Key Points
MARCH5 is crucial in MM, with expression levels closely correlated to disease progression, overall survival, and progression-free survival.
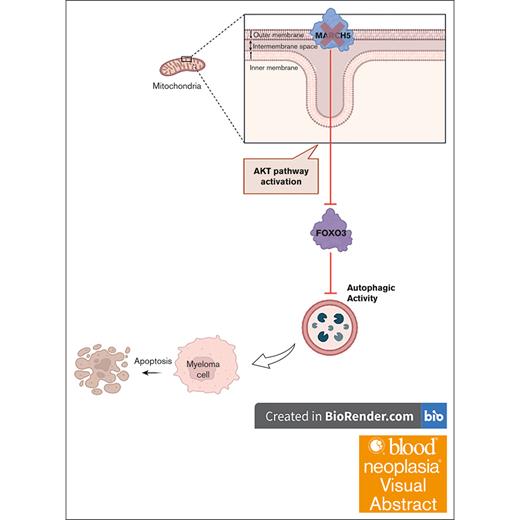
MARCH5-knockdown compromises MM cell viability because of its regulation of autophagy through AKT-mediated degradation of FOXO3.
Visual Abstract
Multiple myeloma (MM) stands as a formidable blood malignancy, necessitating innovative therapeutic approaches. Excessive immunoglobulin production within myeloma cells leads to a buildup of toxic proteins, and autophagy plays a crucial role in their survival by degrading toxic aggregates and generating energy. Membrane-associated RING finger protein 5 (MARCH5) is an E3-ligase positioned at the outer mitochondrial membrane and has been shown to regulate autophagy by competing for MicroRNA 30a (MIR30A). Given the fundamental significance of autophagy in promoting the survival of myeloma cells, coupled with the regulatory role of MARCH5 in autophagic activity, we hypothesized that MARCH5 plays an essential function in MM and holds a pivotal position in the pathogenesis and progression of MM. We identified MARCH5’s unique dependencies in MM cells by analyzing the Cancer Dependency Map, thereby establishing its significance in MM biology. Examining various data sets, including CoMMpass (Clinical Outcomes in Multiple Myeloma to Personal Assessment of Genetic Profile Study) and HOVON (Haemato-Oncology Foundation for Adults in the Netherlands), demonstrated a correlation between MARCH5 expression and patient outcomes. Knockdown of MARCH5 revealed a substantial reduction in MM cell viability, which was associated with a decrease in autophagic activity. Mechanistically, we unraveled a novel MARCH5/AKT/FOXO3 axis, wherein MARCH5 regulates autophagy through the Protein Kinase B (AKT)-mediated degradation of Forkhead Box O3 (FOXO3). Compromised MM cell viability observed with MARCH5 knockdown was recapitulated in FOXO3 knockdown experiments, validating the pivotal role of FOXO3 in mediating MARCH5’s effects. In conclusion, this research highlights the crucial role of MARCH5 in MM, and the identified MARCH5/AKT/FOXO3 axis enhances our understanding of MM biology and provides a foundation for developing targeted therapies.
Introduction
Multiple myeloma (MM) is the second-highest prevalent blood malignancy, accounting for 1% of all cancer types and 10% of all blood cancers.1 MM progresses through various stages. The initial phase of the disease, known as monoclonal gammopathy of undetermined significance, is characterized by a low rate of plasma cell (PC) expansion, a low immunoglobulin load, and the absence of apparent symptoms.2,3 The middle phase, known as smoldering MM, is characterized by a higher immunoglobulin load, but the individual remains asymptomatic.4 Once patients begin to experience symptoms, the condition is known as MM, which can be either intramedullary or extramedullary.1,5 The extramedullary phase correlates with a poor prognosis, particularly the widespread stage of PC leukemia in which considerable quantities of malignant PCs are in the blood circulation.6 Although recent therapeutic strategies have shown encouraging outcomes, myeloma remains a fatal malignancy, and identifying novel targets for treatment purposes is of utmost importance.
MM cells produce large quantities of immunoglobulin, which leads to a considerable accumulation of toxic unfolded or misfolded proteins in the PC.7 To combat these harmful substances, MM cells employ various strategies, such as activating the unfolded protein response (UPR) and initiating the autophagy process.8 Therefore, the UPR and autophagy are crucial for the survival of myeloma cells by degrading the toxic aggregates and simultaneously providing a source of energy.9,10 Studies have proven that inhibiting autophagy through BCL2 Interacting Protein 1 (BECLIN1)-knockdown (KD) or administering autophagy inhibitors, such as 3-methyladenine and chloroquine, induces apoptosis in myeloma cells.11,12
Membrane-associated RING finger protein 5 (MARCH5) is an E3 ligase located on the outer mitochondrial membrane where it plays pivotal roles within cells by regulating mitochondrial fission dynamics,13-15 maintaining mitochondrial quality control,16 and mediating mitophagy.17,18 Research has indicated that a lack of MARCH5 exacerbates the disease condition by interfering with mitochondrial activity and making an individual more vulnerable to stress.19 MARCH5 RNA regulates autophagy by competing for MIR30A and regulating the expression of SMAD2 (Mothers Against Decapentaplegic Homolog2) and ATG5 (Autophagy Related 5).20 SMAD2 is a repressor upstream of the BECLIN1 promoter region,21 and ATG5 is an essential gene involved in the expansion of the phagophoric membrane.22 Concerning the crucial role of autophagy in myeloma cell survival and the importance of MARCH5 as the autophagy regulator, we hypothesized that MARCH5 plays an essential role in MM malignancy and is a potential therapeutic target.
In this study, we investigated the MARCH5 gene effect across different cancer types and evaluated its correlation with MM disease progression, overall survival (OS), and progression-free survival (PFS). In addition, we have clarified MARCH5’s biologic effects and underlying mechanisms.
Materials and methods
Myeloma cell lines culture and collection of TMA samples
MM cell lines were grown in RPMI1640 (Biowest), supplemented with 1% penicillin-streptomycin (Gibco) and 10% fetal bovine serum (Biowest), as previously described.23
In addition, we collected 6 slides of tissue microarray (TMA) samples from the tissue repository section of the Department of Pathology, National University Hospital (NUH), Singapore. The National Healthcare Group (NHG) Domain Specific Review Board approved the study protocol under reference number 2022/00637. The study complied with the Human Biomedical Research Act regulatory requirements.
IHC
Immunohistochemistry (IHC) staining was performed on 4 μm formalin-fixed paraffin-embedded (FFPE) tissue sections with the Bond Max autostainer (Leica Biosystems). Sections were stained with anti-MARCH5 primary antibody (Invitrogen PA5-25584) and secondary antibodies from the Bond Refine Detection Kit (Leica Biosystems). After that, the slides were imaged using the Zeiss Axioscan 7 slide scanner (Zeiss, Oberkochen, Germany). The images were analyzed by a trained pathologist who scored the proportion of MARCH5+ staining.
mIHC
Multiplex IHC (mIHC) staining was performed on 4μm FFPE tonsil (n = 1) and MM TMA (n = 75) sections. Briefly, FFPE tissue sections were subjected to repeated cycles of heat-induced epitope retrieval in the Bond Max autostainer (Leica Biosystems), followed by the addition of anti-MARCH5 primary antibody (Invitrogen PA5-25584), anti-CD138 primary antibody (Dako M7228), polymeric horseradish peroxidase-conjugated secondary antibodies from the Bond Refine Detection Kit (Ready-to-use; Leica Biosystems), and Opal tyramide signal amplification (Akoya Biosciences), as previously described.24 Spectral DAPI (4′,6-diamidino-2-phenylindole) (Akoya Biosciences) was then applied as the nuclear counterstain. The mIHC images were captured using the Zeiss Axioscan 7 Slide Scanner (Zeiss) and analyzed on HALO v3.6 (Indica Labs, Albuquerque, NM) to produce a score reflecting the staining proportion of MARCH5+, CD138+, and MARCH5+CD138+ in each sample, as previously described.25
Lentivirus infection and vectors
pLV-U6-hMARCHF5-shRNA#2 and pLV-U6-hMARCHF5-shRNA#3 vectors and pLV-Exp-EF1A-Mutant FOXO3:IRES:EGFP overexpressing vector were designed and ordered from Vector Builder. Lentiviruses were generated via co-transfection of 293Ts with packaging vectors and either short hairpin RNA (shRNA) vectors or Lentiviral KnockOut plasmid with a puromycin resistance gene (pLKO1-puro) using the FuGENE 6 transfection agent (Promega). The lentivirus supernatant was filtered before infecting the cell lines via centrifugal infection. After 72 hours, Green Fluorescent Protein (GFP)- or mCherry-positive cells were sorted using a BD FACSAria II or puromycin (Sigma) was used to select the infected cells.
Western blotting
Cell pellets were lysed in 1× Radioimmunoprecipitation Assay (RIPA) buffer and incubated on ice for 30 minutes. Following the sonication step and bicinchoninic acid (Thermo Fisher Scientific) protein measurement, equal protein concentrations were loaded to perform sodium dodecyl sulfate polyacrylamide gel electrophoresis using 4% to 20% Tris-Glycine gels (BioRad). The blots were blocked using 5% bovine serum albumin in 0.1% tris-buffered saline in Tween 20. The blots were then incubated with primary antibodies against MARCH5 (06-1036, Millipore), FOXO3 (2497, Cell Signaling), Microtubule-associated protein 1A/1B-light chain 3 beta (LC3B) (ab192890, Abcam), BECLIN1 (ab210498, Abcam), B-cell lymphoma 2 (BCL2) (ab32124, Abcam), Inositol-requiring enzyme 1 alpha (IRE1α) (3294, Cell Signaling), X-box binding protein 1 (XBP-1s) (47134, Cell Signaling), Phosphorylated Protein Kinase B (P-AKT) (9271, Cell Signaling), AKT (4691, Cell Signaling) and β-Actin-HRP (Horseradish peroxidase) (A5441, Sigma). The membranes were washed, incubated with secondary antibodies, and then imaged using the ChemiDoc imaging system.
Cell growth and viability assay
The cells were collected and washed twice with cold PBS. Next, the cells were resuspended in Magnetic-Activated Cell Sorting (MACS) buffer containing 50 μg/mL propidium iodide (PI) and incubated in the dark on ice for 15 minutes for adequate staining. After incubation, the stained cells were analyzed using a BD LSR II flow cytometer, and at least 10 000 events were collected per sample. The collected data were analyzed using FlowJo to distinguish between viable and nonviable cells based on PI fluorescence. Cell viability was further confirmed using trypan blue exclusion with an automated cell counter (CellDrop BF Automated Cell Counter, DeNovix Inc, Wilmington, DE). In addition, cells were plated in 96-well plates, and using the CellTiter Glo kit (Promega), the adenosine triphosphate (ATP) content and the number of metabolically active cells were determined using a luminescent assay.
Annexin-V/Hoechst staining
Annexin-V staining was performed as previously described.26 In brief, after knocking down the MARCH5/FOXO3 genes using shRNA KD, cells were collected and suspended in an annexin V binding buffer (BD Bioscience). A mixture containing Hoechst 33258 dye (H3569, Life Technologies) and annexin V-APC (Allophycocyanin) (A35110, Invitrogen) was used to resuspend the cell pellets. Analysis of the apoptotic proportion was performed using a BD LSR II.
RNA isolation and quantitative PCR analysis
RNA isolation was performed using the RNeasy Mini Kit (Qiagen). Complementary DNA was produced from extracted RNA using EVO Script Universal (07912455001, Roche). The quantitative real-time polymerase chain reaction (qPCR) was carried out on the QuantStudio 3 Real-Time PCR system (Thermo Fisher Scientific) using PowerUp Sybr Green (A25742, Invitrogen). Details of the primers are listed in supplemental Table 1. The comparative cycle threshold methodology was used to determine the relative expression of genes, and the results were normalized against β-actin.
Analysis of bulk RNA-seq data
Total RNA from KMS18 cells was extracted using the RNeasy Mini Kit (Qiagen). The library was constructed at BGI Tech Solutions in Hong Kong. The Spliced Transcripts Alignment (STAR) pipeline27 was used to align the raw bulk RNA sequencing (RNA-seq) reads (FASTQ files) to the human reference genome (hg38). Subsequently, subread packages and Sam tools were used to count the transcripts once the BAM files were merged. The downstream analysis of the generated count matrix was performed using the R package DEseq2.27 Briefly, the DESeq2 object was created using the DESeqDataSetFromMatrix function. Next, the negative binomial distribution was performed to infer differentially expressed genes (DEGs) with the scramble group set as the reference, and the DEGs list was used to explore upregulated or downregulated biologic pathways across the 2 groups.
Biologic pathway analysis
The difference size (DS) score was calculated for DEGs by multiplying the log2 fold change by the −log10 adjusted P value. Then, the DEGs were ranked based on their DS score. Next, the top 1000 upregulated genes (with positive DS scores) and top 1000 downregulated genes (with negative DS scores) were imported into the MetaScape database28 to perform biologic pathway analysis.
Pathway gene set score quantification
The lists of genes for each enriched pathway of interest were downloaded from the Molecular Signatures Database. Next, the gene set score (average expression) was calculated for each gene list using the normalized count of each sample. To statistically compare gene set scores between the 2 experimental groups, the Welch 2-sample t test was used. A P value <.05 was considered statistically significant.
Cyto-ID staining
A Cyto-ID kit (Enzo ENZ-51031-K200) was used to determine the formation of autophagosomes via a green dye.29 Cells were collected and washed with 1× assay buffer. Subsequently, the cell pellets were resuspended in 1× assay buffer including diluted Cyto-ID solution, and the mixture was incubated at room temperature for 30 minutes while covered. Using the BD LSR II flow cytometer, the average fluorescence intensity of the samples was calculated.
Immunoprecipitation and coimmunoprecipitation analysis
Protein A Dynabeads were blocked in 0.5% bovine serum albumin in phosphate-buffered saline, followed by overnight incubation with primary antibody and immunoglobulin G. The next day, cells were lysed in NP40 lysis buffer (25 mM tris-HCl [pH 7.4], 150 mM NaCl, 1 mM EDTA, 1% NP-40, 5% glycerol, 1% protease inhibitor, H2O) and incubated with the mixture of protein A Dynabeads and antibodies. The beads with immunoprecipitated proteins were eluted in Laemmli buffer, followed by western blot (WB) analysis. The same protocol was applied for the coimmunoprecipitation analysis of the Hemagglutinin (HA)-tagged MARCH5 vector.
Statistical analyses
All data are presented as the mean ± standard deviation of 3 independent experiments. Student 2-tailed paired t tests were used to compare the groups. Statistical significance was considered as a P value of .05.
Results
MARCH5 is among the strong determinants enriched in MM and correlates with MM disease progression, OS, and PFS
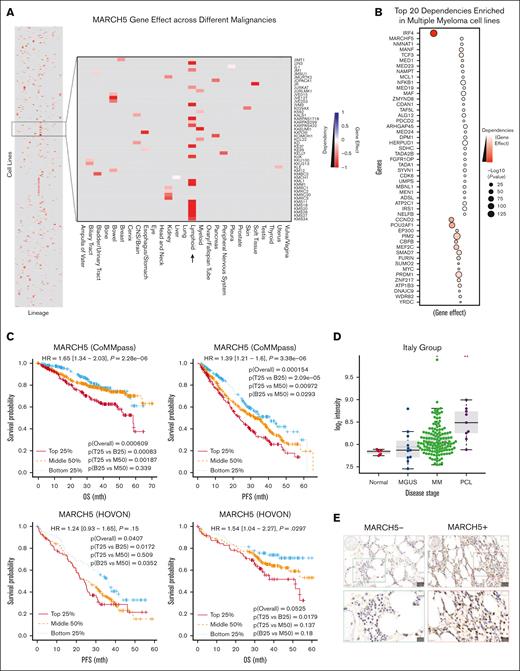
To determine cancer dependency on the MARCH5 gene, we analyzed the most recently released data set from the Cancer Dependency Map (DepMap) and extracted the CRISPR 23Q2 public data set.30-33 We found that MARCH5 seems to have a much lower gene effect size in several MM cell lines when compared with other malignancies, indicating a more essential role in MM. In addition, we observed that MARCH5 is among the top 20 dependencies enriched in MM cell lines, further confirming the essential role of MARCH5 in myeloma cells (Figure 1A-B).
Progressive increase in MARCH5 gene expression along the course of disease progression to myeloma and strong correlation between MARCH5 and survival in MM. (A) MARCH5 gene effect across different cancer types. MARCH5 has a much lower gene effect size in several MM cell lines when compared with other malignancies, indicating that it serves a more essential role in MM than in other cancer types. (B) MARCH5 is among the top 20 dependencies enriched in MM cell lines. (C) Strong correlation between MARCH5 and patient survival in MM. The results of the association between MARCH5 and survival in MM are shown for the CoMMpass and HOVON data sets. The univariate Cox regression results that used MARCH5 expression profiles are shown at the top of each plot and log-rank test results overall and between any 2 of 3 patient groups (top 25% [T25], middle 50% ([M59], and bottom 25% [B25]) based on MARCH5 expression levels are also shown. (D) Progressive increase in MARCH5 gene expression along the course of disease progression to myeloma (and beyond) in the Italy data set. Significance, determined using the Wilcoxon’s test, between the respective disease stages and normal is indicated at the top of each stage as follows: ∗.01 < P ≤ .05; ∗∗.001 < P ≤ .01. (E) IHC staining was performed using anti-MARCH5 antibodies on the TMA slides collected from the tissue repository section of the Department of Pathology, NUH, Singapore. (F) Representative images of MM tissue stained using mIHC staining (MARCH5, green; CD138, red; DAPI, blue), displaying regions with no MARCH5 expression and regions with MARCH5+, CD138+, and MARCH5+CD138+ expressions. Images in the top row have a original magnification of 100×, whereas the images in the bottom row are a selected zoomed-in region with an original magnification of 300×. (G) The mIHC-based quantification of the average proportion of MARCH5+ cells in a MM TMA cohort (n = 75) in comparison with that in a tonsil sample (n = 1). NUH, National University Hospital.
Progressive increase in MARCH5 gene expression along the course of disease progression to myeloma and strong correlation between MARCH5 and survival in MM. (A) MARCH5 gene effect across different cancer types. MARCH5 has a much lower gene effect size in several MM cell lines when compared with other malignancies, indicating that it serves a more essential role in MM than in other cancer types. (B) MARCH5 is among the top 20 dependencies enriched in MM cell lines. (C) Strong correlation between MARCH5 and patient survival in MM. The results of the association between MARCH5 and survival in MM are shown for the CoMMpass and HOVON data sets. The univariate Cox regression results that used MARCH5 expression profiles are shown at the top of each plot and log-rank test results overall and between any 2 of 3 patient groups (top 25% [T25], middle 50% ([M59], and bottom 25% [B25]) based on MARCH5 expression levels are also shown. (D) Progressive increase in MARCH5 gene expression along the course of disease progression to myeloma (and beyond) in the Italy data set. Significance, determined using the Wilcoxon’s test, between the respective disease stages and normal is indicated at the top of each stage as follows: ∗.01 < P ≤ .05; ∗∗.001 < P ≤ .01. (E) IHC staining was performed using anti-MARCH5 antibodies on the TMA slides collected from the tissue repository section of the Department of Pathology, NUH, Singapore. (F) Representative images of MM tissue stained using mIHC staining (MARCH5, green; CD138, red; DAPI, blue), displaying regions with no MARCH5 expression and regions with MARCH5+, CD138+, and MARCH5+CD138+ expressions. Images in the top row have a original magnification of 100×, whereas the images in the bottom row are a selected zoomed-in region with an original magnification of 300×. (G) The mIHC-based quantification of the average proportion of MARCH5+ cells in a MM TMA cohort (n = 75) in comparison with that in a tonsil sample (n = 1). NUH, National University Hospital.
To gain deeper insight into the clinical importance of MARCH5 in MM, we assessed its association with survival in the CoMMpass and HOVON data sets. The HOVON data set comprises microarray data from Dutch and German researchers and contains data from 264 newly diagnosed patients with MM who participated in the HOVON65/GMMG-HD4 (German Multiple Myeloma Study Group-HD4) clinical trial. CoMMpass contains bulk RNA-seq data of 795 newly diagnosed patients with MM who participated in the CoMMpass study. Interestingly, MARCH5 expression was highly associated with OS and PFS in the CoMMpass data set; however, its expression was associated with OS but not with PFS in the HOVON data set (Figure 1C). Moreover, the change in MARCH5 gene expression along the course of disease progression to myeloma has been assessed in the Italian group, which is a microarray data set composed of PCs of 5 nonsymptomatic individuals, 11 patients with monoclonal gammopathy of undetermined significance, 133 patients with MM, and 9 patients with PC leukemia. As shown in Figure 1D, MARCH5 expression progressively increased up to MM (and beyond). The results of the Cox regression analysis across MM data sets and the specific P values of comparison can be found in supplemental Table 1. To further evaluate MARCH5 expression levels among MM samples, we first performed IHC staining using an anti-MARCH5 antibody on the TMA slides, confirming the expression of MARCH5 in the MM samples (Figure 1E). Next, we performed mIHC staining on TMA samples to investigate MARCH5 expression in the malignant PCs (Figure 1F). Representative images from the mIHC staining showed distinct areas with varying MARCH5 expression levels. The images depict regions with no MARCH5 expression and areas with Membrane-Associated RING-CH5 positive (MARCH5+), Cluster of Differentiation 138 positive (CD138+), and coexpressing MARCH5 and CD138 (MARCH5+CD138+) cells. Quantitative analysis of MARCH5 expression across the MM TMA cohort showed a higher average proportion of MARCH5+ cells when compared with a tonsil sample (Figure 1G). Collectively, these findings suggest that MARCH5 may play a role in the formation and progression of MM and might be a potential therapeutic target.
MARCH5 KD suppressed MM cell growth and induced apoptosis
Regarding the previously mentioned findings, we were interested in verifying the dependency of MM cells on MARCH5. Referring to the Genevestigator website,34 we selected 3 MM cell lines with high MARCH5 expression levels, including the KMS11, KMS18, and RPMI8226 cell lines, and we confirmed their high MARCH5 expression at the protein level in comparison with additional MM lines (supplemental Figure 1A). To analyze the biologic roles of MARCH5 in MM cells, MARCH5 KD was performed using a lentiviral shRNA-KD system. Successful MARCH5 KD was confirmed by qPCR and WB techniques as shown in Figure 2A-B and supplemental Figure 1B-C. Following MARCH5 KD, there was a significant decrease in cell viability in the KD groups when analyzed by PI staining (Figure 2C). Live cells are impenetrable to PI, and PI can only penetrate cells with compromised membranes, and therefore it stains dead cells.35 The cell viability was further confirmed by an automated cell counter (Figure 2D; supplemental Figure 1D) and the CellTiter-Blue cell viability assay (Figure 2E). It was reported that MARCH5 KD in normal human dermal fibroblasts (NHDFs) lowered the intracellular ATP levels and hindered the proper assembly of type I collagen.36 Another study revealed that MARCH5 KD in fibroblasts led to a decline in mitochondrial quality, as evidenced by a reduction in ATP synthesis.37 We used the CellTiter-Glo Luminescent assay, which determines the number of viable cells based on quantitation of the ATP present, and the results showed a significant decrease in the ATP levels and growth of the MARCH5 KD groups (Figure 2F; supplemental Figure 1E). In addition, the apoptosis analysis by annexin V staining revealed an enhanced apoptotic fraction in the KD groups when compared with the scramble control (Figure 2G; supplemental Figure 1F). The MM.1s cell line exhibits slightly lower MARCH5 expression than the other 3 cell lines (KMS11, KMS18, and RPMI8226). In addition, MM.1s shows a relatively higher gene effect than these lines (Gene effect: KMS18, −1.42; KMS11, −1.05; RPMI8226, −0.923; MM.1s, −0.323). The gene effect score indicates the dependency of a cell line on a specific gene, so we investigated whether the higher gene effect in MM.1s is related to resistance against MARCH5 KD. Interestingly, although MARCH5-KD in the KMS11, KMS18, and RPMI8226 cell lines led to reduced myeloma viability, MARCH5 KD in MM.1s did not affect cell viability or apoptosis when compared with the scramble control (supplemental Figure 1G-J). The WB analysis showed a downregulation of the antiapoptotic BCL2, confirming the observed increase in the apoptotic fraction (Figure 3A). This downregulation of BCL2 was further confirmed by a significant decrease in the RNA-seq analysis (supplemental Figure 3). MARCH5 is a critical regulator of Bcl-2 Antagonist/Killer (BAK) activity, and the absence of MARCH5 led to the activation of BAK.38 Consistent with this finding, we observed increased proapoptotic BAK1 levels in the pathway analysis of RNA-seq samples. However, the other proapoptotic members of the BCL2 family, including BAX and BH3-interacting domain death agonist (BID), showed a less significant or nonsignificant decrease in the RNA-seq analysis (supplemental Figure 3). Notably, NDUFA4L2 (NADH dehydrogenase [ubiquinone] 1 alpha subcomplex, 4-like 2), a tumor promoter,39,40 was downregulated in the MARCH5 KD group. In contrast, tumor suppressors RASSF6 (Ras association domain family member 6)41 and EHF (ETS homologous factor)42 were upregulated in MARCH5 KD (supplemental Figure 4D). The downregulation of tumor promoters, along with the upregulation of tumor suppressors, might partially explain the observed decrease in cell viability following MARCH5 KD.
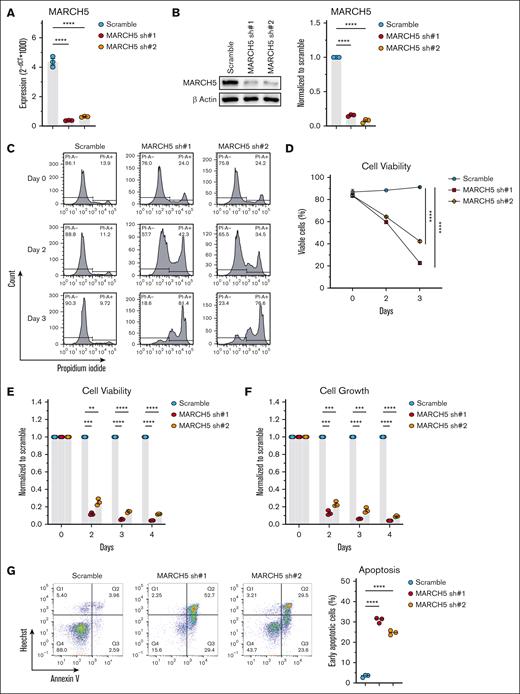
Compromised cell viability upon MARCH5 KD. (A) Messenger RNA and (B) protein expression of MARCH5 72 hours after shRNA KD. MARCH5 KD in KMS11 cells significantly decreased cell viability, as measured by (C) PI staining, (D) cell counter, and (E) CellTiter-Blue. (F) MARCH5 KD significantly decreased cell growth, as analyzed by the CellTiter-Glo Luminescent assay. (G) March5 KD significantly increased the apoptotic fraction, as examined by annexin V staining. Data are shown as mean ± standard error of the mean of 3 independent experiments. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001.
Compromised cell viability upon MARCH5 KD. (A) Messenger RNA and (B) protein expression of MARCH5 72 hours after shRNA KD. MARCH5 KD in KMS11 cells significantly decreased cell viability, as measured by (C) PI staining, (D) cell counter, and (E) CellTiter-Blue. (F) MARCH5 KD significantly decreased cell growth, as analyzed by the CellTiter-Glo Luminescent assay. (G) March5 KD significantly increased the apoptotic fraction, as examined by annexin V staining. Data are shown as mean ± standard error of the mean of 3 independent experiments. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001.
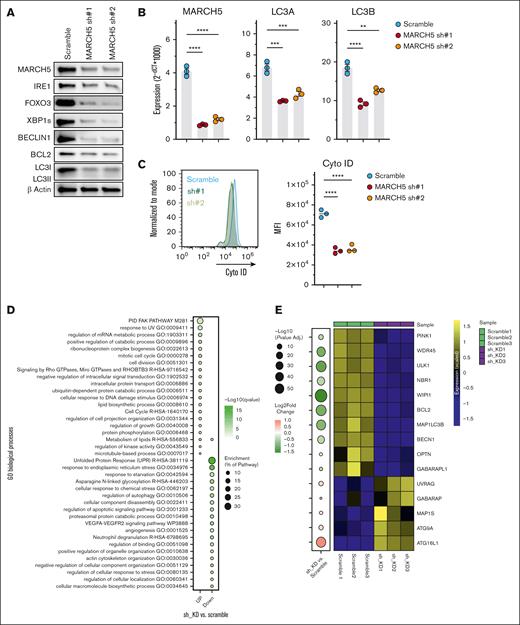
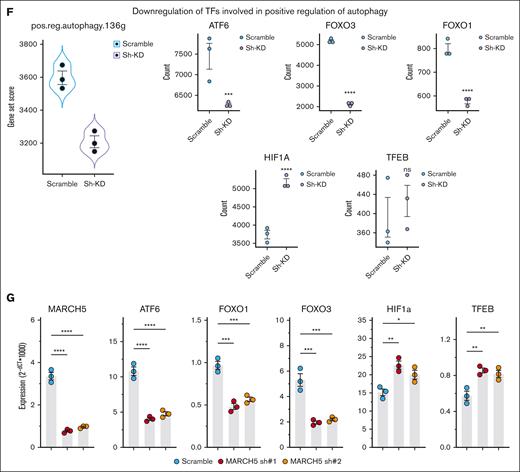
Decreased autophagic activity upon MARCH5 KD. Downregulation of central players during autophagy as demonstrated by (A) WB and (B) qPCR. (C) Decreased autophagosome formation in the MARCH5 KD group as determined by Cyto-ID staining. (D) RNA-seq and Gene Set Enrichment Analysis (GSEA) analysis. GSEA analysis of DEGs in MARCH5 KD vs scramble groups. (E) Central players in the initiation or autophagosome formation/maturation show significant downregulation in the RNA-seq analysis. Data are shown as mean ± standard error of the mean of 3 independent experiments. (F) ATF6, FOXO1, and FOXO3 are among 5 transcription factors involved in the positive regulation of autophagy, and their expression was significantly decreased in the MARCH5 KD group vs the scramble group in RNA-seq sample analysis, whereas HIF1a and TFEB, other positive autophagy regulators, were upregulated. (G) The observed expression patterns of transcription factors in the RNA-seq analysis were confirmed by qPCR. The data are shown as mean ± standard error of the mean of 3 independent experiments. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001.
Decreased autophagic activity upon MARCH5 KD. Downregulation of central players during autophagy as demonstrated by (A) WB and (B) qPCR. (C) Decreased autophagosome formation in the MARCH5 KD group as determined by Cyto-ID staining. (D) RNA-seq and Gene Set Enrichment Analysis (GSEA) analysis. GSEA analysis of DEGs in MARCH5 KD vs scramble groups. (E) Central players in the initiation or autophagosome formation/maturation show significant downregulation in the RNA-seq analysis. Data are shown as mean ± standard error of the mean of 3 independent experiments. (F) ATF6, FOXO1, and FOXO3 are among 5 transcription factors involved in the positive regulation of autophagy, and their expression was significantly decreased in the MARCH5 KD group vs the scramble group in RNA-seq sample analysis, whereas HIF1a and TFEB, other positive autophagy regulators, were upregulated. (G) The observed expression patterns of transcription factors in the RNA-seq analysis were confirmed by qPCR. The data are shown as mean ± standard error of the mean of 3 independent experiments. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001.
MARCH5 KD is associated with decreased autophagy and reduced survival signals
To elucidate the mechanism behind the compromised MM cell viability following MARCH5 KD and considering its regulatory function in the regulation of autophagy, BECLIN1 and LC3, central players in the formation and maturation of autophagosomes,43-45 were analyzed using WB and qPCR (Figure 3A and B, respectively and supplemental Figure 2A-B). Significantly decreased expression of Beclin1 and LC3 in the MARCH5 KD group vs the scramble control suggests diminished autophagic activity. Next, Cyto-ID staining was used to assess the autophagic activity further. Cyto-ID dye selectively labels the autolysosomes formed within the autophagy process. As shown in Figure 3C and supplemental Figure 2C-D, there was a significant reduction in staining in the KD groups, confirming the reduced autophagic activity. To further clarify the underlying mechanisms, we submitted KMS18, the cell line with higher MARCH5 expression (supplemental Figure 4A-B), to RNA-seq. By performing a MetaScape analysis on the RNA-seq samples, regulation of the autophagy pathway seemed to be among the top 6 pathways that were downregulated in the MARCH5 KD groups (Figure 3D), which was consistent with our earlier findings and provided additional evidence on the importance of MARCH5 in the regulation of autophagy in myeloma cells. We then performed a pathway analysis on the RNA-seq samples. In line with the earlier findings, most critical players in the autophagy process were downregulated, supporting the reduced autophagy observed upon MARCH5 KD. The expression pattern of autophagy-related genes in Figure 3E shows that central genes involved in every stage of autophagy were downregulated. For instance, Unc-51 Like Autophagy Activating Kinase 1 (ULK1) is essential for initiating phagophore generation,46 and Optineurin is an autophagy inducer.47 The scramblase Autophagy Related 9A (ATG9A) enables the expansion of the phagophore membrane.48 WD Repeat Domain Phosphoinositide-Interacting Protein 1 (WIPI1) promotes phagophore maturation,49 whereas the Gamma-Aminobutyric Acid Receptor-Associated Protein (GABARAP) subfamily is essential for autophagosome maturation.50 In addition, our pathway analysis of the RNA-seq samples revealed that positive autophagy regulators were significantly decreased in MARCH5 KD cells, whereas negative autophagy regulators decreased slightly (supplemental Figure 6).
Along with the reduced autophagy, there was a significant decrease in the gene and protein levels of inositol-requiring enzyme 1α (IRE1) and spliced form of X-box binding protein 1 (XBP1s) (Figure 3A; supplemental Figure 5A and B). UPR is essential for maintaining homeostasis when toxic misfolded proteins accumulate.51,52 The most conserved branch of the 3 UPR signaling pathways is the IRE1-XBP1 pathway that is critical to sustaining ER homeostasis53 and enhancing the survival signals.54 In addition, IRE1 is involved in the induction of autophagy.55 Therefore, the dependency of myeloma cells on autophagy, apart from the extra accumulated unfolded proteins (characteristics of MM cells), suggests that the compromised viability upon MARCH5 KD is a consequence of the reduction in the survival signals of IRE1-XBP1s and the reduction in autophagic activity.
FOXO3 KD also suppressed MM cell growth through autophagy
Although the regulatory function of MARCH5 in the autophagy process has been determined previously,20 the transcription factors involved in its mechanism of action within autophagy remain unknown. The regulation of autophagy by the FOXO family of transcription factors is well known.56 This family has 4 members, namely FOXO1, FOXO3, FOXO4, and FOXO6. Three of them (FOXO1, FOXO3, and FOXO4) have their activity primarily controlled by the phosphorylation of AKT.
Our pathway analysis of the RNA-seq data unveiled the downregulation of 136 positive autophagy regulators, including several transcription factors such as ATF6, FOXO1, and FOXO3, confirmed by qPCR (Figure 3F-G). ATF6 is a transcription factor that responds to endoplasmic reticulum stress by translocating to the Golgi compartment. There it undergoes cleavage by Site-1 protease (S1P) and S2P. The cleaved N-terminal cytosolic domain of ATF6 then moves into the nucleus where it binds to ATF/cAMP (Cyclic Adenosine Monophosphate) response elements. This binding activates target genes including GRP78/BiP (Glucose-Regulated Protein 78/Binding Immunoglobulin Protein), CHOP (C/EBP Homologous Protein), and XBP-1. Subsequently, ATF6 indirectly modulates autophagy or apoptosis through XBP-1 and CHOP.57 FOXO3 was the first member of the FOXO family to be recognized as a transcriptional controller for various essential autophagy genes (BECN1, LC3, ULK1, ULK2, BNIP3, and VPS34).58-60
Another family member, FOXO1, was also identified as a transcriptional regulator for various autophagy genes. Nonetheless, FOXO1 also triggers autophagy through a transcription-independent mechanism. Under conditions such as oxidative stress or serum starvation, FOXO1 becomes acetylated in the cytosol and subsequently binds to Atg7. This interaction promotes the induction of autophagy by directly engaging with crucial regulators involved in autophagosome biogenesis.61 Studies have demonstrated that FOXO3 plays a role in regulating the expression of FOXO1. Therefore, a KD of FOXO3 will lead to a concurrent reduction in the expression levels of FOXO1.62 Among the reduced transcription factors in our RNA-seq pathway analysis, FOXO3 had the most significant adjusted P value (2.26E−84) and the highest DS score (−247.2798943). Considering the regulatory effect of FOXO3 on the key autophagy players and decreased autophagy upon MARCH5 KD, we thought that decreased autophagy might be caused by FOXO3 downregulation. Therefore, we generated 2 shRNA targeting FOXO3 and performed a lentiviral infection. Interestingly, similar to MARCH5 KD, FOXO3 KD cells had a significant decrease in viability and growth and a significant increase in the apoptotic proportion associated with decreased autophagy (Figure 4). These results suggest that the biologic effects of MARCH5 KD might happen through its interaction with FOXO3.
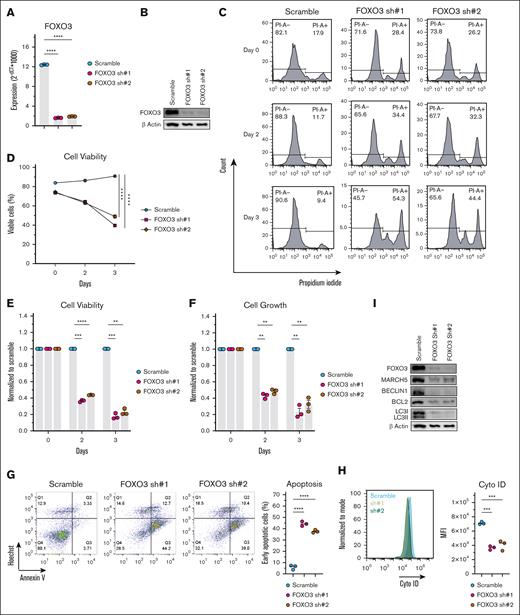
FOXO3 KD in KMS-11 cells showed a similar phenotype as MARCH5 KD. (A) Messenger RNA and (B) protein expression of FOXO3 72 hours after shRNA KD. FOXO3 KD in KMS11 cells significantly decreased cell viability, as examined by (C) PI staining, (D) cell counter, and (E) CellTiter-Blue. (F) FOXO3 KD significantly decreased cell growth as measured by the CellTiter-Glo Luminescent assay. (G) FOXO3 KD significantly enhanced the apoptotic fraction as analyzed by annexin V staining. Reduced autophagic activity was demonstrated by Cyto-ID staining (H) and WB analysis (I). Data are shown as mean ± standard error of the mean of 3 independent experiments. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001.
FOXO3 KD in KMS-11 cells showed a similar phenotype as MARCH5 KD. (A) Messenger RNA and (B) protein expression of FOXO3 72 hours after shRNA KD. FOXO3 KD in KMS11 cells significantly decreased cell viability, as examined by (C) PI staining, (D) cell counter, and (E) CellTiter-Blue. (F) FOXO3 KD significantly decreased cell growth as measured by the CellTiter-Glo Luminescent assay. (G) FOXO3 KD significantly enhanced the apoptotic fraction as analyzed by annexin V staining. Reduced autophagic activity was demonstrated by Cyto-ID staining (H) and WB analysis (I). Data are shown as mean ± standard error of the mean of 3 independent experiments. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001.
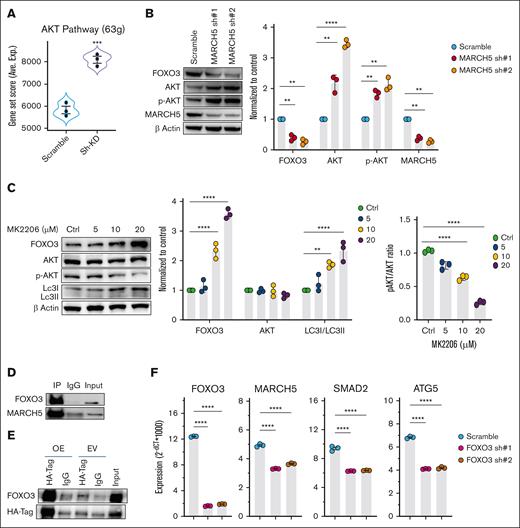
MARCH5 regulates autophagy through the AKT-FOXO3 pathway
The FOXO family members represent key downstream targets in the PI3K/AKT pathway,63,64 and FOXO proteins are inhibited by the activated PI3K/AKT signaling. These processes can be activated directly to phosphorylate FOXOs, which can then be sequestered in the cytoplasm and degraded through the ubiquitin-proteasome pathway.64-66 We asked whether the FOXO3 downregulation in the MARCH5 KD group is related to the AKT signaling pathway. Therefore, we performed a pathway analysis in the RNA-seq sample and found that 63 genes involved in the AKT pathway were upregulated in the MARCH5 KD groups (Figure 5A). Next, we performed a WB analysis and observed an upregulation of AKT and p-Akt in the MARCH5 KD groups, whereas there was a decrease in the FOXO3 level (Figure 5B). This finding indicates that upon MARCH5 KD, AKT signaling was activated, which reduced the FOXO3 level. To confirm the AKT regulatory effect on FOXO3 levels, we assessed the AKT drug inhibitory effect on the FOXO3 protein level using MK-2206, a solid and specific Akt inhibitor.67 As shown in Figure 5C, after treatment of cells with increasing concentrations of MK-2206 for 24 hours, p-AKT was inhibited. In contrast, FOXO3 level increased in a dose-dependent manner and was associated with an increase in the LC3I level, indicating enhanced autophagic activity. Altogether, these findings reveal a MARCH5/AKT/FOXO3 axis as a novel mechanism involved in the regulation of autophagy in myeloma cells.
MARCH5 regulation of autophagy through AKT-FOXO3 signaling. (A) Upregulation of the genes involved in the AKT pathway in the MARCH5 KD group vs the scramble group in the pathway analysis of the RNA-seq sample. (B) Upregulation of AKT and p-Akt, along with a decrease in the FOXO3 level in the MARCH5 KD groups. (C) Inhibition of p-AKT upon treatment of cells with increasing concentrations of MK-2206 for 24 hours leads to a dose-dependent increase in FOXO3 and LC3I levels. (D) Protein interaction of MARCH5 with FOXO3 as demonstrated by immunoprecipitation. (E) FOXO3 interaction with HA-tag vector targeting MARCH5 as demonstrated by coimmunoprecipitation. (F) Decreased MARCH5, SMAD2, and ATG5 expression following FOXO3 KD. Data are shown as mean ± standard error of the mean of 3 independent experiments. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001.
MARCH5 regulation of autophagy through AKT-FOXO3 signaling. (A) Upregulation of the genes involved in the AKT pathway in the MARCH5 KD group vs the scramble group in the pathway analysis of the RNA-seq sample. (B) Upregulation of AKT and p-Akt, along with a decrease in the FOXO3 level in the MARCH5 KD groups. (C) Inhibition of p-AKT upon treatment of cells with increasing concentrations of MK-2206 for 24 hours leads to a dose-dependent increase in FOXO3 and LC3I levels. (D) Protein interaction of MARCH5 with FOXO3 as demonstrated by immunoprecipitation. (E) FOXO3 interaction with HA-tag vector targeting MARCH5 as demonstrated by coimmunoprecipitation. (F) Decreased MARCH5, SMAD2, and ATG5 expression following FOXO3 KD. Data are shown as mean ± standard error of the mean of 3 independent experiments. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001.
MARCH5 interaction with FOXO3 contributes to compromised myeloma cell viability
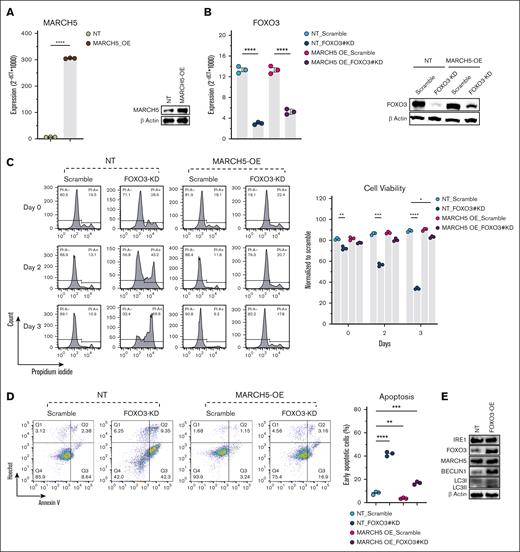
AKT activation phosphorylates FOXO3 to be degraded by the ubiquitin-proteasome pathway.64-66 These findings led us to investigate the potential interaction between MARCH5 and FOXO3. Hence, we performed an immunoprecipitation by pulling down the MARCH5 protein (Figure 5D). Interestingly, FOXO3 was immunoprecipitated with MARCH5. Next, we designed an HA-tagged MARCH5 vector and performed a coimmunoprecipitation assay (Figure 5E). Consistently, upon HA-tagged MARCH5 pulldown, FOXO3 was co-immunoprecipitated, further supporting their interaction. In addition, WB (Figure 3A) and qPCR (Figure 3G) analysis of MARCH5 KD cells revealed a significant decrease in the FOXO3 protein and gene expression levels. Conversely, in the FOXO3 KD group, MARCH5 expression decreased at both the protein and gene levels (Figures 4I and 5F). This mutual effect of MARCH5/FOXO3 might be negative feedback, thereby reducing their expression to a greater extent. In addition, regarding MARCH5 regulation of SMAD2 and ATG5,20 we conducted a qPCR analysis and observed a decrease in SMAD2 and ATG5 genes in MARCH5 KD cells (Figure 5F). Moreover, we performed a STRING (Search Tool for the Retrieval of Interacting Genes/Proteins) analysis68 and observed a network of interactions consisting of FOXO3, SMAD2, ATG5, and AKT (supplemental Figure 7A). This finding was confirmed by the GeneMANIA database69 (supplemental Figure 7B). Based on the regulatory effect of FOXO3 on the key autophagy players61 and considering myeloma cells’ dependency on autophagy,9,10 we concluded that the compromised cell viability caused by MARCH5 KD happened through its interaction with FOXO3, which led to the reduction in autophagy and, consequently, the accumulation of toxic organelles and proteins and the reduction in amino acids and fatty acids essential for the synthesis of macromolecules. To ascertain whether the observed phenotype in myeloma cells following MARCH5 KD is primarily attributable to the consequent reduction in autophagic activity caused by FOXO3 KD rather than potential off-target effects, we conducted the following experiments. Initially, we established MARCH5 overexpressing cells, as illustrated in Figure 6A. Subsequently, we performed FOXO3 KD in these MARCH5 overexpressing cells, as depicted in Figure 6B. This experiment allowed us to assess if the observed phenotypic changes caused by the reduced autophagic activity could be rescued or reversed by MARCH5 overexpression. This approach helped to validate the functional relationship between MARCH5 and FOXO3 and provided insights into their cooperative role in MM pathogenesis.
MARCH5-overexpression (OE) in KMS11 cells rescued the compromised viability caused by FOXO3 KD. Initially, MARCH5 was overexpressed in KMS11 cells as confirmed by (A) qPCR and WB. Next, FOXO3 KD was performed in these cells (MARCH5 overexpressing KMS11 cells), as confirmed by qPCR and WB (B). (C) FOXO3 KD in MARCH5 nontargeting (NT) cells showed a decreased viability, whereas FOXO3 KD in MARCH5 OE cells showed enhanced cell viability, as analyzed by PI staining. (D) Enhanced apoptosis in MARCH5-NT-FOXO3-KD cells vs a decrease in apoptosis in MARCH5-OE-FOXO3-KD cells, as analyzed by annexin V staining. (E) Upregulation of the autophagic activity following FOXO3 OE. The data are shown as mean ± standard error of the mean of 3 independent experiments. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001.
MARCH5-overexpression (OE) in KMS11 cells rescued the compromised viability caused by FOXO3 KD. Initially, MARCH5 was overexpressed in KMS11 cells as confirmed by (A) qPCR and WB. Next, FOXO3 KD was performed in these cells (MARCH5 overexpressing KMS11 cells), as confirmed by qPCR and WB (B). (C) FOXO3 KD in MARCH5 nontargeting (NT) cells showed a decreased viability, whereas FOXO3 KD in MARCH5 OE cells showed enhanced cell viability, as analyzed by PI staining. (D) Enhanced apoptosis in MARCH5-NT-FOXO3-KD cells vs a decrease in apoptosis in MARCH5-OE-FOXO3-KD cells, as analyzed by annexin V staining. (E) Upregulation of the autophagic activity following FOXO3 OE. The data are shown as mean ± standard error of the mean of 3 independent experiments. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001.
As anticipated, FOXO3 KD in the nontargeting cells led to a notable decrease in cell viability. In contrast, FOXO3 KD in MARCH5 overexpressing cells was associated with a discernible enhancement in viability, as illustrated in Figure 6C. This observation was further corroborated by the annexin V staining results, depicted in Figure 6D.
The rescue of viability upon FOXO3 KD in MARCH5 overexpressing cells supports the notion that the MARCH5-mediated regulation of autophagy significantly influenced the observed cellular phenotype. In addition to this, we induced FOXO3 overexpression in myeloma cells to assess its impact on autophagic activity. Subsequently, we conducted a WB analysis to evaluate the expression levels of BECLIN1 and LC3I/II. Interestingly, our findings revealed a notable increase in the expression of these autophagy-related proteins following FOXO3 overexpression. Moreover, consistent with our expectations, upon FOXO3 overexpression, we observed an elevation in the levels of IRE1 and MARCH5, which were initially downregulated in our preliminary experiments (Figure 6E). Collectively, these results provide compelling evidence that the phenotypic changes observed in myeloma cells after MARCH5 KD are primarily the consequence of the concurrent reduction in autophagic activity.
In addition to the role of MARCH5 and FOXO3 in regulating autophagy, our study shed light on the potential involvement of MARCH5 and FOXO3 in peroxisomal function. MARCH5 has been shown previously to be dual-organelle localized and to interact with various peroxisomal proteins to regulate pexophagy.70 Our WB analysis revealed a downregulation of catalase, the most prevalent enzyme with an important role in hydrogen peroxide (H2O2) metabolism within peroxisomes,71,72 in both MARCH5 KD and FOXO3 KD cells when compared with their respective controls (supplemental Figure 8). This finding suggests a potential regulatory role of MARCH5 and FOXO3 in peroxisomal biogenesis or function. Although the primary focus of our investigation was on the effects of MARCH5 and FOXO3 KD on autophagy, this observation underscores the intricate interplay between different cellular processes and the need for further exploration of the cross talk between MARCH5, FOXO3, and peroxisomes. Future studies that elucidate the mechanism of this interaction could provide valuable insights into the broader regulatory networks that govern cellular homeostasis and disease pathogenesis.
Discussion
MM is a highly prevalent blood malignancy with limited therapeutic options. The identification of novel therapeutic targets is essential to improve patient outcomes. This study investigated the biologic effects of MARCH5 and its underlying mechanisms within the context of MM. The examination of various data sets, including the Italy, CoMMpass, and HOVON data sets, demonstrated a significant correlation between MARCH5 expression and patient outcomes. The KD of MARCH5 led to suppressed MM cell growth and increased apoptosis. In line with our study, Haili Tang et al noted that MARCH5 was upregulated in breast cancer cell lines and tumor tissues and played an oncogenic role in these cases.73 Although the therapeutic potential of MARCH5 in acute myeloid leukemia has been demonstrated previously,74 our findings emphasize the potential of MARCH5 to be a therapeutic target in MM.
Mechanistically, MARCH5 KD was associated with a decrease in autophagy that was mediated through the interaction with FOXO3. Our discovery of FOXO3 as a mediator of MARCH5’s effects on autophagy aligns with existing research that implicates FOXO transcription factors in the regulation of autophagy.56 Autophagy is critical for MM cell survival, because it helps to degrade toxic protein aggregates and provides a source of energy.9,10 Inhibition of autophagy induces apoptosis in myeloma cells,11 and MARCH5 regulates autophagy.20 In addition, MARCH5 KD was associated with a downregulation in IRE1 and XBP1, which are critical components of the UPR pathway. The reduced survival signals provided by IRE1-XBP1 and the compromised autophagy collectively contributed to the decreased viability of MM cells upon MARCH5 KD. Although we observed downregulation of IRE-1/XBP1 in MARCH5 KD KMS11 cells, contrary to our findings, Takeda et al found that in mouse embryonic fibroblasts, MARCH5 enhanced K63-linked chain ubiquitylation of IRE1a at lysine 481 to prevent IRE1 hyperoligomerization.75 The observed controversy may be attributed to myeloma cell biology. MM cells inherently exhibit heightened levels of endoplasmic reticulum stress because of increased protein synthesis rates and disease-specific factors.7 The baseline activation of the UPR in myeloma cells may establish a unique cellular context that potentially influences the response to MARCH5 KD. The preexisting state of UPR activation in these cells could modulate the mechanisms that govern IRE1-XBP1s, highlighting the importance of considering disease-specific factors when interpreting the impact of MARCH5 depletion on UPR signaling in the context of MM. To understand the underlying mechanisms of MARCH5 in regulating autophagy, we explored the potential involvement of transcription factors. Pathway analysis revealed a downregulation of several transcription factors involved in autophagy regulation, including ATF6, FOXO1, and FOXO3. Among these, FOXO3 showed the most significant downregulation. FOXO3 KD in MM cells led to a similar phenotype as observed in MARCH5 KD. These findings suggest that the biologic effects of MARCH5 KD are mediated through its interaction with FOXO3. Indeed, immunoprecipitation experiments confirmed the interaction between MARCH5 and FOXO3. These results support that MARCH5 promotes MM cell survival by interacting with FOXO3. Furthermore, we identified the MARCH5/AKT/FOXO3 axis as a crucial pathway in regulating autophagy in MM cells. The activation of AKT, a central mediator of cell survival and proliferation, has been recognized in various cancers.76 Although we observed an induction of myeloma cell apoptosis upon MARCH5 KD, one of the main proliferative pathways (p-AKT) was upregulated. This apparent paradox can be explained by several factors. AKT, conventionally associated with cell survival, has been reported to have a dual role, including proapoptotic functions under certain conditions. Current evidence suggests that the prosurvival function of AKT can be overridden or converted to a proapoptotic function. This conversion can occur in the presence of specific chemotherapeutic agents and cellular conditions that alter AKT signaling, which leads to the induction of apoptosis. Prolonged treatment with chemotherapeutic agents, hyperactivation of AKT kinase activity, nuclear retention, or unbalanced phosphorylation of the AKT protein can convert its function to proapoptotic.77 Increased intracellular oxidation stimulated by chronic AKT hyperactivity also contributes to this switch. Through intricate feedback interactions, downstream effects on Mechanistic Target of Rapamycin (mTOR) and Cell Division Cycle 2 (cdk-2) alter the balance between prosurvival and proapoptotic functions. In contrast, upstream regulators like caveolin-1 can stimulate the proapoptotic function of AKT.77 In the context of this study, the upregulation of AKT we observed could be attributed to several of these factors. MARCH5 KD might have triggered compensatory mechanisms to maintain cellular homeostasis under stress conditions, which led to the activation of AKT as a survival response. However, this initial prosurvival signaling might be overridden by prolonged or altered AKT activity, potentially shifting toward a proapoptotic role. This shift could be influenced by specific conditions within the MM cell environment, such as increased oxidative stress or interactions with other signaling pathways. Therefore, the dual role of AKT, as both a survival and apoptotic factor, provides a plausible explanation for the observed upregulation of AKT in our study despite the induction of apoptosis through MARCH5 KD. Further investigation into the temporal dynamics and specific conditions that lead to this switch could provide deeper insights into the underlying mechanisms.
In conclusion, this study presents compelling evidence for the essential role of MARCH5 in MM, and its role in disease progression, autophagy regulation, and interaction with the AKT-FOXO3 pathway makes it a promising candidate for further investigations.
Acknowledgments
The authors thank Malini Rethnam, Spencer Yang Chong, Takayoshi Matsumura, Shi Hao Tan, Chartsiam Tipgomut, and Hossein Tabatabaeian for their comments and suggestions.
This research received support from the Agency of Science, Technology, and Research Singapore and the Ministry of Health’s National Medical Research Council STaR Investigator Award (NMRC/STaR/024/2019) awarded to T.S.
Authorship
Contribution: H.B., A.K., H.T., A.W., and T.S. designed and analyzed the research; H.B. carried out and analyzed the experiments and wrote the manuscript; A.K. performed the RNA seq analysis; T.-H.C. and W.J.C. performed the bioinformatic analysis of the clinical datasets; J.Y., Y.X., F.W., L.Y.C., and Z.W.N. performed the IHC/mIHC staining and prepared the IHC/mIHC figure; S.-B.N. and T.P. contributed essential samples; T.S. supervised the project, acquired funding, and revised the manuscript; all authors gave their approval for the final version of the manuscript to be submitted.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Toshio Suda, Institute of Hematology, Blood Diseases Hospital, Chinese Academy of Medical Sciences & Peking Union Medical College, 288 Nanjing Rd, Tianjin 300020, China; email: sudato@keio.jp.
References
Author notes
Data are available on request from the corresponding author, Toshio Suda (sudato@keio.jp).
The full-text version of this article contains a data supplement.

![Progressive increase in MARCH5 gene expression along the course of disease progression to myeloma and strong correlation between MARCH5 and survival in MM. (A) MARCH5 gene effect across different cancer types. MARCH5 has a much lower gene effect size in several MM cell lines when compared with other malignancies, indicating that it serves a more essential role in MM than in other cancer types. (B) MARCH5 is among the top 20 dependencies enriched in MM cell lines. (C) Strong correlation between MARCH5 and patient survival in MM. The results of the association between MARCH5 and survival in MM are shown for the CoMMpass and HOVON data sets. The univariate Cox regression results that used MARCH5 expression profiles are shown at the top of each plot and log-rank test results overall and between any 2 of 3 patient groups (top 25% [T25], middle 50% ([M59], and bottom 25% [B25]) based on MARCH5 expression levels are also shown. (D) Progressive increase in MARCH5 gene expression along the course of disease progression to myeloma (and beyond) in the Italy data set. Significance, determined using the Wilcoxon’s test, between the respective disease stages and normal is indicated at the top of each stage as follows: ∗.01 < P ≤ .05; ∗∗.001 < P ≤ .01. (E) IHC staining was performed using anti-MARCH5 antibodies on the TMA slides collected from the tissue repository section of the Department of Pathology, NUH, Singapore. (F) Representative images of MM tissue stained using mIHC staining (MARCH5, green; CD138, red; DAPI, blue), displaying regions with no MARCH5 expression and regions with MARCH5+, CD138+, and MARCH5+CD138+ expressions. Images in the top row have a original magnification of 100×, whereas the images in the bottom row are a selected zoomed-in region with an original magnification of 300×. (G) The mIHC-based quantification of the average proportion of MARCH5+ cells in a MM TMA cohort (n = 75) in comparison with that in a tonsil sample (n = 1). NUH, National University Hospital.](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodneoplasia/1/4/10.1016_j.bneo.2024.100046/2/m_bneo_neo-2024-000185-gr1fg.jpeg?Expires=1765039303&Signature=BzhlI36SCys8om-iff4sSIJuqc9gdsEArDRITIBoHXZeDHEJ9S130DlZ4tB6ccyT2SLGKh6yx9JAwmZSOCka0WgGA1AzzgECYRTOSywBP24YCuK0tzy-HqZky3o3wERKBw1z7RZLxBFJ-uM8fypgN~mxecb2bNbmHkrAmgcpMcOPeY-~DgQljzmphvq8mPbT0v-9~yBr0cuGixJqgb9~sCFH7KHhEpUlfnD3htivYCwNkL6Cil0xpKqqvNnn2yqVVYBgt36J8wn2O8GFhpe7HV7Zze7AUREIo~UZ3Rcf-9Kd8pKVaLq0lqxdkKaYSlhXK0pAa6MgbzOP5QHNCKQiRA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)