenitors was associated with reduced apoptosis.22 To translate those findings to malignant hematopoiesis, we now show here that mouse Dnmt3a-null T-ALL cells are also resistant to apoptosis. This phenomenon is conserved in humans because samples from patients with primary T-ALL with DNMT3A mutations are resistant to cell death induced by certain chemotherapeutics. Mechanistically, we show this prosurvival phenotype is driven by enhanced JAK/STAT and identify BIRC5 as a specific genetic dependency and therapeutic vulnerability of DNMT3A-mutant T-ALL cells. These data provide a critical first step toward novel target precision medicine approaches for this patient group.
Methods
Mice and transplantation
The institutional animal care and use committee at Washington University approved all animal procedures. Transplant recipient mice (C57Bl/6 CD45.1, The Jackson Laboratory, no. 002014) received a split dose of irradiation (11 Gy) ∼4 hours apart. Patient-derived xenograft (PDX) models were generated by transplanting cells from patients with primary T-ALL into 6- to 8-week-old NOD-scid IL2Rgammanull (The Jackson Laboratory, no. 005557) via tail vein injection in a volume of 200 μL with 27-gauge U-100 insulin syringes (EasyTouch, no. 08496-2755-01). Ruxolitinib (RUX) was administered in chow formulation (2 g RUX/1 kg chow; Incyte, no. INCB018424).
Human T-ALL samples
Samples from patients with T-ALL were obtained with written consent in accordance with the Declaration of Helsinki protocol. Because all patient samples were deidentified and the study team had no access to individual patient health information, the Washington University institutional review board and human research protection office determined this to be a nonhuman study. Deidentified samples were cultured in vitro in StemSpan SFEM II Media (StemCell Technologies, no. 09655) supplemented with penicillin-streptomycin (50 U/mL), human stem cell factor (50 ng/mL), human interleukin-2 (IL-2; 50 U/mL), and human IL-7 (10 ng/mL).
Drug treatments
Approximately 1 × 105 cells were seeded in 48-well cell culture plates together with the different drugs. Cells were incubated for 48hours in a 5% CO2 incubator at 37⁰C, then cell viability was assessed using annexin-V flow cytometry (Invitrogen, no. 509279) with Sytox blue cell stain (ThermoFisher, no. S34857). For YM155 (Sepantronium Bromide) treatment, 2 × 106 primary human T-ALL cells were seeded in 6-well tissue culture plate wells and incubated with YM155 (Selleckchem, no. S1130) at a concentration of 1 μM for 48 hours, then assayed for viability as described above.
Statistics
Student t test and 1-way, and 2-way analysis of variance were used for statistical comparisons as appropriate. Survival curves were analyzed using a Mantel-Cox log-rank test. Significance is indicated using the following convention: ∗P < .05, ∗∗P < .01, ∗∗∗P < .001, and ∗∗∗∗P < .0001. All graphs represent mean ± standard error of the mean.
Results
DNMT3A mutations are founding events in T-ALL and alter DNA methylation
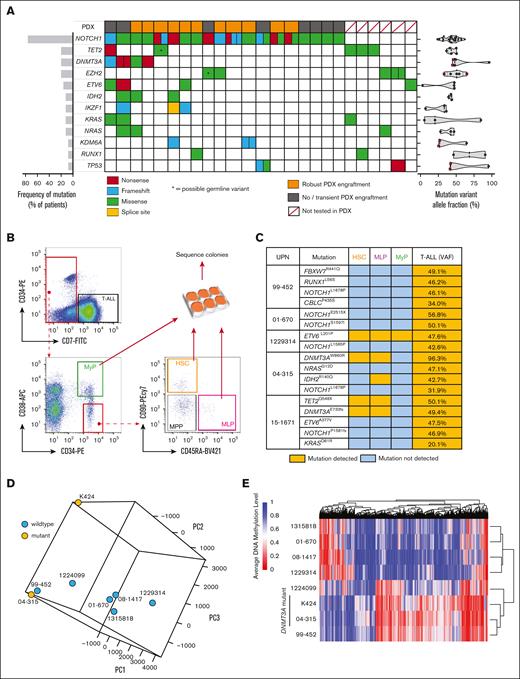
We determined the mutational spectrum of a cohort of samples from 25 adult patients with T-ALL through targeted genomic sequencing (supplemental Table 1). As expected, NOTCH1 variants were the predominant oncogenic lesion with 19 of 25 (76.0%) patients harboring mutations in NOTCH1, with 5 of 19 of these patients having multiple NOTCH1 variants (Figure 1A). The next most recurrently mutated genes were the epigenetic regulators TET2, DNMT3A, and EZH2 (Figure 1A). DNMT3A mutations co-occurred with NOTCH1 mutations in this patient cohort as has been reported by other groups.17 The variant allele fraction (VAF) of DNMT3A mutations in patients with T-ALL was consistently ∼50% (Figure 1A), suggestive of heterozygous mutations found in all the tumor cells, presenting DNMT3A mutation as a founding event in T-ALL as in AML23 and myelodysplastic syndromes.19 As has previously been reported for T-ALL, 1 patient had compound DNMT3A mutations (DNMT3AQ606X VAF = 45.9%; DNMT3AW305X VAF = 44.8%). We attempted to establish PDXs to produce banks of primary cells for mechanistic study (Figure 1A; supplemental Figure 1A).
DNMT3A mutations are founding events in T-ALL and alter DNA methylation. (A) Genomic summary of the T-ALL cohort showing incidence of recurrently mutated genes, engraftment in PDXs, and VAFs. (B) Schematic for flow cytometric purification of HSPC populations from the BM of patients with T-ALL. (C) Summary of human HSPC-derived colony sequencing showing detection of different somatic variants in individual populations. (D) Principle component analysis of DNA methylation profiles of patients with T-ALL by whole-genome bisulfite sequencing. (E) Clustering of T-ALL samples based on differentially methylated regions.
DNMT3A mutations are founding events in T-ALL and alter DNA methylation. (A) Genomic summary of the T-ALL cohort showing incidence of recurrently mutated genes, engraftment in PDXs, and VAFs. (B) Schematic for flow cytometric purification of HSPC populations from the BM of patients with T-ALL. (C) Summary of human HSPC-derived colony sequencing showing detection of different somatic variants in individual populations. (D) Principle component analysis of DNA methylation profiles of patients with T-ALL by whole-genome bisulfite sequencing. (E) Clustering of T-ALL samples based on differentially methylated regions.
The VAF of NOTCH1 mutations was highly variable, suggesting NOTCH1 may act as a founding mutation or cooperating mutation in T-ALL, as has been suggested by recent studies.24,25 To definitively establish the ontogeny of mutations in T-ALL, hematopoietic stem and progenitor cell (HSPC) subsets were isolated from patient BM specimens by flow cytometry. Colonies were generated from hematopoietic stem cells (HSCs; CD7− CD38− CD34+ CD45RA− CD90+), mixed lineage progenitors (CD7− CD38− CD34+ CD45RA+ CD90−), and myeloid progenitors (CD7− CD38+ CD34+) for mutation sequencing analysis (Figure 1B). The abundance of these HSPCs (including multipotent progenitors; CD7− CD38− CD34+ CD45RA− CD90−) in samples from patients with T-ALL were highly variable (supplemental Figure 1B-C). Targeted sequencing was performed for the mutations identified in bulk T-ALL cells in each of the HSPC populations (supplemental Figure 1D). NOTCH1 mutations were never observed in HSPCs (Figure 1C). Conversely, the DNMT3A mutations identified in the bulk T-ALL for each patient were detected in HSPCs, indicating that they are an early event in T-ALL oncogenesis. This suggests that in these cases, T-ALL likely evolves from underlying clonal hematopoiesis (CH) from primitive HSPCs that have acquired DNMT3A mutations. Mutations in other genes commonly associated with CH were also observed in HSPC populations such as TET2, IDH2, and ETV6 (Figure 1C).
Because DNMT3A is a DNA methyltransferase enzyme, DNA methylation profiles were examined in PDX-derived hCD45+hCD7+ T-ALL cells by whole-genome bisulfite sequencing. Principle component analysis did not strictly group the DNMT3A-mutant samples based on global DNA methylation patterns (Figure 1D). However, analysis of the most variable CpGs did stratify the DNMT3A-mutant patients together (supplemental Figure 2A). When DNMT3A-mutant samples were grouped together, analysis of differentially methylated regions further segregated these patients (Figure 1E) and identified hypomethylation at important loci implicated in leukemogenesis such as TERT and the HOX locus (supplemental Figure 2B-C). As in AML,26 it appears that DNMT3A mutations do not lead to massive alteration of the DNA methylome in patients with T-ALL (supplemental Table 2).
Order of mutational acquisition in T-ALL development
Although DNMT3A mutations were found in HSPCs from patients with T-ALL, in some cases the VAF of NOTCH1 mutations was higher than DNMT3A mutations in individual patients (supplemental Table 1). This brings into question the relevance of order of mutational acquisition in the pathological development of T-ALL. We previously used conditional knockout mice to elucidate a tumor suppressor function for Dnmt3a in T-cell transformation. Mx1-Cre:Dnmt3afl/+ (Dnmt3aHET) and Mx1-Cre:Dnmt3afl/fl (Dnmt3aKO) along with Mx1-Cre:Dnmt3a+/+ (control) mice were treated with polyinosinic:polycytidylic acid (pIpC) to induce deletion of Dnmt3a in hematopoietic cells. BM progenitors were then transduced with a retrovirus expressing NICD, an oncogenic model that recapitulates many features of human T-ALL,10 and transplanted into recipient mice to reveal a much shorter latency to T-ALL development in a Dnmt3a-deficient background.22 We leveraged this model to test whether the order of mutational acquisition influences T-ALL development. BM progenitor cells from control, Dnmt3aHET, and Dnmt3aKO mice were transduced with NICD retrovirus, and 500 transduced HSPCs (CD45.2+ green fluorescent protein [GFP]+ lineage− Sca-1+ c-Kit+) were transplanted with a radioprotective dose of 3.0 × 105 BM competitor cells. Once GFP+ engraftment reached an average of 10%, recipients were injected with pIpC to induce deletion of Dnmt3a after expression of NICD. Results showed that, as in our previous study, genetic deletion of Dnmt3a in an existing Notch1-mutant background also accelerated T-ALL development (Figure 2A).
Order of mutational acquisition in T-ALL development. (A) Kaplan-Meier plot of mice that received transplantation with control, Dnmt3aHET-, and Dnmt3aKO-NICD–expressing cells and injected with pIpC 5 weeks after transplant. (B) Schematic for generation of 2-hit Dnmt3a loss-of-function NICD gain-of-function genetic mouse models. (C) Western blot showing expression levels of NICD from doxycycline-induced RTN mice with comparison with levels resulting from retroviral NICD expression. (D) Kaplan-Meier plot showing time to morbidity of RTN and RTN3a mice injected with pIpC and secondarily induced for NICD expression with doxycycline chow. (E) Kaplan-Meier plot showing time to morbidity of RTN and RTN3a mice induced for NICD expression with doxycycline chow then secondarily injected with pIpC. (F) Summary of in vivo DNMT3A CRISPR experiments with wild-type DNMT3A T-ALL specimens showing secondary transplant blood engraftment (6-8 weeks after transplant), time to morbidity, final disease burden at euthanasia, and protein levels of DNMT3A in T-ALL blasts at euthanasia.
Order of mutational acquisition in T-ALL development. (A) Kaplan-Meier plot of mice that received transplantation with control, Dnmt3aHET-, and Dnmt3aKO-NICD–expressing cells and injected with pIpC 5 weeks after transplant. (B) Schematic for generation of 2-hit Dnmt3a loss-of-function NICD gain-of-function genetic mouse models. (C) Western blot showing expression levels of NICD from doxycycline-induced RTN mice with comparison with levels resulting from retroviral NICD expression. (D) Kaplan-Meier plot showing time to morbidity of RTN and RTN3a mice injected with pIpC and secondarily induced for NICD expression with doxycycline chow. (E) Kaplan-Meier plot showing time to morbidity of RTN and RTN3a mice induced for NICD expression with doxycycline chow then secondarily injected with pIpC. (F) Summary of in vivo DNMT3A CRISPR experiments with wild-type DNMT3A T-ALL specimens showing secondary transplant blood engraftment (6-8 weeks after transplant), time to morbidity, final disease burden at euthanasia, and protein levels of DNMT3A in T-ALL blasts at euthanasia.
Varying NOTCH1 mutations in T-ALL drive downstream oncogenic signaling to various strengths,11 which can have important consequences for disease outcomes.27 Retroviral NICD leads to supraphysiological expression levels. To establish a more physiological model, we generated “2-hit” mice that allow independent induction of mutations, with Dnmt3a inactivation controlled through Cre/lox recombination and expression of NICD regulated by doxycycline induction (Figure 2B). Mx1-Cre:Dnmt3afl/fl mice were crossed to mice with the reverse tetracycline transactivator (rtTA) protein knocked in to the Rosa26 locus (Rosa26rtTA), whereas NICD (amino acids 1749-2293) is expressed downstream of a tetracycline responsive element,28 acting as a “TetON” system. Level of NICD expression can be titrated by different concentrations of doxycycline in rodent chow (Figure 2C). We chose 200 ppm as a low dose to mimic “weak” NOTCH1 mutations, and 1250 ppm as a high dose to model “strong” NOTCH1 mutations.11 Rosa26rtTA; tetO_NICD; Mx1-Cre; Dnmt3a+/+ (RTN control) and Rosa26rtTA; tetO_NICD; Mx1-Cre; Dnmt3afl/fl (RTN3a) mice were treated with pIpC to inactivate Dnmt3a, and then 6-weeks later 2.0 × 106 BM cells were transplanted into irradiated recipient mice. Doxycycline was administered 6-weeks after transplant through chow to activate NICD expression. Latency to T-ALL was reduced in RTN3a mice compared with control RTN mice regardless of doxycycline dose (Figure 2D). To reverse the mutation order in this model, 2.0 × 106 BM cells from RTN and RTN3a mice were transplanted into irradiated mice. Recipients were placed on “low-dose” doxycycline chow 6-week after transplant to induce NICD expression, and then were injected with pIpC 10 weeks later. Consistent with all prior models, latency to T-ALL was accelerated in the absence of Dnmt3a (Figure 2E). Cumulatively, these data indicate that the order of NOTCH1 and DNMT3A mutation acquisition is not important for the development of T-ALL, just that the 2 mutations be present in the same initiating cell.
To validate DNMT3A as a T-ALL tumor suppressor in human cells, guide RNAs (gRNAs) were designed to inactivate DNMT3A by CRISPR/CRISPR–associated protein 9 (CRISPR/Cas9) genome targeting in cells from patients with primary T-ALL with wild-type DNMT3A. Two previously validated gRNAs that target human DNMT3A29 were used alongside a negative control gRNA that targets the inert AAVS1 locus. gRNA/Cas9 ribonucleoprotein complexes were nucleofected into primary human T-ALL cells and 48 hours after nucleofection, 2.5 × 105 targeted cells were transplanted into NOD-scid IL2Rgammanull mice. Generally, no significant differences were observed between AAVS1 and DNMT3A gRNA-targeted groups across different patient samples as assessed by leukemic burden in the blood, BM, and spleen of moribund mice or time to morbidity (supplemental Figure 3). Analysis of VAFs showed that the frequency of DNMT3A-edited cells tended to increase over the course of the transplant (supplemental Figure 3), suggesting a potential competitive advantage. However, DNMT3A protein could still be detected in the leukemic blasts of moribund mice (supplemental Figure 3), suggesting that the timeframe of these experiments may not have been sufficient to observe dramatic differences in survival. Secondary transplantation was performed and, in this setting, DNMT3A-targeted cells showed much more rapid engraftment in the peripheral blood, significantly shorter time to morbidity, and almost complete elimination of DNMT3A protein (Figure 2F).
JAK/STAT signaling is activated in DNMT3A-mutant T-ALL
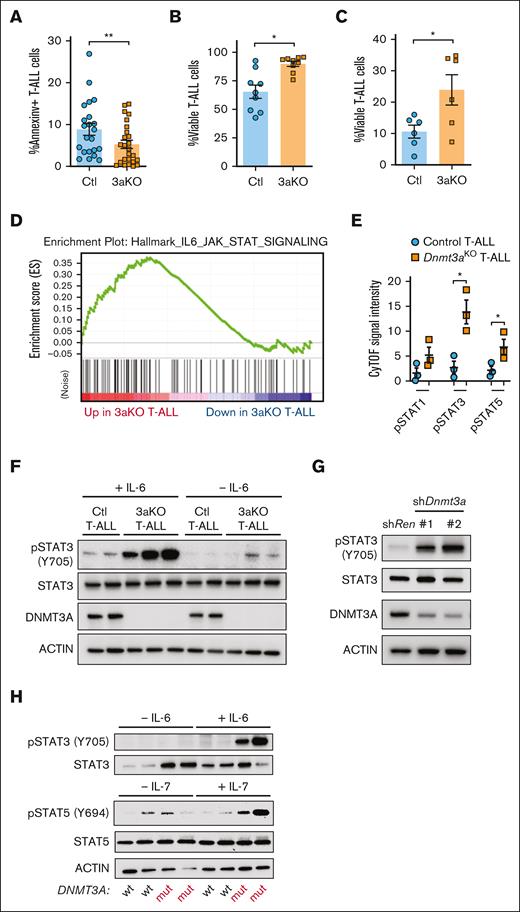
We previously identified that loss of Dnmt3a in mouse T-cell progenitors lead to developmental arrest and reduced apoptosis.22 Annexin-V staining showed apoptosis was also lower in Dnmt3aKO T-ALL cells (Figure 3A). With additional stress of a 6-day in vitro culture, Dnmt3aKO T-ALL cells also showed a survival advantage with OP9-DL1 coculture (Figure 3B) or without stromal cell support (Figure 3C).
JAK/STAT signaling in development of Dnmt3a-mutant T-ALL. (A) Percentage of annexin-V+ (apoptotic) NICD-expressing control (Ctl) or Dnmt3aKO (3aKO) T-ALL blasts freshly isolated from moribund recipient mice. (B) Proportion of viable T-ALL cells of indicated genotypes after 6-day in vitro coculture on OP9-DL1 cells. (C) Proportion of viable T-ALL cells of indicated genotypes after 6-day in vitro culture without stromal support. (D) GSEA plot showing enrichment of JAK/STAT gene expression in mouse Dnmt3aKO T-ALL cells. (E) Cytometry by time of flight (CyTOF) analysis showing levels of phosphorylated Stat proteins in mouse T-ALL cells of indicated genotypes. (F) Western blot of control (Ctl) and Dnmt3aKO (3aKO) T-ALL cells after in vitro stimulation with IL-6. (G) Western blot of MOHITO cells transduced with control (Renilla) or Dnmt3a short hairpin RNAs. (H) Western blot of human T-ALL cells after in vitro stimulation with IL-6 or IL-7.
JAK/STAT signaling in development of Dnmt3a-mutant T-ALL. (A) Percentage of annexin-V+ (apoptotic) NICD-expressing control (Ctl) or Dnmt3aKO (3aKO) T-ALL blasts freshly isolated from moribund recipient mice. (B) Proportion of viable T-ALL cells of indicated genotypes after 6-day in vitro coculture on OP9-DL1 cells. (C) Proportion of viable T-ALL cells of indicated genotypes after 6-day in vitro culture without stromal support. (D) GSEA plot showing enrichment of JAK/STAT gene expression in mouse Dnmt3aKO T-ALL cells. (E) Cytometry by time of flight (CyTOF) analysis showing levels of phosphorylated Stat proteins in mouse T-ALL cells of indicated genotypes. (F) Western blot of control (Ctl) and Dnmt3aKO (3aKO) T-ALL cells after in vitro stimulation with IL-6. (G) Western blot of MOHITO cells transduced with control (Renilla) or Dnmt3a short hairpin RNAs. (H) Western blot of human T-ALL cells after in vitro stimulation with IL-6 or IL-7.
To identify transcriptional signatures associated with this prosurvival phenotype, we examined gene expression (RNA-sequencing) data comparing Dnmt3aKO and control T-ALL blasts.22 Gene set enrichment analysis (GSEA; supplemental Figure 4) revealed 1 of the pathways most significantly different between the genotypes was JAK/STAT signaling, which was elevated in the Dnmt3aKO background (Figure 3D). Cytometry by time of flight (CyTOF) mass cytometry identified higher levels of phosphorylated STAT proteins in Dnmt3aKO T-ALLs (Figure 3E). Increased pSTAT3 was confirmed in freshly isolated leukemic Dnmt3aKO T-ALL blasts, and Dnmt3aKO T-ALL blasts were hypersensitive to IL-6 stimulation (Figure 3F). Introduction of short hairpin RNAs targeting Dnmt3a in Mouse Hematopoietic Interleukin-dependent cell line of T-cell Origin (MOHITO) cells (mouse IL-7–dependent CD4+CD8+ T-cell line) showed increased pSTAT3 (Figure 3G). This isogenic system suggests that increased JAK/STAT signaling is a direct effect of Dnmt3a loss-of-function in premalignant T-cells. Human T-ALL specimens with DNMT3A mutations also showed hypersensitive JAK/STAT signaling in response to cytokine stimulation (Figure 3H).
JAK/STAT signaling in development of Dnmt3a-mutant T-ALL
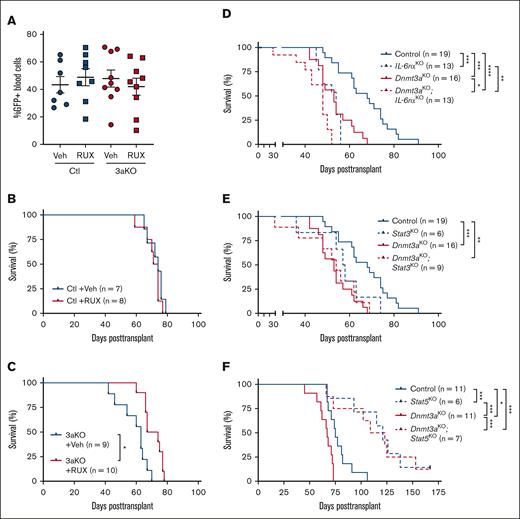
Given the importance of JAK/STAT signaling in T-ALL30,31 and therapy resistance,32 we hypothesized that enhanced activity of this pathway might underlie the increased pathogenesis of Dnmt3a-mutant T-ALL. To determine if mitigation of this signaling pathway influenced Dnmt3a-mutant T-ALL, control and Dnmt3aKO BM progenitor cells from pIpC-induced mice were transduced with NICD retrovirus and transplanted. Mice were randomized based on 5-week peripheral blood T-ALL burden (Figure 4A) to receive the JAK/STAT inhibitor RUX or vehicle control chow. Although RUX had no effect on development of control T-ALL (Figure 4B), mice engrafted with Dnmt3aKO T-ALL showed a significant survival benefit with RUX treatment (Figure 4C).
JAK/STAT signaling in development of Dnmt3a-mutant T-ALL. (A) Engraftment levels of NICD-expressing Control (Ctl) or Dnmt3aKO (3aKO) T-ALL cells in recipient mice before treatment with RUX or vehicle (veh) chow. (B) Kaplan-Meier plot of mice transplanted with control NICD-expressing cells treated with RUX or vehicle (veh) chow. (C) Kaplan-Meier plot of mice transplanted with Dnmt3aKO NICD-expressing cells treated with RUX or vehicle (veh) chow. (D) Kaplan-Meier plot of mice transplanted with NICD-expressing cells of indicated genotypes with and without IL-6rα. (E) Kaplan-Meier plot of mice transplanted with NICD-expressing cells of indicated genotypes with and without Stat3. (F) Kaplan-Meier plot of mice transplanted with NICD-expressing cells of indicated genotypes with and without Stat5.
JAK/STAT signaling in development of Dnmt3a-mutant T-ALL. (A) Engraftment levels of NICD-expressing Control (Ctl) or Dnmt3aKO (3aKO) T-ALL cells in recipient mice before treatment with RUX or vehicle (veh) chow. (B) Kaplan-Meier plot of mice transplanted with control NICD-expressing cells treated with RUX or vehicle (veh) chow. (C) Kaplan-Meier plot of mice transplanted with Dnmt3aKO NICD-expressing cells treated with RUX or vehicle (veh) chow. (D) Kaplan-Meier plot of mice transplanted with NICD-expressing cells of indicated genotypes with and without IL-6rα. (E) Kaplan-Meier plot of mice transplanted with NICD-expressing cells of indicated genotypes with and without Stat3. (F) Kaplan-Meier plot of mice transplanted with NICD-expressing cells of indicated genotypes with and without Stat5.
To determine whether JAK/STAT signaling is also important for clonal expansion of nontransformed Dnmt3a-mutant HSCs, competitive HSC transplantation was performed. A total of 200 HSCs (CD45.2+ lineage− Sca-1+ c-Kit+ CD48− CD150+) from Vav-Cre:Dnmt3a+/+ (Control), Vav-Cre:Dnmt3afl/+ (Dnmt3aHET), Vav-Cre:Dnmt3afl/fl (Dnmt3aKO), and Vav-Cre:Dnmt3aR878H/+ (Dnmt3aR878; mouse homolog of DNMT3AR882H) were transplanted into lethally irradiated mice in competition with 2.5 × 105 congenic (CD45.1) BM cells. Recipient mice of each HSC genotype were randomized to receive either RUX or vehicle control chow between weeks 5 to 9 and 11 to 15 after transplant (with a 2-week drug “break” in the interval). RUX had no significant effect on donor-derived peripheral blood chimerism (supplemental Figure 5A), BM cellularity (supplemental Figure 5B), BM engraftment (supplemental Figure 5C), or self-renewal of donor-derived HSCs in the BM within each genotype (supplemental Figure 5D). To determine whether JAK/STAT inhibition had any long-term impact on HSC function, 200 donor-derived HSCs were purified from primary recipients and transferred into secondary hosts along with 2.5 × 105 fresh competitor BM cells. In this setting, there were some genotype-specific differences. Prior exposure to RUX enhanced overall engraftment of Dnmt3aHET cells in the peripheral blood and BM without affecting HSC self-renewal, whereas clonal expansion of Dnmt3aKO HSCs was mitigated by prior JAK/STAT inhibition (supplemental Figure 5E-H). This suggests that JAK/STAT signaling may also regulate some of the phenotypes associated with DNMT3A-mutant CH.
Because Dnmt3aKO T-ALL cells were hypersensitive to IL-6, and both pSTAT3 and pSTAT5 were elevated, we crossed mice with inactivating floxed alleles of IL-6rα,33,Stat3,34 and Stat535 to Mx1-Cre:Dnmt3afl/fl mice. Resulting double-mutant strains were injected with pIpC to induce deletion of floxed alleles. After 6-weeks recovery, there were no significant differences in HSC numbers in the BM (supplemental Figure 6). NICD retroviral transduction and transplantation assays were performed as described above. Loss of IL-6rα and Stat3 in a Dnmt3aKO background did not rescue the latency to T-ALL (Figure 4D-E). In contrast, loss of Stat5 significantly impeded development of T-ALL, however there was no genotype selectivity (Figure 4F). Thus, although JAK/STAT signaling inhibition can mitigate the survival advantage of Dnmt3aKO T-ALL, it is likely other pathways are also involved.
DNMT3A mutations in T-ALL convey a survival benefit and chemotherapy resistance
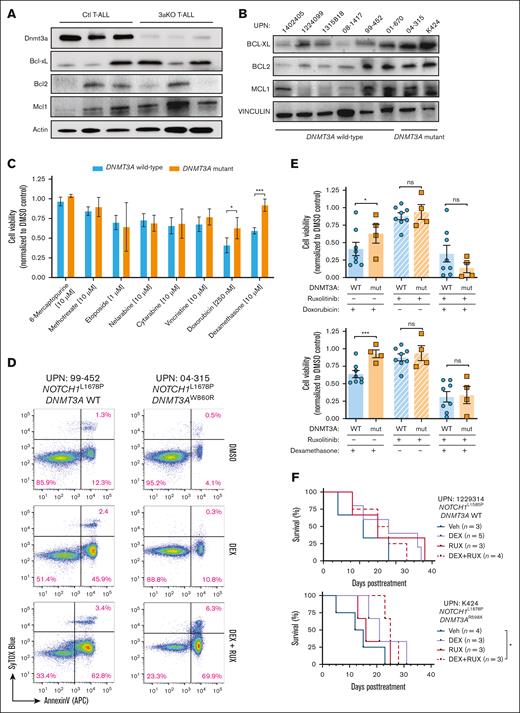
JAK/STAT signaling can promote survival of T-ALL cells by upregulating antiapoptotic proteins. There was heterogeneous upregulation of BCL2 Apoptosis Regulator (BCL2), BCL2 Like 1 (BCL-xL), and MCL1 Apoptosis Regulator, BCL2 Family Member (MCL-1) in mouse (Figure 5A) and human (Figure 5B) T-ALL with DNMT3A mutations. We hypothesized apoptotic resistance may underlie the inferior response of patients with DNMT3A-mutant T-ALL to chemotherapy.18 Primary T-ALL specimens (Figure 1A) were subjected to a panel of agents that form the backbone of conventional T-ALL chemotherapy36; 48-hours later, cell viability was assessed. In general, DNMT3A-mutant T-ALL showed similar sensitivity to most chemotherapeutics compared with patient samples with wild-type DNMT3A but were resistant to apoptosis induced by doxorubicin and dexamethasone (DEX; Figure 5C). We repeated the drug treatment experiments in the presence of RUX, which resensitized cells from patients with DNMT3A-mutant T-ALL to apoptosis (Figure 5D), confirming JAK/STAT signaling potentiates the chemotherapy resistance phenotype of DNMT3A-mutant T-ALL (Figure 5E). To determine whether this may sensitize DNMT3A-mutant T-ALL cells in vivo, PDXs were established and treated with agents once human cell engraftment averaged 10%. Although treatment with DEX or RUX alone had no significant effect on DNMT3A-mutant T-ALL, combination therapy was able to significantly prolong survival of recipient mice (Figure 5F).
DNMT3A mutations in T-ALL convey a survival benefit and chemotherapy resistance. (A) Western blot of freshly isolated control (Ctl) and Dnmt3aKO (3aKO) NICD-expressing T-ALL cells. (B) Western blot of primary human T-ALL cells showing expression of antiapoptotic proteins. (C) Cell viability of primary human T-ALL cells after 48 hours in vitro exposure to indicated chemotherapeutics normalized to DMSO control for each patient sample. (D) Representative flow cytometry plots showing annexin-V staining of T-ALL cells after exposure to DEX with/without RUX. (E) Cumulative cell viability of DNMT3A wild-type (WT) and mutant (mut) T-ALL cells after 48 hours in vitro exposure to indicated drug combinations normalized to DMSO control for each patient sample. (F) Kaplan-Meier plots of representative DNMT3A WT (UPN 1229314) and DNMT3A mutant (UPN K424) T-ALL specimens xenografted into NOD-scid IL2Rgammanull (NSG) mice and then treated with indicated agents once T-ALL burden reached 10% of the peripheral blood.
DNMT3A mutations in T-ALL convey a survival benefit and chemotherapy resistance. (A) Western blot of freshly isolated control (Ctl) and Dnmt3aKO (3aKO) NICD-expressing T-ALL cells. (B) Western blot of primary human T-ALL cells showing expression of antiapoptotic proteins. (C) Cell viability of primary human T-ALL cells after 48 hours in vitro exposure to indicated chemotherapeutics normalized to DMSO control for each patient sample. (D) Representative flow cytometry plots showing annexin-V staining of T-ALL cells after exposure to DEX with/without RUX. (E) Cumulative cell viability of DNMT3A wild-type (WT) and mutant (mut) T-ALL cells after 48 hours in vitro exposure to indicated drug combinations normalized to DMSO control for each patient sample. (F) Kaplan-Meier plots of representative DNMT3A WT (UPN 1229314) and DNMT3A mutant (UPN K424) T-ALL specimens xenografted into NOD-scid IL2Rgammanull (NSG) mice and then treated with indicated agents once T-ALL burden reached 10% of the peripheral blood.
BIRC5 supports the survival advantage of DNMT3A-mutant T-ALL cells
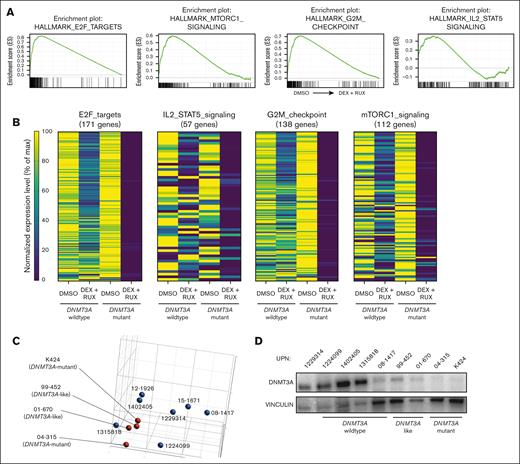
Glucocorticoids such as DEX play an essential role in the treatment of ALL and glucocorticoid resistance is an adverse prognostic factor.37 Activation of the JAK/STAT pathway is known to induce glucocorticoid resistance in patients with T-ALL.30,32 Our data suggest DNMT3A mutations may be a previously unrecognized mechanism that increases JAK/STAT signaling in T-ALL. To identify important genes and pathways that might support this phenotype, RNA-sequencing gene expression analysis was performed on T-ALL cells after 24 hours exposure to DEX (10 μM) in the presence or absence of the JAK/STAT inhibitor RUX (10 μM). GSEA of DNMT3A-mutant T-ALL samples comparing the dimethyl sulfoxide (DMSO) control with DEX + RUX treatment identified 4 differential gene sets: “E2F targets I,” “MTORC1 signaling,” “G2M checkpoint,” and “IL2 / STAT5 signaling”; all downregulated after DEX + RUX treatment (Figure 6A). Although some aspects of these gene sets were also downregulated in cells from patients with wild-type DNMT3A treated with these drugs, the magnitude was much greater in DNMT3A-mutant samples (Figure 6B). Principle component analysis of the control DMSO-treated cells to compare the global transcriptomes of the patient cells at baseline identified 2 DNMT3A-wild-type T-ALL samples that clustered with the DNMT3A-mutant samples (Figure 6C), which we designated “DNMT3A-mutant–like.” Western blot analysis showed patients with DNMT3A-mutant–like disease also had very low expression of DNMT3A, similar to patients with genetic mutations in DNMT3A (Figure 6D). DEX treatment of PDX-derived patient cells showed that DNMT3A-mutant–like samples were highly sensitive to the drug in the presence of RUX, comparable with cells from patients with DNMT3A-mutant T-ALL (Figure 6E). Thus, low DNMT3A expression in patients with T-ALL by heterogeneous mechanisms may confer drug resistance through JAK/STAT pathway activation.
BIRC5 supports the survival advantage of DNMT3A-mutant T-ALL cells. (A) GSEA plots showing significantly different gene sets in DNMT3A-mutant T-ALL specimens after 24-hour treatment with DEX + RUX (all downregulated after treatment). (B) Expression levels of genes within significantly different gene sets comparing WT DNMT3A and DNMT3A-mutant samples after 24 hours exposure to indicated treatments. (C) Principle component analysis of DMSO-treated control T-ALL gene expression profiles from patients with T-ALL by RNA sequencing. (D) Western blot showing DNMT3A levels in indicated T-ALL specimens. (E) Cell viability of wild-type DNMT3A (WT), DNMT3A-mutant–like (like) and DNMT3A-mutant (mut) T-ALL cells after 48 hours in vitro exposure to indicated drug combinations normalized to DMSO control for each patient sample. (F) Normalized expression level of BIRC5 in primary T-ALL cells after 24 hours exposure to indicated treatments. Individual patient specimens are denoted by the same color. (G) Western blot showing protein levels of BIRC5 in indicated samples from patients with T-ALL. (H) Western blot analysis of CUTTL1 cells after CRISPR/Cas9-mediated gene targeting of DNMT3A. (I) Western blot showing protein levels of BIRC5 in a representative WT DNMT3A T-ALL specimen after CRISPR/Cas9 targeting with indicated gRNAs. (J) Western blot for BIRC5 levels in specimens from patients with T-ALL after 48 hours treatment with indicated agents.
BIRC5 supports the survival advantage of DNMT3A-mutant T-ALL cells. (A) GSEA plots showing significantly different gene sets in DNMT3A-mutant T-ALL specimens after 24-hour treatment with DEX + RUX (all downregulated after treatment). (B) Expression levels of genes within significantly different gene sets comparing WT DNMT3A and DNMT3A-mutant samples after 24 hours exposure to indicated treatments. (C) Principle component analysis of DMSO-treated control T-ALL gene expression profiles from patients with T-ALL by RNA sequencing. (D) Western blot showing DNMT3A levels in indicated T-ALL specimens. (E) Cell viability of wild-type DNMT3A (WT), DNMT3A-mutant–like (like) and DNMT3A-mutant (mut) T-ALL cells after 48 hours in vitro exposure to indicated drug combinations normalized to DMSO control for each patient sample. (F) Normalized expression level of BIRC5 in primary T-ALL cells after 24 hours exposure to indicated treatments. Individual patient specimens are denoted by the same color. (G) Western blot showing protein levels of BIRC5 in indicated samples from patients with T-ALL. (H) Western blot analysis of CUTTL1 cells after CRISPR/Cas9-mediated gene targeting of DNMT3A. (I) Western blot showing protein levels of BIRC5 in a representative WT DNMT3A T-ALL specimen after CRISPR/Cas9 targeting with indicated gRNAs. (J) Western blot for BIRC5 levels in specimens from patients with T-ALL after 48 hours treatment with indicated agents.
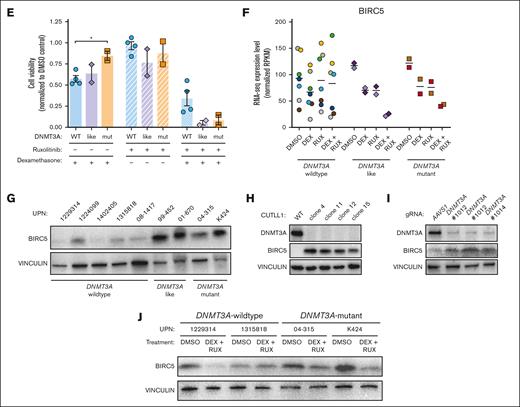
One gene that was identified in multiple GSEA analyses was BIRC5. BIRC5 (also called Survivin) is a member of the inhibitor of apoptosis family of negative regulatory proteins that prevent apoptotic cell death.38 BIRC5 was somewhat downregulated in patients with DNMT3A-mutant and DNMT3A-like T-ALL when treated with either DEX or RUX alone, but dramatically suppressed by the combination therapy, a trend not observed in patient samples without DNMT3A mutations (Figure 6F). Western blot confirmed the upregulation of BIRC5 in DNMT3A-mutant samples (Figure 6G). Because patients contain a heterogeneous mix of mutations, isogenic clones were created after CRISPR/Cas9 targeting of DNMT3A in the CUTTL1 T-ALL cell line. Western blot confirmed that elimination of DNMT3A coincided with increased BIRC5 (Figure 6H). Furthermore, BIRC5 was also heterogeneously increased in DNMT3A-wildtype T-ALL specimens after CRISPR/Cas9 targeting for DNMT3A (Figure 6I), and DEX + RUX treatment reduced BIRC5 protein levels (Figure 6J). Although the expression differences were not obviously linked to changes in DNA methylation (supplemental Figure 7), cumulatively these data show that BIRC5 is increased in T-ALL cells as a direct consequence of DNMT3A loss of function.
BIRC5 as a precision medicine target for DNMT3A-mutant T-ALL
We hypothesized that BIRC5 might specifically support the prosurvival phenotype of DNMT3A-mutant T-ALL. Patient T-ALL cells were nucleofected with small interfering RNAs against human BIRC5 (supplemental Figure 8A). Exposure of cells from patients with wild-type DNMT3A T-ALL to BIRC5 small interfering RNA had minimal effect on cell viability (Figure 7A-B). In contrast, BIRC5 inhibition induced significant apoptosis in DNMT3A-mutant T-ALL cells (Figure 7A-B). Sepantronium bromide (YM155) has been identified to inhibit BIRC5 expression at the messenger RNA and protein level.39 Treatment of JURKAT cells with YM155 inhibited BIRC5 expression (supplemental Figure 8B) and DNMT3A-knockout JURKAT cells showed increased sensitivity to YM155 (supplemental Figure 8C). A drug concentration gradient using primary T-ALL cells identified a dose that showed differential effects in DNMT3A-mutant cells (supplemental Figure 8D). Treatment of T-ALL cells with 1 μM YM155 for 48 hours revealed a dramatic decrease in viability of DNMT3A-mutant cells with minimal impact on wild-type DNMT3A cells (Figure 7C-D). To determine whether YM155 might synergize with JAK/STAT inhibition, primary cells were cultured for 48 hours with 10 μM DEX and 10 μM RUX with or without 1 μM YM155. There was no synergistic effect (supplemental Figure 8E), suggesting that BIRC5 is likely a direct target of DEX plus RUX inhibition in primary T-ALL cells.
BIRC5 as a precision medicine target for DNMT3A-mutant T-ALL. (A) Cell viability of indicated T-ALL specimens (normalized to negative control small interfering [siRNA]) 48 hours after nucleofection with BIRC5 siRNA. (B) Compiled normalized cell viability averaged for individual WT DNMT3A and DNMT3A-mutant T-ALL samples after nucleofection with BIRC5 siRNA. (C) Cell viability of indicated T-ALL specimens (normalized to DMSO control) after 48 hours exposure to YM155. (D) Compiled normalized cell viability averaged for individual WT DNMT3A and DNMT3A-mutant T-ALL samples after 48 hours exposure to YM155. (E) Kaplan-Meier plots of NSG mice xenografted with indicated T-ALL specimens after CRISPR/Cas9 genome targeting with indicated gRNAs. (F) VAF of CRISPR edits from indicated gRNAs in T-ALL blasts from peripheral blood (6-8 weeks after transplant) and BM of moribund mice (euthanized) normalized to initial targeting efficiency 48 hours after nucleofection (initial) at time of transplant.
BIRC5 as a precision medicine target for DNMT3A-mutant T-ALL. (A) Cell viability of indicated T-ALL specimens (normalized to negative control small interfering [siRNA]) 48 hours after nucleofection with BIRC5 siRNA. (B) Compiled normalized cell viability averaged for individual WT DNMT3A and DNMT3A-mutant T-ALL samples after nucleofection with BIRC5 siRNA. (C) Cell viability of indicated T-ALL specimens (normalized to DMSO control) after 48 hours exposure to YM155. (D) Compiled normalized cell viability averaged for individual WT DNMT3A and DNMT3A-mutant T-ALL samples after 48 hours exposure to YM155. (E) Kaplan-Meier plots of NSG mice xenografted with indicated T-ALL specimens after CRISPR/Cas9 genome targeting with indicated gRNAs. (F) VAF of CRISPR edits from indicated gRNAs in T-ALL blasts from peripheral blood (6-8 weeks after transplant) and BM of moribund mice (euthanized) normalized to initial targeting efficiency 48 hours after nucleofection (initial) at time of transplant.
To test the role of BIRC5 in DNMT3A-mutant T-ALL in vivo, we targeted the locus for genetic inactivation using CRISPR/Cas9. Three gRNAs targeting human BIRC5 were designed, and most efficacious gRNA used for in vivo studies (supplemental Figure 8F). Across samples from patients with T-ALL, either wild-type and mutant for DNMT3A, genetic inhibition of BIRC5 did not alter overall time to morbidity (Figure 7E) or disease burden compared with AAVS1-targeted cells (supplemental Figure 8G). However, although samples from patients with wild-type DNMT3A were still able to expand in the absence of BIRC5, BIRC5-targeted DNMT3A-mutant cells were rapidly outcompeted in vivo (supplemental Figure 8H). This severe competitive disadvantage (Figure 7F) supports the hypothesis that BIRC5 is a specific genetic dependency of DNMT3A-mutant T-ALL and targeting BIRC5 in this subset of patients may be an effective precision medicine strategy.
Discussion
In T-ALL, DNMT3A mutations frequently co-occur with NOTCH1 mutations and predict poor clinical outcomes.16-18 Although this mutational profile represents a minor fraction of adult patients with T-ALL overall, it represents a major population of difficult-to-treat cases with poor outcomes. Our studies highlight new clinical approaches for these patients. Results here show that 1 of the major functional consequences of DNMT3A mutations in patients with T-ALL is to potentiate JAK/STAT signaling, leading to enhanced survival. Aberrant JAK/STAT signaling has previously been recognized as a potential pathogenic mechanism in T-ALL.30 The pathway is accentuated by activating genetic mutations in IL7R, JAK1, JAK3, and STAT5 in 20% to 30% of T-ALL cases.5 Although clinical trials with RUX in relapsed/refractory acute leukemia have produced modest results, these trials did not consider genetics to select the most appropriate patient population that would benefit from such therapy. We propose that more appropriate selection of adult patients with T-ALL for JAK/STAT inhibition could produce significant clinical benefit. In addition to genetic mutations in DNMT3A, we show that DNMT3A suppression in T-ALL cells by alternative mechanisms (Figure 6D) also confers sensitivity to RUX (Figure 6E), potentially expanding the T-ALL population that may benefit from JAK/STAT inhibition. A complicating factor could arise from our observation that JAK/STAT inhibition may also have mutation-specific effects on underlying CH clones with DNMT3A mutations (supplemental Figure 5). However, genomic studies of patients with myelofibrosis treated long-term with RUX as standard of care has not identified clear selection biases of preleukemic clones with DNMT3A mutations.40-42
An unresolved question arising from these studies is how do DNMT3A mutations activate JAK/STAT signaling in T-ALL cells? We were unable to identify obvious DNA methylation alterations that could explain this (Figure 1E) and although expression of upstream regulators was increased in murine Dnmt3aKO T-ALL cells (supplemental Figure 4B), levels of the core components was largely similar to control T-All cells, with the exception of Ptpn6 (supplemental Figure 4C). Ptpn6 encodes the tyrosine phosphatase SHP-1 that negatively regulates STAT signaling.43 It is possible that downregulation of Ptpn6 leads to the inability to dephosphorylate activated STATs and potentiates the signaling in Dnmt3aKO T-ALL cells. These mechanisms remain to be explored in future studies.
Mechanistically, our data show that DNMT3A loss of function upregulates BIRC5 in T-ALL cells. BIRC5 inhibits cell death via both the extrinsic and intrinsic apoptotic pathways and suppresses caspase activity.44,45 It has been shown that inhibition of BIRC5 can induce tumor cell apoptosis and enhance sensitivity to chemotherapeutics or other apoptotic stimuli.46 Our data show that BIRC5 is upregulated in DNMT3A-mutant T-ALL specimens, and these patient cells are exquisitely sensitive to BIRC5 inhibition. This positions BIRC5 as a specific genetic dependency in DNMT3A-mutant T-ALL. Although there has been clinical interest in targeting this protein, there are currently no specific inhibitors available. Clinical efforts are ongoing to produce more specific BIRC5 inhibitors, and our results indicate that patients with DNMT3A-mutant T-ALL would be an ideal patient population to trial such agents.
Cumulatively, these studies reveal that DNMT3A mutations promote survival and chemotherapy resistance in cells from patients with T-ALL and identify BIRC5 as a specific genetic dependency of DNMT3A-mutant T-ALL cells. These data provide a critical first step toward a novel target for precision medicine approaches for this patient group with poor outcomes.
Acknowledgments
The authors thank all members of the Challen laboratory for critical discussions and support. The authors thank the Histology and Morphometry Core at the Washington University Musculoskeletal Research Center for slide processing and imaging. For supplying critical reagents, the authors thank Ross Levine (Memorial Sloan Kettering Cancer Center) for MOHITO cells, Rajendra Apte (Washington University School of Medicine) for Stat3fl/fl mice, and Stacey Rentschler (Washington University School of Medicine) for tetO_NICD mice. The authors thank the Alvin J. Siteman Cancer Center at Washington University for use of the Siteman Flow Cytometry Core, Tissue Procurement Core, and Immunomonitoring Laboratory, supported, in part, by National Cancer Institute (NCI) grant CA91842 and National Institutes of Health (NIH) WLC6313040077. The Immunomonitoring Laboratory is also supported by the Andrew M. and Jane M. Bursky Center for Human Immunology and Immunotherapy Programs. The authors thank the Genome Technology Access Center and McDonnell Genome Institute at Washington University for genomic analysis, partially supported by NCI grant CA91842 and by NIH grant UL1TR000448. Support for procurement of human samples was provided by an NIH/NCI SPORE in Leukemia grant (P50 CA171963; principle investigator: D. Link). G.A.C. was a scholar of the Leukemia and Lymphoma Society.
G.A.C. was supported by the National Heart, Lung, and Blood Institute (HL147978), the National Cancer Institute (CA236819) and the National Institute of Diabetes and Digestive and Kidney Diseases (DK124883), the Leukemia and Lymphoma Society (6667-23), and the American Cancer Society (CSCC-RSG-23-991417-01-CSCC). T.M.P. was a fellow of the Leukemia and Lymphoma Society. A.L.Y. was supported by National Heart, Lung, and Blood Institute (T32HL007088) and the ASH Research Training Award for Fellows. J.R.E. was supported by the National Library of Medicine (LM013096).
This publication is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Authorship
Contribution: G.A.C. conceptualized and designed the study, acquired funding, performed project administration, supervised the study, and prepared the manuscript. W.M.B.D., W.C.W., N.I., W.H., I.X.R., A.K., H.B., T.G., and G.A.C. performed experimentation and data acquisition; T.M.P., A.L.Y., J.R.E., and G.A.C. performed data analysis; E.S.W., A.P.W., M.C.S., H.C., and A.F.C. provided resources and reagents.
Conflict-of-interest disclosure: A.L.Y. has performed consulting for BioGenerator; and is a cofounder, chief executive officer, and shareholder of Pairidex, Inc. T.M.P. has performed consulting for Pillar Patient Advocates, Silence Therapeutics, and the Myeloproliferative neoplasm research foundation (MPNRF). M.C.S. and H.C. are employees of Incyte Research Institute. G.A.C. has performed consulting and received research funding from Incyte, Ajax Therapeutics, and ReNAgade Therapeutics Management; and is a cofounder, member of the scientific advisory board, and shareholder of Pairidex, Inc. The remaining authors declare no competing financial interests.
The current affiliation for W.C.W. is DEM BioPharma, Cambridge, MA.
Correspondence: Grant A. Challen, Division of Oncology, Department of Medicine, Washington University School of Medicine, 660 Euclid Ave, St. Louis, MO 63110; email: grantchallen@wustl.edu.
References
Author notes
Whole-genome bisulfite sequencing and RNA-sequencing data of patients with T-ALL are available at dbGAP under accession phs003623.v1.p1
All data are available in the main text or the supplemental Materials.
The full-text version of this article contains a data supplement.


![BIRC5 as a precision medicine target for DNMT3A-mutant T-ALL. (A) Cell viability of indicated T-ALL specimens (normalized to negative control small interfering [siRNA]) 48 hours after nucleofection with BIRC5 siRNA. (B) Compiled normalized cell viability averaged for individual WT DNMT3A and DNMT3A-mutant T-ALL samples after nucleofection with BIRC5 siRNA. (C) Cell viability of indicated T-ALL specimens (normalized to DMSO control) after 48 hours exposure to YM155. (D) Compiled normalized cell viability averaged for individual WT DNMT3A and DNMT3A-mutant T-ALL samples after 48 hours exposure to YM155. (E) Kaplan-Meier plots of NSG mice xenografted with indicated T-ALL specimens after CRISPR/Cas9 genome targeting with indicated gRNAs. (F) VAF of CRISPR edits from indicated gRNAs in T-ALL blasts from peripheral blood (6-8 weeks after transplant) and BM of moribund mice (euthanized) normalized to initial targeting efficiency 48 hours after nucleofection (initial) at time of transplant.](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodneoplasia/1/4/10.1016_j.bneo.2024.100040/2/m_bneo_neo-2024-000314-gr7.jpeg?Expires=1764613204&Signature=jn8Le2nsHMLHCZ2w55X4IbYiiaYE77e~RmV66VKKvLCfXvuwqwEIoM-k4oS-IHZI5gx0wKyPGRCDhHzgNpyXeLaC95BsFSDk5SDfC6HkTNj~7zUBdeln~l1h80RvV0CTzUckJy0rjwxRVIO3GLSQxdPm0Y2kIUYOJefiSZO5xgxwxbP2ZqTsU7yYoDj7-En0qrNuPOFa6AvJX4KzuvFfRyEhen6b4Y2IaBWUL--8-4tvAfnCavhse76HAM5-3NJR4CQIew~attbESa9Ib6IZWCoWCqGbMsJlwRrXOiA40IJCNi7LbBALzhKpgqSRFhv9E-fi78G9btpNYdkcUoOy0g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)