Key Points
In a phase 1b study of relapsed/refractory myeloma, the anti-CD38 antibody mezagitamab was well tolerated and showed preliminary activity.
At the recommended phase 2 dose of 600 mg, the overall response rate was 47%, and the median duration of response was 22.1 months.
Visual Abstract
This phase 1b trial aimed to determine the safety, tolerability, and preliminary efficacy of mezagitamab, a subcutaneously administered anti-CD38 monoclonal antibody, in patients with relapsed/refractory multiple myeloma (RRMM). Eligible patients had received ≥3 prior lines of treatment, including an immunomodulatory drug (IMiD), a proteasome inhibitor (PI), and a steroid, or ≥2 prior lines in which 1 included a PI + IMiD, and were refractory or intolerant to ≥1 IMiD and ≥1 PI. Fifty patients were enrolled: 44 received mezagitamab monotherapy (dose-escalating cohorts at 45-1200 mg) and 6 received mezagitamab 300 mg in combination with pomalidomide plus dexamethasone. Patients received mezagitamab weekly for 8 doses, every other week for 8 doses, and monthly thereafter. No dose-limiting toxicities were reported with single-agent mezagitamab, and the recommended phase 2 dose was determined as 600 mg. The most common drug-related treatment-emergent adverse events (TEAEs) were fatigue in the monotherapy cohort (9/44 patients) and neutropenia in the combination cohort (4/6 patients); neutropenia was the only drug-related grade ≥3 TEAE to occur in >1 patient. No infusion reactions occurred, and 4 injection-site reactions were reported. Three patients discontinued treatment due to TEAEs. Among the 22 patients receiving 600 mg mezagitamab, the overall response rate was 47%, and the median duration of response was 22.1 months. Mezagitamab outcomes were comparable to those reported with other anti-CD38 therapies in patients with advanced RRMM. Further development of mezagitamab in myeloma is not planned, but studies are underway in autoimmune conditions. This trial was registered at www.ClinicalTrials.gov as #NCT03439280.
Introduction
Despite treatment advances, relapsed/refractory multiple myeloma (RRMM) remains incurable, and there is considerable scope to improve the prognosis for patients in whom prior treatments have failed.1
Mezagitamab (TAK-079) is a fully human, immunoglobulin G1 (IgG1) monoclonal antibody (mAb) with high affinity for CD38.2 CD38 is a cell-surface molecule that is highly expressed on malignant cells in hematologic cancers (including MM), antibody-producing plasma cells, plasmablasts, and natural killer cells, and is induced on subsets of activated T cells and B cells.3 In addition, pathogenic cells related to autoimmune diseases such as IgA nephropathy or systemic lupus erythematosus often express CD38.4-9 Mezagitamab has been shown to deplete human tumors and immune cells expressing CD38 through antibody-dependent cellular cytotoxicity, antibody-dependent phagocytosis, complement-dependent cytotoxicity, and apoptosis.2,8,10 Through these mechanisms of action, mezagitamab may hold promise for treating hematologic malignancies (particularly MM and autoimmune diseases).
Mezagitamab binds to a partially distinct epitope of CD38 and possesses a different binding profile than other anti-CD38 therapeutic mAbs. This formulation supports IV and subcutaneous (SC) administration, with the SC route being selected for further clinical development. A low drug volume (200 mg/2 mL; supplemental Methods) may help reduce the treatment administration burden for patients and health care providers.
In a first-in-human study conducted in healthy individuals, treatment with mezagitamab resulted in a dose-dependent reduction of natural killer cells and plasmablasts after single-dose IV/SC administration (at escalating doses), with no unexpected and unwanted clinical or hematologic effects observed.11 The highest single SC dose administered in the healthy volunteer study was 0.6 mg/kg.11 In view of its potential in CD38-expressing hematologic malignancies, the present phase 1b trial aimed to determine the safety, tolerability, efficacy, pharmacokinetics (PK), pharmacodynamics, and immunogenicity of single-agent mezagitamab in patients with RRMM. The study later added a cohort for mezagitamab in combination with pomalidomide and dexamethasone (PomDex), an approved regimen for patients with RRMM treated previously with a proteasome inhibitor (PI) and an immunomodulatory drug (IMiD).12 The primary study results are reported.
Methods
Patient eligibility
Eligible patients were aged ≥18 years with measurable RRMM, as defined by the International Myeloma Working Group criteria.13,14 For single-agent mezagitamab, patients had received ≥3 prior lines of treatment, including an IMiD, a PI, and a steroid, or ≥2 prior lines, in which 1 included a combination of a PI and an IMiD. Patients also had to be refractory or intolerant to ≥1 IMiD and ≥1 PI. Prior exposure to an anti-CD38 agent, including refractoriness to anti-CD38 mAb, was allowed provided that there was a 180-day washout period before study start. For mezagitamab in combination with PomDex, patients had received ≥2 prior lines, including lenalidomide and a PI, and had disease progression (PD) on or within 60 days of completing their last therapy.
Patients who had previously undergone allogeneic stem cell transplantation were not eligible. See supplemental Methods for the full eligibility and exclusion criteria.
Study design and treatment
This was an open-label, multicenter, phase 1b study. A phase 2a portion was planned but, due to strategic decisions unrelated to safety, the sponsor decided not to open this part of the study. In phase 1, a 3 + 3 dose-escalation scheme was used to determine the maximum tolerated dose (MTD) and recommended phase 2 dose (RP2D) of single-agent mezagitamab, based on the occurrence of cycle 1 dose-limiting toxicities (DLTs). Cohort expansion was permitted to further inform the selection of RP2D. Once the RP2D had been selected, additional patients were enrolled for dose confirmation (dose-confirmation cohort), including up to 6 patients who were refractory to an anti-CD38 mAb and ∼12 patients who were anti-CD38 mAb naïve. Lastly, a separate cohort was opened to evaluate the combination of mezagitamab with PomDex.
In the monotherapy cohorts, after receiving premedication (which included dexamethasone), patients received mezagitamab in 28-day cycles via SC administration at a starting fixed dose of 45 mg. Up to 6 dose levels were planned at a maximum dose of 1800 mg. Mezagitamab doses were administered as SC injections up to a maximum volume of 2 mL per injection (ie, 200 mg/2 mL). For dose levels ≥300 mg, multiple SC injections were required to administer the full dose. Mezagitamab was given on days 1, 8, 15, and 22 of cycles 1 and 2, days 1 and 15 of cycles 3 to 6, and on day 1 from cycle 7 onwards. In the mezagitamab and PomDex combination cohort, patients received mezagitamab 300 mg per the same dosing schedule. In the combination cohort, oral pomalidomide (4 mg) was administered daily on days 1 to 21, whereas oral dexamethasone (40 mg) was given on days 1, 8, 15, and 22 of a 28-day cycle. All patients were treated until PD, unacceptable toxicities, or patient withdrawal. Patients who derive clinical benefits, as agreed upon by the sponsor’s study physician, could continue the study treatment. Pre- and postdose medications to mitigate against potential hypersensitivity infusion-related reactions and supportive therapies consistent with optimal patient care were available, as required (supplemental Methods).
This trial was performed in accordance with the Declaration of Helsinki, Good Clinical Practice regulations, and relevant local and national guidelines. The study documentation, including the protocol, was approved by the institutional review board of each site. Patients provided written informed consent.
Data were analyzed using ICON, Raleigh, NC (start of the study to March 2019) and Pharmaceutical Product Development (PPD), part of Thermo Fisher Scientific, Wilmington, NC (March 2019 to the end of the study).
DLTs and dose escalation
DLTs were assessed during cycle 1 of the study treatment (supplemental Figure 1). With some specific exceptions (supplemental Methods), DLTs were defined as grade 4 laboratory abnormalities, grade ≥3 nonhematologic treatment-emergent adverse events (TEAEs), grade ≥4 hematologic TEAEs, or incomplete recovery from treatment-related toxicity causing a >2-week delay in the next scheduled injection before the initiation of cycle 2. MTD was defined as the highest dose in a cohort of 6 patients, in which ≤1 patient experienced DLT. An RP2D dose below the MTD was identified based on an assessment of all available data.
In the combination cohort, 6 patients were initially enrolled. Although dose expansion (at the initial dose) or dose de-escalation was planned based on the occurrence of DLTs, it did not proceed after the decision not to open the phase 2 portion of the study.
Objectives
The primary objective was to determine the safety and tolerability of mezagitamab as monotherapy and in combination with PomDex. The secondary objectives were to evaluate the MTD/RP2D, immunogenicity, PK, and preliminary clinical activity of mezagitamab. An exploration of potential biomarkers was also undertaken.
Assessments
TEAEs were monitored throughout and for ∼30 days after the last dose of the study drug or until the start of subsequent alternative anticancer therapy. Grading of TEAEs was according to the National Cancer Institute’s Common Terminology Criteria for Adverse Events, version 4.03. Disease response was assessed by investigators at screening and the start of each cycle according to the International Myeloma Working Group uniform response criteria,15 with all response categories requiring 2 consecutive assessments to be considered confirmed, and bone marrow aspirate required for confirmation of a complete response. Responses were assessed at each time point individually, instead of carrying over the best response until PD. Best overall responses, as reported here, did not require 2 consecutive assessments, and included a combination of confirmed and unconfirmed responses. Patients who discontinued treatment for reasons other than PD were followed up for progression-free survival (PFS) every 4 weeks from the end-of-treatment visit until PD, death, the start of subsequent anticancer therapy, study termination, or 12 months after discontinuation, whichever occurred first. Follow-up for overall survival was every 12 weeks after documented PD until death, loss to follow-up, withdrawal of consent, or study termination.
Blood samples were obtained throughout cycles 1 and 2 for PK analysis, with the sampling frequency decreasing in cycles 3 to 10, as described in supplemental Methods. PK parameters were estimated from the serum concentration-time profiles using noncompartmental methods with Phoenix WinNonlin. Antidrug antibody testing was performed on blood samples taken before dosing on day 1 of each cycle, with an additional sample taken on day 15 of cycle 1.
Statistical analyses
Approximately 60 patients were planned to be enrolled based on the 3 + 3 dose-escalation and dose-confirmatory study design. Continuous data were summarized using descriptive statistics. Categorical data were summarized as the number and percentage of patients. Time-to-event data were analyzed using the Kaplan-Meier method. No formal statistical analyses were planned.
The safety population included all patients who received ≥1 dose of mezagitamab. The DLT-evaluable population (used to determine RP2D/MTD) included all patients who had received cycle 1 doses of mezagitamab and had completed cycle 1 procedures or experienced a DLT in this first cycle. The response-evaluable population included all patients with measurable disease at the baseline and at ≥1 posttreatment evaluation. The PK analysis set included patients from the safety analysis set with sufficient dosing data and mezagitamab concentration-time data to permit the calculation of PK parameters. Patients from the safety population with a baseline and ≥1 postbaseline sample assessment for antidrug antibodies were included in the immunogenicity analysis set.
Results
Patients
Between 20 April 2018, and 26 January 2021, 50 patients were enrolled in the study at 7 sites in the United States, with 44 patients receiving mezagitamab monotherapy (45 mg, n = 4; 135 mg, n = 3; 300 mg, n = 12; 600 mg, n = 22; and 1200 mg, n = 3) and 6 receiving mezagitamab 300 mg in combination with PomDex. All 50 patients discontinued the study treatment, including 4 who benefited from study treatment at the time of study closure and entered a posttrial access study. The main reason for study discontinuation was PD (n = 40). One patient discontinued the treatment because of AEs (supplemental Table 1).
The patient characteristics are presented in Table 1. The median age was 66 years (range, 50-85); 66% of patients were male; 62% and 18% of patients reported their race as White and Black or African American, respectively. A total of 56% of the patients had International Staging System stage II/III disease. Among the 48 patients with baseline cytogenetic testing, 31% had high-risk cytogenetic abnormalities (defined as del 17, t[4:14], t[14:16]).
Patient demographics, disease characteristics, and treatment history at baseline (all enrolled patients)
| . | Mezagitamab cohort . | ||||||
|---|---|---|---|---|---|---|---|
| 45 mg (n = 4) . | 135 mg (n = 3) . | 300 mg (n = 12) . | 600 mg (n = 22) . | 1200 mg (n = 3) . | PomDex (n = 6) . | Total (N = 50) . | |
| Median age, y (range) | 64.5 (53-75) | 69.0 (64-74) | 67.5 (56-76) | 67.0 (59-85) | 64.0 (50-72) | 64.0 (52-74) | 66.0 (50-85) |
| Male sex, n (%) | 2 (50) | 2 (67) | 10 (85) | 14 (64) | 3 (100) | 2 (33) | 33 (66) |
| Race, n (%) | |||||||
| White | 1 (25) | 0 | 9 (75) | 15 (68) | 3 (100) | 3 (50) | 31 (62) |
| Black or African American | 2 (50) | 2 (67) | 0 | 3 (14) | 0 | 2 (33) | 9 (18) |
| Other | 0 | 0 | 2 (17) | 1 (5) | 0 | 0 | 3 (6) |
| Not reported | 1 (25) | 1 (33) | 1 (8) | 3 (14) | 0 | 1 (17) | 7 (14) |
| ECOG performance status, n (%) | |||||||
| 0 | 2 (50) | 1 (33) | 5 (42) | 2 (9) | 0 | 3 (50) | 13 (26) |
| 1 | 2 (50) | 2 (67) | 7 (58) | 19 (86) | 3 (100) | 3 (50) | 36 (72) |
| 2 | 0 | 0 | 0 | 1 (5) | 0 | 0 | 1 (2) |
| Median time from initial diagnosis, mo (range) | 108.3 (29.4-129.9) | 96.1 (43.1-165.3) | 70.8 (30.5-163.8) | 95.3 (16.0-272.2) | 83.1 (50.7-147.1) | 110.4 (25.7-384.7) | 94.8 (16.0-384.7) |
| MM type at initial diagnosis, n (%) | |||||||
| IgG | 4 (100) | 3 (100) | 6 (50) | 15 (68) | 3 (100) | 5 (83) | 36 (72) |
| IgA | 0 | 0 | 5 (42) | 6 (27) | 0 | 1 (17) | 12 (24) |
| Light chain only | 0 | 0 | 1 (8) | 1 (5) | 0 | 0 | 2 (4) |
| ISS stage at study entry, n (%) | |||||||
| I | 3 (75) | 1 (33) | 5 (42) | 6 (27) | 2 (67) | 1 (17) | 18 (36) |
| II | 0 | 1 (33) | 3 (25) | 10 (45) | 1 (33) | 2 (33) | 17 (34) |
| III | 1 (25) | 0 | 4 (33) | 4 (18) | 0 | 2 (33) | 11 (22) |
| Missing∗ | 0 | 1 (33) | 0 | 2 (10) | 0 | 1 (17) | 4 (8) |
| Median corrected calcium, mmol/L (range) | 2.3 (2.3-2.4) | 2.3 (2.2-2.3) | 2.4 (2.2-2.8) | 2.3 (2.2-2.6) | 2.3 (2.2-2.6) | 2.3 (2.2-2.4) | 2.3 (2.2-2.8) |
| Median hemoglobin, g/L (range) | 125 (106-138) | 118 (104-126) | 124.5 (83-153) | 121 (89-145) | 107 (107-122) | 119 (110-152) | 121 (83-153) |
| Median creatinine clearance, mL/min (range) | 83 (44-116) | 71 (52-77) | 86 (30-134) | 81 (31-143) | 97 (91-117) | 107 (56-144) | 86 (30-144) |
| Creatinine clearance category, n (%) | |||||||
| 30 to <60 mL/min | 1 (25) | 1 (33) | 3 (25) | 6 (27) | 0 | 1 (17) | 12 (24) |
| 60 to <90 mL/min | 1 (25) | 2 (67) | 3 (25) | 8 (36) | 0 | 1 (17) | 15 (30) |
| ≥90 mL/min | 2 (50) | 0 | 6 (50) | 8 (36) | 3 (100) | 4 (67) | 23 (46) |
| Lytic bone lesions, n (%) | n = 3 | n = 3 | n = 11 | n = 21 | n = 3 | n = 6 | N = 47 |
| Yes | 3 (100) | 2 (67) | 7 (64) | 12 (57) | 1 (33) | 2 (33) | 27 (57) |
| No | 0 | 1 (33) | 4 (36) | 8 (38) | 2 (67) | 4 (67) | 19 (40) |
| Indeterminate | 0 | 0 | 0 | 1 (5) | 0 | 0 | 1 (2) |
| Plasmacytomas, n (%) | n = 1 | n = 0 | n = 2 | n = 5 | n = 1 | n = 0 | N = 9 |
| Yes | 0 (9) | 0 | 0 | 1 (20) | 1 (100) | 0 | 2 (22) |
| No | 1 (100) | 0 | 2 (100) | 4 (80) | 0 | 0 | 7 (78) |
| Chromosomal aberrations, n (%) | n = 4 | n = 3 | n = 11 | n = 21 | n = 3 | n = 6 | N = 48 |
| High-risk cytogenetics† | 0 | 1 (33) | 4 (36) | 6 (29) | 1 (33) | 3 (50) | 15 (31) |
| Standard-risk cytogenetics | 4 (100) | 2 (67) | 7 (64) | 15 (71) | 2 (67) | 3 (50) | 33 (69) |
| Median number of prior lines of therapy, (range) | 4.0 (2-7) | 3.0 (3-5) | 3.0 (2-12) | 3.0 (2-16) | 4 (3-4) | 4 (2-5) | 3.5 (2-16) |
| Prior treatment, n (%) | |||||||
| Autologous stem cell transplant | 4 (100) | 3 (100) | 10 (83) | 17 (77) | 3 (100) | 6 (100) | 43 (86) |
| Alkylating agent | 4 (100) | 3 (100) | 12 (100) | 20 (91) | 3 (100) | 6 (100) | 48 (96) |
| Anti-CD38 antibody | 1 (25) | 0 | 3 (25) | 7 (32) | 0 | 0 | 11 (22) |
| IMiD | 4 (100) | 3 (100) | 12 (100) | 22 (100) | 3 (100) | 6 (100) | 50 (100) |
| PI | 4 (100) | 3 (100) | 12 (100) | 22 (100) | 3 (100) | 6 (100) | 50 (100) |
| Refractory status, n (%)‡ | |||||||
| To anti-CD38 antibody | 0 | 0 | 1 (8) | 5 (23) | 0 | 0 | 6 (12) |
| To either an IMiD or a PI | 4 (100) | 3 (100) | 10 (83) | 22 (100) | 3 (100) | 5 (83) | 47 (94) |
| To both an IMiD and a PI | 3 (75) | 1 (33) | 8 (67) | 17 (77) | 0 | 1 (17) | 30 (60) |
| To last line of therapy | 4 (100) | 2 (67) | 10 (83) | 18 (82) | 3 (100) | 4 (67) | 41 (82) |
| To any line of therapy | 4 (100) | 3 (100) | 10 (83) | 22 (100) | 3 (100) | 5 (83) | 47 (94) |
| . | Mezagitamab cohort . | ||||||
|---|---|---|---|---|---|---|---|
| 45 mg (n = 4) . | 135 mg (n = 3) . | 300 mg (n = 12) . | 600 mg (n = 22) . | 1200 mg (n = 3) . | PomDex (n = 6) . | Total (N = 50) . | |
| Median age, y (range) | 64.5 (53-75) | 69.0 (64-74) | 67.5 (56-76) | 67.0 (59-85) | 64.0 (50-72) | 64.0 (52-74) | 66.0 (50-85) |
| Male sex, n (%) | 2 (50) | 2 (67) | 10 (85) | 14 (64) | 3 (100) | 2 (33) | 33 (66) |
| Race, n (%) | |||||||
| White | 1 (25) | 0 | 9 (75) | 15 (68) | 3 (100) | 3 (50) | 31 (62) |
| Black or African American | 2 (50) | 2 (67) | 0 | 3 (14) | 0 | 2 (33) | 9 (18) |
| Other | 0 | 0 | 2 (17) | 1 (5) | 0 | 0 | 3 (6) |
| Not reported | 1 (25) | 1 (33) | 1 (8) | 3 (14) | 0 | 1 (17) | 7 (14) |
| ECOG performance status, n (%) | |||||||
| 0 | 2 (50) | 1 (33) | 5 (42) | 2 (9) | 0 | 3 (50) | 13 (26) |
| 1 | 2 (50) | 2 (67) | 7 (58) | 19 (86) | 3 (100) | 3 (50) | 36 (72) |
| 2 | 0 | 0 | 0 | 1 (5) | 0 | 0 | 1 (2) |
| Median time from initial diagnosis, mo (range) | 108.3 (29.4-129.9) | 96.1 (43.1-165.3) | 70.8 (30.5-163.8) | 95.3 (16.0-272.2) | 83.1 (50.7-147.1) | 110.4 (25.7-384.7) | 94.8 (16.0-384.7) |
| MM type at initial diagnosis, n (%) | |||||||
| IgG | 4 (100) | 3 (100) | 6 (50) | 15 (68) | 3 (100) | 5 (83) | 36 (72) |
| IgA | 0 | 0 | 5 (42) | 6 (27) | 0 | 1 (17) | 12 (24) |
| Light chain only | 0 | 0 | 1 (8) | 1 (5) | 0 | 0 | 2 (4) |
| ISS stage at study entry, n (%) | |||||||
| I | 3 (75) | 1 (33) | 5 (42) | 6 (27) | 2 (67) | 1 (17) | 18 (36) |
| II | 0 | 1 (33) | 3 (25) | 10 (45) | 1 (33) | 2 (33) | 17 (34) |
| III | 1 (25) | 0 | 4 (33) | 4 (18) | 0 | 2 (33) | 11 (22) |
| Missing∗ | 0 | 1 (33) | 0 | 2 (10) | 0 | 1 (17) | 4 (8) |
| Median corrected calcium, mmol/L (range) | 2.3 (2.3-2.4) | 2.3 (2.2-2.3) | 2.4 (2.2-2.8) | 2.3 (2.2-2.6) | 2.3 (2.2-2.6) | 2.3 (2.2-2.4) | 2.3 (2.2-2.8) |
| Median hemoglobin, g/L (range) | 125 (106-138) | 118 (104-126) | 124.5 (83-153) | 121 (89-145) | 107 (107-122) | 119 (110-152) | 121 (83-153) |
| Median creatinine clearance, mL/min (range) | 83 (44-116) | 71 (52-77) | 86 (30-134) | 81 (31-143) | 97 (91-117) | 107 (56-144) | 86 (30-144) |
| Creatinine clearance category, n (%) | |||||||
| 30 to <60 mL/min | 1 (25) | 1 (33) | 3 (25) | 6 (27) | 0 | 1 (17) | 12 (24) |
| 60 to <90 mL/min | 1 (25) | 2 (67) | 3 (25) | 8 (36) | 0 | 1 (17) | 15 (30) |
| ≥90 mL/min | 2 (50) | 0 | 6 (50) | 8 (36) | 3 (100) | 4 (67) | 23 (46) |
| Lytic bone lesions, n (%) | n = 3 | n = 3 | n = 11 | n = 21 | n = 3 | n = 6 | N = 47 |
| Yes | 3 (100) | 2 (67) | 7 (64) | 12 (57) | 1 (33) | 2 (33) | 27 (57) |
| No | 0 | 1 (33) | 4 (36) | 8 (38) | 2 (67) | 4 (67) | 19 (40) |
| Indeterminate | 0 | 0 | 0 | 1 (5) | 0 | 0 | 1 (2) |
| Plasmacytomas, n (%) | n = 1 | n = 0 | n = 2 | n = 5 | n = 1 | n = 0 | N = 9 |
| Yes | 0 (9) | 0 | 0 | 1 (20) | 1 (100) | 0 | 2 (22) |
| No | 1 (100) | 0 | 2 (100) | 4 (80) | 0 | 0 | 7 (78) |
| Chromosomal aberrations, n (%) | n = 4 | n = 3 | n = 11 | n = 21 | n = 3 | n = 6 | N = 48 |
| High-risk cytogenetics† | 0 | 1 (33) | 4 (36) | 6 (29) | 1 (33) | 3 (50) | 15 (31) |
| Standard-risk cytogenetics | 4 (100) | 2 (67) | 7 (64) | 15 (71) | 2 (67) | 3 (50) | 33 (69) |
| Median number of prior lines of therapy, (range) | 4.0 (2-7) | 3.0 (3-5) | 3.0 (2-12) | 3.0 (2-16) | 4 (3-4) | 4 (2-5) | 3.5 (2-16) |
| Prior treatment, n (%) | |||||||
| Autologous stem cell transplant | 4 (100) | 3 (100) | 10 (83) | 17 (77) | 3 (100) | 6 (100) | 43 (86) |
| Alkylating agent | 4 (100) | 3 (100) | 12 (100) | 20 (91) | 3 (100) | 6 (100) | 48 (96) |
| Anti-CD38 antibody | 1 (25) | 0 | 3 (25) | 7 (32) | 0 | 0 | 11 (22) |
| IMiD | 4 (100) | 3 (100) | 12 (100) | 22 (100) | 3 (100) | 6 (100) | 50 (100) |
| PI | 4 (100) | 3 (100) | 12 (100) | 22 (100) | 3 (100) | 6 (100) | 50 (100) |
| Refractory status, n (%)‡ | |||||||
| To anti-CD38 antibody | 0 | 0 | 1 (8) | 5 (23) | 0 | 0 | 6 (12) |
| To either an IMiD or a PI | 4 (100) | 3 (100) | 10 (83) | 22 (100) | 3 (100) | 5 (83) | 47 (94) |
| To both an IMiD and a PI | 3 (75) | 1 (33) | 8 (67) | 17 (77) | 0 | 1 (17) | 30 (60) |
| To last line of therapy | 4 (100) | 2 (67) | 10 (83) | 18 (82) | 3 (100) | 4 (67) | 41 (82) |
| To any line of therapy | 4 (100) | 3 (100) | 10 (83) | 22 (100) | 3 (100) | 5 (83) | 47 (94) |
PomDex was administered in combination with mezagitamab 300 mg (per the product label).
ECOG, Eastern Cooperative Oncology Group; ISS, International Staging System.
Percentages may not add up to 100% because of rounding.
Defined as del [17], t[4:14], and t[14:16] (analyzed locally by fluorescence in situ hybridization or conventional cytogenetics [karyotype], according to local standards).
Defined as a <25% reduction in M-protein (response to stable disease during prior therapy) or PD during treatment or within 60 days after the last dose of prior therapy.
Among all patients, the median number of prior therapies was 3.5 (range, 2-16). All patients had received a prior IMiD and PI, and 22% had received a prior anti-CD38 antibody; 94% of the patients were refractory to an IMiD or PI, 60% to both an IMiD and a PI, and 12% were refractory to an anti-CD38 antibody (Table 1).
DLTs and MTD
Among all patients enrolled, 41 were evaluable for DLTs. No DLTs occurred in the monotherapy cohorts, whereas 1 patient treated in the combination cohort experienced a DLT of grade 4 neutropenia. Although no MTD was identified, 600 mg was selected as the RP2D for mezagitamab monotherapy based on the assessment of all available data, particularly the duration of response (DOR) and receptor occupancy data (NM. Walton, F. Hong, C. M, VA. Nguyen, DT. Berg, R. McComb, T. Bo, J. Estevam, and S. McDonnell, manuscript in preparation).
Safety
All the enrolled patients were included in the safety population. The median number of treatment cycles was 5 (range, 1-34) and the median relative dose intensity was 100% (range, 80%-100%) at the data cutoff (4 January 2022), with 4 patients continuing study treatment as part of a posttrial access program.
In general, mezagitamab was well tolerated as monotherapy and with PomDex, with no unexpected toxicities (Tables 2 and 3). The most common any-grade hematologic TEAEs were neutropenia (defined as either neutropenia or decreased neutrophil count) in 12 (24%) patients and anemia in 8 (16%) patients; neutropenia was more common in the PomDex combination cohort than in the monotherapy cohort. The most common nonhematologic TEAE was fatigue in 15 (30%) patients and occurred at similar rates in the combination and monotherapy cohorts. Overall, 50% of TEAEs were grade ≥3 in intensity, with higher rates (100%) in the PomDex combination cohort than in the monotherapy cohort (43%). Neutropenia, anemia, and hypertension were the most common grade ≥3 TEAEs, occurring in 7 (14%), 3 (6%), and 3 (6%) patients, respectively (Table 3). Overall, 27 (54%) patients experienced TEAEs reported as drug-related (Table 2), the most common of which were fatigue in the monotherapy cohort (n = 9 [20%]) and neutropenia in the combination cohort (n = 4 [67%]; Table 4). Neutropenia was the only drug-related grade ≥3 TEAE to occur in >1 patient in any cohort. TEAEs resulted in treatment discontinuation for all study drugs in 3 patients (Table 2).
Overview of TEAEs (safety population)
| Patients, n (%) . | Mezagitamab cohort . | ||||||
|---|---|---|---|---|---|---|---|
| 45 mg (n = 4) . | 135 mg (n = 3) . | 300 mg (n = 12) . | 600 mg (n = 22) . | 1200 mg (n = 3) . | PomDex (n = 6) . | Total (N = 50) . | |
| Any TEAE | 4 (100) | 3 (100) | 12 (100) | 22 (100) | 3 (100) | 6 (100) | 50 (100) |
| Grade ≥3 TEAE | 2 (50) | 1 (33) | 3 (25) | 12 (55) | 1 (33) | 6 (100) | 25 (50) |
| Drug-related TEAE | 2 (50) | 2 (67) | 5 (42) | 12 (55) | 2 (67) | 4 (67) | 27 (54) |
| Drug-related grade ≥3 TEAE | 1 (25) | 0 | 1 (8) | 3 (14) | 0 | 3 (50) | 8 (16) |
| Serious TEAE | 0 | 0 | 1 (8) | 8 (36) | 1 (33) | 2 (33) | 12 (24) |
| Drug-related serious TEAE | 0 | 0 | 0 | 1 (5)∗ | 0 | 0 | 1 (2) |
| TEAE resulting in study drug dose modification | 1 (25) | 1 (33) | 1 (8) | 11 (50) | 1 (33) | 6 (100) | 21 (42) |
| TEAE resulting in study drug dose reduction | 0 | 1 (33) | 1 (8) | 6 (27) | 0 | 6 (100) | 14 (28) |
| TEAE resulting in study drug discontinuation | 0 | 0 | 0 | 2 (9)† | 0 | 3 (50) | 5 (10) |
| TEAE resulting in discontinuation from all study drugs | 0 | 0 | 0 | 2 (9) | 0 | 1 (17) | 3 (6) |
| On-study deaths | 0 | 0 | 0 | 1 (5) | 0 | 1 (17) | 2 (4) |
| Patients, n (%) . | Mezagitamab cohort . | ||||||
|---|---|---|---|---|---|---|---|
| 45 mg (n = 4) . | 135 mg (n = 3) . | 300 mg (n = 12) . | 600 mg (n = 22) . | 1200 mg (n = 3) . | PomDex (n = 6) . | Total (N = 50) . | |
| Any TEAE | 4 (100) | 3 (100) | 12 (100) | 22 (100) | 3 (100) | 6 (100) | 50 (100) |
| Grade ≥3 TEAE | 2 (50) | 1 (33) | 3 (25) | 12 (55) | 1 (33) | 6 (100) | 25 (50) |
| Drug-related TEAE | 2 (50) | 2 (67) | 5 (42) | 12 (55) | 2 (67) | 4 (67) | 27 (54) |
| Drug-related grade ≥3 TEAE | 1 (25) | 0 | 1 (8) | 3 (14) | 0 | 3 (50) | 8 (16) |
| Serious TEAE | 0 | 0 | 1 (8) | 8 (36) | 1 (33) | 2 (33) | 12 (24) |
| Drug-related serious TEAE | 0 | 0 | 0 | 1 (5)∗ | 0 | 0 | 1 (2) |
| TEAE resulting in study drug dose modification | 1 (25) | 1 (33) | 1 (8) | 11 (50) | 1 (33) | 6 (100) | 21 (42) |
| TEAE resulting in study drug dose reduction | 0 | 1 (33) | 1 (8) | 6 (27) | 0 | 6 (100) | 14 (28) |
| TEAE resulting in study drug discontinuation | 0 | 0 | 0 | 2 (9)† | 0 | 3 (50) | 5 (10) |
| TEAE resulting in discontinuation from all study drugs | 0 | 0 | 0 | 2 (9) | 0 | 1 (17) | 3 (6) |
| On-study deaths | 0 | 0 | 0 | 1 (5) | 0 | 1 (17) | 2 (4) |
PomDex was given in combination with mezagitamab 300 mg (administered per the product label).
A patient with a past medical history of diverticulitis was hospitalized for diverticulitis.
A patient discontinued therapy and died after 1 dose of mezagitamab due to respiratory failure secondary to Haemophilus influenzae sepsis related to complications for underlying progressing myeloma. The second patient contracted severe acute respiratory syndrome coronavirus 2 and died of COVID-19.
Most frequently reported TEAEs, all grades (occurring in 10% or more of patients overall) and grade 3 or higher (occurring in 5% or more of patients overall) (safety population)
| n (%) . | Mezagitamab cohort . | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 45 mg (n = 4) . | 135 mg (n = 3) . | 300 mg (n = 12) . | 600 mg (n = 22) . | 1200 mg (n = 3) . | PomDex (n = 6) . | Total (N = 50) . | ||||||||
| Any grade . | Grade ≥3 . | Any grade . | Grade ≥3 . | Any grade . | Grade ≥3 . | Any grade . | Grade ≥3 . | Any grade . | Grade ≥3 . | Any grade . | Grade ≥3 . | Any grade . | Grade ≥3 . | |
| Any TEAEs | 4 (100) | 2 (50) | 3 (100) | 1 (33) | 12 (100) | 3 (25) | 22 (100) | 12 (55) | 3 (100) | 1 (33) | 6 (100) | 6 (100) | 50 (100) | 25 (50) |
| Hematologic | ||||||||||||||
| Anemia | 0 | 0 | 1 (33) | 0 | 3 (25) | 1 (8) | 4 (18) | 2 (9) | 0 | 0 | 0 | 0 | 8 (16) | 3 (6) |
| Neutropenia∗ | 1 (25) | 0 | 0 | 0 | 1 (8) | 0 | 2 (9) | 1 (5) | 1 (33) | 0 | 3 (50) | 2 (33) | 8 (16) | 3 (6) |
| Neutrophil count decreased∗ | 1 (25) | 1 (25) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 (50) | 3 (50) | 4 (8) | 4 (8) |
| Nonhematologic | ||||||||||||||
| Fatigue | 1 (25) | 0 | 1 (33) | 0 | 4 (33) | 0 | 5 (23) | 0 | 2 (67) | 0 | 2 (33) | 1 (17) | 15 (30) | 1 (2) |
| URT infection | 0 | 0 | 1 (33) | 0 | 2 (17) | 0 | 7 (32) | 0 | 1 (33) | 0 | 2 (33) | 0 | 13 (26) | 0 |
| Insomnia | 0 | 0 | 1 (33) | 1 (33) | 3 (25) | 0 | 5 (23) | 0 | 0 | 0 | 1 (17) | 0 | 10 (20) | 1 (2) |
| Back pain | 0 | 0 | 0 | 0 | 4 (33) | 0 | 6 (27) | 1 (5) | 0 | 0 | 0 | 0 | 10 (20) | 1 (2) |
| Arthralgia | 3 (75) | 0 | 1 (33) | 0 | 3 (25) | 0 | 2 (9) | 1 (5) | 0 | 0 | 0 | 0 | 9 (18) | 1 (2) |
| Headache | 0 | 0 | 1 (33) | 0 | 4 (33) | 0 | 2 (9) | 0 | 0 | 0 | 1 (17) | 0 | 8 (16) | 0 |
| Diarrhea | 1 (25) | 0 | 1 (33) | 0 | 1 (8) | 0 | 3 (14) | 0 | 1 (33) | 0 | 1 (17) | 0 | 8 (16) | 0 |
| Nausea | 1 (25) | 0 | 0 | 0 | 3 (25) | 0 | 3 (14) | 0 | 0 | 0 | 1 (17) | 0 | 8 (16) | 0 |
| Hypertension | 1 (25) | 0 | 1 (33) | 0 | 0 | 0 | 4 (18) | 3 (14) | 0 | 0 | 0 | 0 | 6 (12) | 3 (6) |
| Pneumonia | 0 | 0 | 0 | 0 | 0 | 0 | 4 (18) | 2 (9) | 0 | 0 | 1 (17) | 0 | 5 (10) | 2 (4) |
| Abdominal pain | 1 (25) | 0 | 0 | 0 | 0 | 0 | 2 (9) | 1 (5) | 1 (33) | 0 | 1 (17) | 0 | 5 (10) | 1 (2) |
| Abdominal distension | 0 | 0 | 1 (33) | 0 | 1 (8) | 0 | 2 (9) | 0 | 1 (33) | 0 | 0 | 0 | 5 (10) | 0 |
| Myalgia | 0 | 0 | 2 (67) | 0 | 1 (8) | 0 | 1 (5) | 0 | 0 | 0 | 1 (17) | 0 | 5 (10) | 0 |
| n (%) . | Mezagitamab cohort . | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 45 mg (n = 4) . | 135 mg (n = 3) . | 300 mg (n = 12) . | 600 mg (n = 22) . | 1200 mg (n = 3) . | PomDex (n = 6) . | Total (N = 50) . | ||||||||
| Any grade . | Grade ≥3 . | Any grade . | Grade ≥3 . | Any grade . | Grade ≥3 . | Any grade . | Grade ≥3 . | Any grade . | Grade ≥3 . | Any grade . | Grade ≥3 . | Any grade . | Grade ≥3 . | |
| Any TEAEs | 4 (100) | 2 (50) | 3 (100) | 1 (33) | 12 (100) | 3 (25) | 22 (100) | 12 (55) | 3 (100) | 1 (33) | 6 (100) | 6 (100) | 50 (100) | 25 (50) |
| Hematologic | ||||||||||||||
| Anemia | 0 | 0 | 1 (33) | 0 | 3 (25) | 1 (8) | 4 (18) | 2 (9) | 0 | 0 | 0 | 0 | 8 (16) | 3 (6) |
| Neutropenia∗ | 1 (25) | 0 | 0 | 0 | 1 (8) | 0 | 2 (9) | 1 (5) | 1 (33) | 0 | 3 (50) | 2 (33) | 8 (16) | 3 (6) |
| Neutrophil count decreased∗ | 1 (25) | 1 (25) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 (50) | 3 (50) | 4 (8) | 4 (8) |
| Nonhematologic | ||||||||||||||
| Fatigue | 1 (25) | 0 | 1 (33) | 0 | 4 (33) | 0 | 5 (23) | 0 | 2 (67) | 0 | 2 (33) | 1 (17) | 15 (30) | 1 (2) |
| URT infection | 0 | 0 | 1 (33) | 0 | 2 (17) | 0 | 7 (32) | 0 | 1 (33) | 0 | 2 (33) | 0 | 13 (26) | 0 |
| Insomnia | 0 | 0 | 1 (33) | 1 (33) | 3 (25) | 0 | 5 (23) | 0 | 0 | 0 | 1 (17) | 0 | 10 (20) | 1 (2) |
| Back pain | 0 | 0 | 0 | 0 | 4 (33) | 0 | 6 (27) | 1 (5) | 0 | 0 | 0 | 0 | 10 (20) | 1 (2) |
| Arthralgia | 3 (75) | 0 | 1 (33) | 0 | 3 (25) | 0 | 2 (9) | 1 (5) | 0 | 0 | 0 | 0 | 9 (18) | 1 (2) |
| Headache | 0 | 0 | 1 (33) | 0 | 4 (33) | 0 | 2 (9) | 0 | 0 | 0 | 1 (17) | 0 | 8 (16) | 0 |
| Diarrhea | 1 (25) | 0 | 1 (33) | 0 | 1 (8) | 0 | 3 (14) | 0 | 1 (33) | 0 | 1 (17) | 0 | 8 (16) | 0 |
| Nausea | 1 (25) | 0 | 0 | 0 | 3 (25) | 0 | 3 (14) | 0 | 0 | 0 | 1 (17) | 0 | 8 (16) | 0 |
| Hypertension | 1 (25) | 0 | 1 (33) | 0 | 0 | 0 | 4 (18) | 3 (14) | 0 | 0 | 0 | 0 | 6 (12) | 3 (6) |
| Pneumonia | 0 | 0 | 0 | 0 | 0 | 0 | 4 (18) | 2 (9) | 0 | 0 | 1 (17) | 0 | 5 (10) | 2 (4) |
| Abdominal pain | 1 (25) | 0 | 0 | 0 | 0 | 0 | 2 (9) | 1 (5) | 1 (33) | 0 | 1 (17) | 0 | 5 (10) | 1 (2) |
| Abdominal distension | 0 | 0 | 1 (33) | 0 | 1 (8) | 0 | 2 (9) | 0 | 1 (33) | 0 | 0 | 0 | 5 (10) | 0 |
| Myalgia | 0 | 0 | 2 (67) | 0 | 1 (8) | 0 | 1 (5) | 0 | 0 | 0 | 1 (17) | 0 | 5 (10) | 0 |
PomDex was given in combination with mezagitamab 300 mg (administered per the product label).
URT, upper respiratory tract.
Numbers for these preferred terms were pooled as neutropenia in the manuscript text.
Drug-related TEAEs occurring in more than 1 patient in any cohort (safety population)
| n (%) . | Mezagitamab cohort . | ||||||
|---|---|---|---|---|---|---|---|
| 45 mg (n = 4) . | 135 mg (n = 3) . | 300 mg (n = 12) . | 600 mg (n = 22) . | 1200 mg (n = 3) . | PomDex (n = 6) . | Total (N = 50) . | |
| Hematologic | |||||||
| Neutropenia∗ | 1 (25) | 0 | 1 (8) | 2 (9) | 1 (33) | 1 (17) | 6 (12) |
| Neutrophil count decreased∗ | 1 (25) | 0 | 0 | 0 | 0 | 3 (50) | 4 (8) |
| Anemia | 0 | 1 (33) | 2 (17) | 1 (5) | 0 | 0 | 4 (8) |
| Leukopenia | 1 (25) | 2 (67) | 0 | 1 (5) | 0 | 0 | 4 (8) |
| Nonhematologic | |||||||
| Fatigue | 1 (25) | 1 (33) | 2 (17) | 4 (18) | 1 (33) | 1 (17) | 10 (20) |
| URT infection | 0 | 0 | 1 (8) | 3 (14) | 1 (33) | 1 (17) | 6 (12) |
| Diarrhea | 0 | 0 | 0 | 2 (9) | 1 (33) | 1 (17) | 4 (8) |
| Nausea | 0 | 0 | 2 (17) | 1 (5) | 0 | 0 | 3 (6) |
| Headache | 0 | 0 | 1 (8) | 2 (9) | 0 | 0 | 3 (6) |
| Pneumonia | 0 | 0 | 0 | 2 (9) | 0 | 0 | 2 (4) |
| Blood alkaline phosphatase increased | 0 | 0 | 0 | 2 (9) | 0 | 0 | 2 (4) |
| n (%) . | Mezagitamab cohort . | ||||||
|---|---|---|---|---|---|---|---|
| 45 mg (n = 4) . | 135 mg (n = 3) . | 300 mg (n = 12) . | 600 mg (n = 22) . | 1200 mg (n = 3) . | PomDex (n = 6) . | Total (N = 50) . | |
| Hematologic | |||||||
| Neutropenia∗ | 1 (25) | 0 | 1 (8) | 2 (9) | 1 (33) | 1 (17) | 6 (12) |
| Neutrophil count decreased∗ | 1 (25) | 0 | 0 | 0 | 0 | 3 (50) | 4 (8) |
| Anemia | 0 | 1 (33) | 2 (17) | 1 (5) | 0 | 0 | 4 (8) |
| Leukopenia | 1 (25) | 2 (67) | 0 | 1 (5) | 0 | 0 | 4 (8) |
| Nonhematologic | |||||||
| Fatigue | 1 (25) | 1 (33) | 2 (17) | 4 (18) | 1 (33) | 1 (17) | 10 (20) |
| URT infection | 0 | 0 | 1 (8) | 3 (14) | 1 (33) | 1 (17) | 6 (12) |
| Diarrhea | 0 | 0 | 0 | 2 (9) | 1 (33) | 1 (17) | 4 (8) |
| Nausea | 0 | 0 | 2 (17) | 1 (5) | 0 | 0 | 3 (6) |
| Headache | 0 | 0 | 1 (8) | 2 (9) | 0 | 0 | 3 (6) |
| Pneumonia | 0 | 0 | 0 | 2 (9) | 0 | 0 | 2 (4) |
| Blood alkaline phosphatase increased | 0 | 0 | 0 | 2 (9) | 0 | 0 | 2 (4) |
PomDex was given in combination with mezagitamab 300 mg (administered per the product label).
URT, upper respiratory tract.
Numbers for these preferred terms were pooled as neutropenia in the manuscript text.
A total of 4 injection-site reactions were reported among the 2112 injections administered (0.2% of injections): 2 reports of mild pruritus, 1 with moderate swelling, and 1 with mild injection-site hemorrhage. No grade ≥1 early or late systemic infusion reactions, hypersensitivity, or cytokine release syndrome reactions were reported across all the dose cohorts.
Serious TEAEs occurred in 24% of the patients; 1 was reported to be drug-related (grade 3 diverticulitis in a patient with a prior history of the condition treated at the 600 mg dose level). The most common serious TEAE was pneumonia, reported in 2 patients.
Two on-study deaths occurred (1 in the 600 mg cohort [PD] and 1 in the combination cohort [COVID-19]), neither of which was related to the study drug.
Immunogenicity
Among the 42 evaluable patients, 2 patients (1 in the 300 mg monotherapy cohort and 1 in the combination cohort) developed low-titer antidrug antibodies at a single time point. No immunogenicity-related TEAEs were reported.
PK
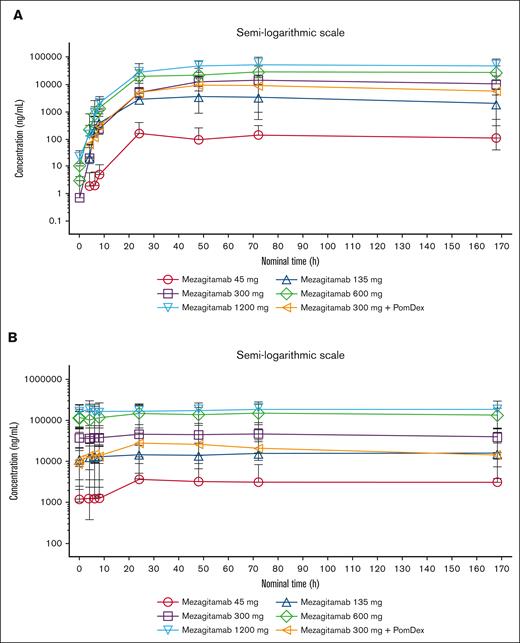
After single- or multiple-dose administration, the maximum serum concentration (Cmax) and area under the concentration-time curve to the last measurable concentration (AUClast) of mezagitamab increased more than proportionally with increasing doses (Figure 1; supplemental Table 2). Median time to peak concentration ranged from 60.18 to 73.22 hours after a single dose and from 58.77 to 71.92 hours after multiple doses.
Mezagitamab pharmacokinetics. Mean serum concentration-time curves after (A) single (cycle 1) and (B) multiple (cycle 2) dosing of SC mezagitamab on a semilogarithmic scale.
Mezagitamab pharmacokinetics. Mean serum concentration-time curves after (A) single (cycle 1) and (B) multiple (cycle 2) dosing of SC mezagitamab on a semilogarithmic scale.
After the administration of mezagitamab 300 mg in combination with PomDex in cycles 1 and 2, mezagitamab mean systemic exposure (Cmax and AUClast) was lower than that of mezagitamab 300 mg alone (by ∼29%-57%). However, moderate to high interpatient variability (coefficient of variation: 34%-101%) and overlaps in mezagitamab systemic exposure were observed across both dose groups after single and multiple SC doses of mezagitamab. Therefore, conclusions about the impact of coadministration of PomDex on systemic exposure to mezagitamab could not be drawn at this time.
Preliminary efficacy
At data cutoff, 21 of 47 response-evaluable patients had an objective response, with a best overall response rate (ORR) of 45%, a very good partial response rate of 9%, and a partial response (PR) rate of 36% (Table 5). Fifteen patients had the best response to PR or better, including 2 patients with a very good partial response and 13 patients with PR (supplemental Figure 2). The confirmed clinical benefit rate (defined as stable disease or better) was 85%. The best ORRs among patients receiving 300 mg, 600 mg, and 1200 mg mezagitamab (doses providing maximal CD38 target saturation) were 42%, 47%, and 33%, respectively.
Summary of efficacy end points
| . | Mezagitamab cohort . | ||||||
|---|---|---|---|---|---|---|---|
| 45 mg (n = 4) . | 135 mg (n = 3) . | 300 mg (n = 12) . | 600 mg (n = 22) . | 1200 mg (n = 3) . | PomDex (n = 6) . | Total (N = 50) . | |
| Best overall response∗ (confirmed/unconfirmed) | n = 4 | n = 3 | n = 12 | n = 19 | n = 3 | n = 6 | N = 47 |
| ORR, n (%) [95% CI] | 1 (25) [0.6-80.6] | 0 | 5 (42) [15.2-72.3] | 9 (47) [24.4-71.1] | 1 (33) [0.8-90.6] | 5 (83) [35.9-99.6] | 21 (45) [30.2-59.9] |
| ≥VGPR, n (%) [95% CI] | 0 | 0 | 1 (8) [0.2-38.5] | 2 (11) [1.3-33.1] | 1 (33) [0.8-90.6] | 0 | 4 (9) [2.4-20.4] |
| sCR or CR, n (%) | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| VGPR, n (%) | 0 | 0 | 1 (8) | 2 (11) | 1 (33) | 0 | 4 (9) |
| PR, n (%) | 1 (25) | 0 | 4 (33) | 7 (37) | 0 | 5 (83) | 17 (36) |
| MR, n (%) | 0 | 1 (33) | 0 | 2 (11) | 0 | 1 (17) | 4 (9) |
| SD, n (%) | 2 (50) | 2 (67) | 5 (42) | 5 (26) | 2 (67) | 0 | 16 (34) |
| PD, n (%) | 1 (25) | 0 | 2 (17) | 3 (16) | 0 | 0 | 6 (13) |
| Responder outcomes (patients with a PR or better) (confirmed† ) | n = 1; PR | n = 0 | n = 5; 1 VGPR, 4 PR | n = 4; PR | n = 1; VGPR | n = 4; PR | N = 15; 2 VGPR, 13 PR |
| Median time to response, mo (range) | 2.1 (2.1-2.1) | – | 1.3 (1.0-3.7) | 1.1 (1.0-1.9) | 1.0 (1.0-1.0) | 2.5 (1.0-4.4) | 1.3 (1.0-4.4) |
| Median DOR, mo (range) | 3.0 (3.0-3.0) | – | 3.7 (1.8-28.0) | 22.1 (10.1-30.2) | NE (27.6∗-27.6∗) | 6.7 (1.2∗-22.7∗) | 11.3 (1.2∗-30.2) |
| PFS | |||||||
| Patients with an event, n (%) | 4 (100) | 2 (67) | 12 (100) | 19 (86) | 2 (67) | 4 (67) | 43 (86) |
| Median, mo (range) | 2.9 (0.7-31.6) | 2.9 (0.3-36.4‡) | 3.5 (2.1-28.6‡) | 8.8 (3.3-23.8‡) | 3.7 (0.3-36.4‡) | ||
| OS | |||||||
| Patients with event, n (%) | 1 (25) | 0 | 7 (58) | 6 (27) | 1 (33) | 1 (17) | 16 (32) |
| Median, mo (range) | 30.4 (10.3-38.4‡) | NE (0.8-36.4‡) | NE (4.9-28.6‡) | NE (3.3-28.1‡) | NE (0.8-44.0‡) | ||
| . | Mezagitamab cohort . | ||||||
|---|---|---|---|---|---|---|---|
| 45 mg (n = 4) . | 135 mg (n = 3) . | 300 mg (n = 12) . | 600 mg (n = 22) . | 1200 mg (n = 3) . | PomDex (n = 6) . | Total (N = 50) . | |
| Best overall response∗ (confirmed/unconfirmed) | n = 4 | n = 3 | n = 12 | n = 19 | n = 3 | n = 6 | N = 47 |
| ORR, n (%) [95% CI] | 1 (25) [0.6-80.6] | 0 | 5 (42) [15.2-72.3] | 9 (47) [24.4-71.1] | 1 (33) [0.8-90.6] | 5 (83) [35.9-99.6] | 21 (45) [30.2-59.9] |
| ≥VGPR, n (%) [95% CI] | 0 | 0 | 1 (8) [0.2-38.5] | 2 (11) [1.3-33.1] | 1 (33) [0.8-90.6] | 0 | 4 (9) [2.4-20.4] |
| sCR or CR, n (%) | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| VGPR, n (%) | 0 | 0 | 1 (8) | 2 (11) | 1 (33) | 0 | 4 (9) |
| PR, n (%) | 1 (25) | 0 | 4 (33) | 7 (37) | 0 | 5 (83) | 17 (36) |
| MR, n (%) | 0 | 1 (33) | 0 | 2 (11) | 0 | 1 (17) | 4 (9) |
| SD, n (%) | 2 (50) | 2 (67) | 5 (42) | 5 (26) | 2 (67) | 0 | 16 (34) |
| PD, n (%) | 1 (25) | 0 | 2 (17) | 3 (16) | 0 | 0 | 6 (13) |
| Responder outcomes (patients with a PR or better) (confirmed† ) | n = 1; PR | n = 0 | n = 5; 1 VGPR, 4 PR | n = 4; PR | n = 1; VGPR | n = 4; PR | N = 15; 2 VGPR, 13 PR |
| Median time to response, mo (range) | 2.1 (2.1-2.1) | – | 1.3 (1.0-3.7) | 1.1 (1.0-1.9) | 1.0 (1.0-1.0) | 2.5 (1.0-4.4) | 1.3 (1.0-4.4) |
| Median DOR, mo (range) | 3.0 (3.0-3.0) | – | 3.7 (1.8-28.0) | 22.1 (10.1-30.2) | NE (27.6∗-27.6∗) | 6.7 (1.2∗-22.7∗) | 11.3 (1.2∗-30.2) |
| PFS | |||||||
| Patients with an event, n (%) | 4 (100) | 2 (67) | 12 (100) | 19 (86) | 2 (67) | 4 (67) | 43 (86) |
| Median, mo (range) | 2.9 (0.7-31.6) | 2.9 (0.3-36.4‡) | 3.5 (2.1-28.6‡) | 8.8 (3.3-23.8‡) | 3.7 (0.3-36.4‡) | ||
| OS | |||||||
| Patients with event, n (%) | 1 (25) | 0 | 7 (58) | 6 (27) | 1 (33) | 1 (17) | 16 (32) |
| Median, mo (range) | 30.4 (10.3-38.4‡) | NE (0.8-36.4‡) | NE (4.9-28.6‡) | NE (3.3-28.1‡) | NE (0.8-44.0‡) | ||
PomDex was given in combination with mezagitamab 300 mg (administered per the product label).
CI, confidence interval; CR, complete response; MR, minimal response; NE, not estimable; OS, overall survival; sCR, stringent complete response; SD, stable disease; VGPR, very good partial response.
Best overall responses did not require 2 consecutive assessments and included a combination of confirmed and unconfirmed responses.
All objective responses required at least 2 consecutive assessments made at any time before the institution of a new therapy to be considered confirmed.
Censored observations.
In the 600 mg (RP2D) cohort, the best ORR in patients who had disease refractory to an IMiD, PI, or both were 56%, 44%, and 53%, respectively. Among patients who were naïve to anti-CD38 therapy, the best ORR in the 600 mg cohort was 54% (confirmed ORR of 31%) and 50% (confirmed ORR of 0%) in patients who were refractory to anti-CD38 therapy. In patients with high-risk cytogenetics, the ORR was similar to that in patients with standard-risk cytogenetics (each 50%). The median time to first response in the RP2D cohort was 1.1 months. In the combination cohort, the best ORR was 83% (confirmed, 67%).
Median DOR was 11.3 months across all dose cohorts, ranging up to 30.2 months among patients in the 600 mg cohort (supplemental Figure 2). The durability of response was a key contributing factor in determining 600 mg as the RP2D with 3.7 and 22.1 months observed in the 300 mg and 600 mg cohorts, respectively (Table 5). The median PFS was 3.7 months for all patients. In the 600 mg cohort, median PFS was 2.9 months overall but differed relative to prior anti-CD38 mAb exposure: 5.8 and 2.1 months in patients naïve or refractory to an anti-CD38 mAb, respectively. At 24 months, 23% of the patients in the 600 mg cohort, regardless of prior exposure to an anti-CD38 regimen, were estimated to be progression free. For the combination cohort, the DOR was 6.7 months and the median PFS was 8.8 months. Both event-driven outcomes in the combination cohort may have been influenced by the small number of patients (n = 6) and an early death after 2 cycles of therapy in a responding patient due to COVID-19 pneumonia. Based on the data cutoff and a median follow-up of 23.8 months, the median overall survival in all patients was not estimable, as less than half of the patients died (n = 16 [32%]). At 2 years, 73% of patients were estimated to be alive.
The reduction in serum M-protein levels was assessed as a biomarker of response. The reduction in serum M-protein in the 300 mg and 600 mg cohorts was comparable, with 4 (40%) and 8 (50%) patients having a ≥50% reduction, whereas 2 and 1 achieved a 100% reduction, respectively. In the combination cohort, 83% of the patients had a ≥50% reduction in serum M-protein, with 3 of those patients achieving a decrease of 75% to 90% (Table 6).
Summary of reduction in M-protein (a biomarker of efficacy; response-evaluable population)
| Patients, n (%) . | Mezagitamab cohort . | ||||||
|---|---|---|---|---|---|---|---|
| 45 mg (n = 4) . | 135 mg (n = 3) . | 300 mg (n = 12) . | 600 mg (n = 19) . | 1200 mg (n = 3) . | PomDex (n = 6) . | Total (N = 47) . | |
| Evaluable patients∗ | 1 (25) | 3 (100) | 10 (83) | 16 (84) | 2 (67) | 6 (100) | 38 (81) |
| 100% reduction | 0 | 0 | 2 (20) | 1 (6) | 0 | 0 | 3 (8) |
| 75% to <90% reduction | 0 | 0 | 0 | 3 (19) | 0 | 3 (50) | 6 (16) |
| 50% to <75% reduction | 0 | 0 | 2 (20) | 4 (25) | 0 | 2 (33) | 8 (21) |
| ≥50% reduction | 0 | 0 | 4 (40) | 8 (50) | 1 (50) | 5 (83) | 18 (47) |
| Patients, n (%) . | Mezagitamab cohort . | ||||||
|---|---|---|---|---|---|---|---|
| 45 mg (n = 4) . | 135 mg (n = 3) . | 300 mg (n = 12) . | 600 mg (n = 19) . | 1200 mg (n = 3) . | PomDex (n = 6) . | Total (N = 47) . | |
| Evaluable patients∗ | 1 (25) | 3 (100) | 10 (83) | 16 (84) | 2 (67) | 6 (100) | 38 (81) |
| 100% reduction | 0 | 0 | 2 (20) | 1 (6) | 0 | 0 | 3 (8) |
| 75% to <90% reduction | 0 | 0 | 0 | 3 (19) | 0 | 3 (50) | 6 (16) |
| 50% to <75% reduction | 0 | 0 | 2 (20) | 4 (25) | 0 | 2 (33) | 8 (21) |
| ≥50% reduction | 0 | 0 | 4 (40) | 8 (50) | 1 (50) | 5 (83) | 18 (47) |
PomDex was given in combination with mezagitamab 300 mg (administered per the product label).
Patients with measurable serum M-protein at baseline and at least 1 postbaseline M-protein assessment.
Discussion
The ubiquitous expression of CD38 by myeloma cells and the emerging efficacy of anti-CD38 agents in patients with RRMM at the time of study initiation provided a rationale for exploring the activity of the investigational anti-CD38 mAb mezagitamab in this patient population.7,16 A wealth of clinical data has since been published and approved indications expanded for these agents, establishing anti-CD38 therapy as an important, recommended option for treating MM.17-21
After the demonstration of the tolerability and selective plasma cytolytic activity of IV/SC mezagitamab in healthy volunteers in a first-in-human study,11 we investigated single-agent SC mezagitamab (in escalating fixed doses of 45-1200 mg) and mezagitamab (at a single dose of 300 mg) in combination with PomDex in patients with RRMM. Eligibility criteria used for this phase 1b study ensured that patients had disease characteristics and treatment histories typical of the RRMM population recruited in similar trials.22-26
Mezagitamab administered SC demonstrated an acceptable safety and tolerability profile up to the highest dose evaluated when administered on the same schedule as the approved anti-CD38 mAb daratumumab.17,18 Only 3 of the 50 enrolled patients discontinued the study treatment because of a TEAE, with most discontinuations attributed to complications of myeloma progression; however, 4 patients (3 were daratumumab naïve; 1 was daratumumab refractory) continued to benefit after ≥30 months of study treatment and entered a posttrial access program.
The most common TEAEs regardless of causality with mezagitamab monotherapy were fatigue, upper respiratory tract infection, insomnia, and back pain, which are commonly reported in patients undergoing treatment for RRMM.27 The most common TEAEs regardless of causality with mezagitamab in combination with PomDex were neutropenia, fatigue, and upper respiratory tract infection, which are listed in the product information for pomalidomide.12 During dose escalation, just 1 DLT was reported (grade 4 neutropenia in the PomDex combination cohort with 300 mg of mezagitamab).
Although the MTD for mezagitamab was not identified, an RP2D of 600 mg was selected for dose confirmation based on the assessment of all clinical data, with the prolonged DOR observed at this dose level being an important contributing factor, along with the safety profile and CD38 target occupancy. Briefly, maximal receptor occupancy values of ≥80% were observed for most doses tested but were only maintained for doses >300 mg when shifted to a biweekly schedule; immune cell depletion patterns were largely consistent with CD38 expression after treatment (NM. Walton, F. Hong, C. M, VA. Nguyen, DT. Berg, R. McComb, T. Bo, J. Estevam, and S. McDonnell, manuscript in preparation).
Based on the mechanism of action and mode of administration, potentially serious systemic infusion-related reactions have been associated with the IV-administered anti-CD38 mAbs daratumumab, isatuximab, and felzartamab28 (formerly known as MOR202; an investigational agent).17,19,29 In this study, no infusion-related reactions were seen after dosing with SC mezagitamab, and local injection-site reactions were uncommon, mild/moderate, and transient. The daratumumab hyaluronidase-fihj SC formulation provides a shorter administration time than the IV formulation; however, injection-site reactions have been reported in 8% of patients and infusion-related reactions have exceeded 10%.18,30 Mezagitamab offers a low treatment administration burden, with low-volume (200 mg/2 mL) SC injections performed in ≤1 minute, indicating the potential for home-based self-administration. Furthermore, unlike the daratumumab SC formulation,18 mezagitamab does not require the addition of hyaluronidase to facilitate drug dispersal and absorption.
Although commonly observed with anti-CD38 therapies, hematologic TEAEs and infections occurring during mezagitamab treatment tended to be mild or moderate in intensity. Neutropenia was the only drug-related grade ≥3 TEAE to occur in >1 patient and was generally manageable with supportive therapy. Neutropenia was less common in the monotherapy cohorts (14%) compared with the combination cohort (100%); the higher rates in the combination cohort were comparable to what is reported with PomDex where neutropenia is the most frequently reported hematologic toxicity of any grade.12 Additionally, no significant (grade 3 or 4) lymphopenia or thrombocytopenia were noted in any patient. The observed TEAEs were generally expected from those reported with the PomDex doublet and were manageable with standard supportive care, including dose modification of pomalidomide as per the product label.12 These observations are in agreement with the pharmacodynamic findings from the first-in-human study, which demonstrated no sustained off-target effects of mezagitamab on red blood cells, platelets, granulocytes, or lymphocytes.11
Early and durable clinical responses were observed with mezagitamab across all dose levels, particularly at the RP2D dose of 600 mg. Notably, at the 600 mg dose, mezagitamab monotherapy demonstrated clinical activity in the overall study population (best ORR, 47%), PI/IMiD-refractory patients (ORR, 53%), and in patients with high-risk cytogenetic features (ORR, 50%). The ORR with mezagitamab monotherapy was higher in patients naïve to anti-CD38 mAb (31%) compared with 0% in refractory patients. The preliminary efficacy of mezagitamab monotherapy reported in this study appears to be similar to that of other anti-CD38 mAbs (0%-41%) in studies involving patients who were naïve to this drug class.18,30,31 Despite mezagitamab binding to a partially distinct epitope, compared with other anti-CD38 therapies and having a 180-day washout period, the lack of response in patients who were refractory to prior anti-CD38 mAb suggests other factors may contribute to efficacy beyond target-mediated effects. Generally, outcomes in patients with MM refractory to CD38 mAb are known to be poor32 and the low response rate in this study is consistent with this finding; the best-confirmed response with mezagitamab monotherapy in refractory patients was durable minimal response. Although only evaluated in a small number of patients, the combination of mezagitamab 300 mg and PomDex provided evidence of potent antimyeloma activity with no clinically significant added toxicity, providing a rationale for including mezagitamab in combination regimens.
Overall, the preliminary treatment efficacy outcomes, both for mezagitamab monotherapy and the PomDex combination, appeared largely comparable with those reported with other anti-CD38 therapies in patients with advanced MM, naïve, or exposed to prior anti-CD38 therapy.
The pharmacokinetic analyses in this study supported a more than dose-proportional increase in systemic exposure with increasing doses of SC mezagitamab. Although a formal assessment of the dose proportionality or bioavailability of SC mezagitamab could not be conducted in the first-in-human healthy volunteer study due to limited serum concentration data, there was some evidence that exposure after IV dosing also increased greater than dose proportionally.11 Indeed, all data collected so far on dose proportionality for both SC- and IV-administered mezagitamab, (including from this study), are consistent with the preclinical pharmacokinetic data in monkeys and with published data on the 2 approved anti-CD38 mAbs, daratumumab and isatuximab, which are both principally cleared by target-mediated elimination.
In summary, the data from this phase 1b trial indicate that SC mezagitamab at doses up to 1200 mg is generally well tolerated and clinically and pharmacodynamically active in patients with RRMM. Although there were no concerns related to safety or lack of efficacy, the sponsor decided not to open the phase 2a portion of the trial due to changes in development priorities and the overall MM treatment landscape. However, mezagitamab monotherapy is being evaluated for CD38-expressing autoimmune diseases, including IgA nephropathy (NCT05174221) and primary immune thrombocytopenia (NCT04278924). This decision is based on promising pharmacodynamic data in human cells and animal models of autoimmune disease,2,8,9 and the encouraging clinical safety and activity shown in human studies to date.11
Acknowledgments
The authors thank the patients and their families, as well as the physicians, nurses, study coordinators, and research staff, for their participation in the trial. Medical writing support for the development of this manuscript, under the direction of the authors, was provided by Luisa Madeira of Ashfield MedComms, an Inizio company, funded by Takeda Pharmaceuticals, USA, Inc., and complied with Good Publication Practice ethical guidelines.33
This study was supported by Millennium Pharmaceuticals, Inc, a wholly owned subsidiary of Takeda Pharmaceutical Company Limited.
Authorship
Contribution: A.Y.K., S.R.P.M., and D.B. designed the research; A.Y.K., K.K.P., R.N., R.W.S., T.L., D.B., and K.E.S.-G. performed the research; A.Y.K., K.K.P., M.M., R.N., R.W.S., and K.E.S.-G. provided study materials or patients; R.W.S., T.L., S.R.P.M., and D.B. collected and assembled the data; all authors had access to the primary clinical trial data; A.Y.K., K.K.P., R.N., Z.Y., T.L., S.R.P.M., and D.B. analyzed and interpreted the data; T.L., S.R.P.M., and D.B. wrote the manuscript; and all authors reviewed and revised the manuscript and approved the final version of the manuscript.
Conflict-of-interest disclosure: A.Y.K. reports ownership of stock/shares with Bristol Myers Squibb (BMS); participation in advisory council or committee with GlaxoSmithKline (GSK); and receiving consulting fees from Sanofi, Pfizer, GSK, Janssen, AbbVie, and AstraZeneca. K.K.P. reports receiving consulting fees from AbbVie Biotherapeutics, Arcellx, BMS, Caribou Biosciences, Cellectis, Genentech, Janssen Biotech, Karyopharm Therapeutics Inc, Merck, Pfizer, Pfizer Canada Inc, and Takeda Oncology; and grants and/or contract funding from Nekta. M.M. reports receiving honoraria from Sanofi, Blood Cancers Today, MashupMD, MJH Life Sciences; consulting fees from Sanofi, Pfizer; grants from Medical College of Wisconsin Clinical and Translation Science Institute (MCW CTSI), Advancing a Healthier Wisconsin Endowment (AHW) KL2 Award; and institutional research funds from Sanofi S.A, GSK, Takeda Pharmaceutical, Ionis Pharmaceuticals, BMS, Celgene Corporation, and Amgen Inc. S.J. reports receiving consulting fees from Janssen, BMS, Karyopharm, Takeda, Sanofi, Caribou, Regeneron, and Legend Biotech. R.N. reports receiving consulting fees from Amgen, BMS, Karyopharm, Janssen, Takeda, and GSK. R.W.S. reports receiving consulting fees from Janssen, Sanofi-Aventis, and Celgene. T.L. and S.R.P.M. report employment and ownership of stock/shares with Takeda. D.B. reports employment and other potential financial relationships (holding a patent) with Takeda. K.E.S.-G. reports ownership of stock/shares with Abbott and AbbVie and other potential financial relationships related to expert witness work with Celgene. Z.Y. declares no competing financial interests.
Correspondence: Amrita Y. Krishnan, Judy and Bernard Briskin Center for Multiple Myeloma Research and Department of Hematology and Hematopoietic Cell Transplantation, City of Hope, 1000 FivePoint, Irvine, CA 92618; email: akrishnan@coh.org.
References
Author notes
The data sets, including the redacted study protocols, redacted statistical analysis plans, and individual participant data supporting the results of the completed study, will be made available after the publication of the final study results within 3 months from initial request to researchers who provide a methodologically sound proposal. The data will be provided after deidentification, in compliance with applicable privacy laws, data protection, and requirements for consent and anonymization.
The full-text version of this article contains a data supplement.