Key Points
We developed and characterized novel humanized CD19 CAR T cells for B-cell malignancies.
Visual Abstract
In this study, we aim to develop a humanized CD19 chimeric antigen receptor (CAR) that matches the potency of the FMC63 CAR and potentially reduces the risk of immunogenicity. The murine FMC63 single-chain variable fragment (scFv) was humanized yielding 2 lead candidate scFvs, VH4vκ1 and 4D5, which exhibit weaker binding affinity than FMC63 scFv. These humanized CD19-scFvs were incorporated into CAR constructs to generate huCD19R(VH4Vκ1) and huCD19R(4D5) CARs, both containing the 41BB costimulatory domain. The antitumor activity of the CAR T cells was assessed against CD19+ and CD19 low-expressing tumors. FMC63 CAR T cells with the same backbone in all studies were used as controls. The results showed that the huCD19R(VH4vκ1) CAR T cells exhibited similar expansion, phenotype, and effector function to the FMC63 CAR upon stimulation with CD19 targets. When the CAR T cells were challenged with CD19-bearing tumors, the huCD19R(VH4vκ1) CAR T cells showed similar proliferation to the FMC63 CAR T cells, whereas the huCD19R(4D5) CAR T cells essentially failed to proliferate. Moreover, the huCD19R(VH4vκ1) CAR T cells exhibited significantly better in vivo antitumor activity than the huCD19R(4D5) CAR T cells when tested against tumors expressing a range of CD19 antigens. Finally, using a hybrid model, we found that the huCD19R(VH4vκ1) T cells had a comparable cytokine secretion profile to that of FMC63 CAR T cells. Furthermore, the huCD19R(VH4vκ1) CAR T cells exhibited efficacy against both CD19+ and engineered CD19 low-expressing tumors. These findings suggest that huCD19R(VH4vκ1) CAR T cells may offer enhanced persistence and represent a promising candidate for clinical translation as a therapy for CD19+ tumors.
Introduction
The adoptive transfer of CD19-targeted chimeric antigen receptor (CD19-CAR) T cells to treat patients with B-cell malignancies has resulted in impressive clinical response. Currently, 4 anti–CD19-CAR T cell products have been approved by the US Food and Drug Administration for clinical use to treat patients with B-cell malignancies.1-3 These products are second-generation CARs containing an anti-CD19 single-chain variable fragment (scFv) derived from a murine monoclonal antibody (FMC63). Despite the initial success of various CD19-CAR T cell products, 50% of patients with acute lymphoblastic leukemia (ALL) relapsed within a year.4,5 One mechanism that contributes to decreased CAR T cell persistence after infusion is the development of humoral and/or cellular immune responses against the CAR. After infusion, a patient’s immune system may mount an anti-CAR response against the mouse-derived scFv and remove the CAR T cells from the patient’s body,6,7 increasing the likelihood of tumor relapse. To overcome these problems, several investigators have redesigned the CD19-specific scFv to decrease the anti-CAR immune response. Thus far, 4 humanized CD19-CAR constructs have been developed. Clinical trials assessing these humanized CD19-CAR T cells demonstrated safety and efficacy in treating ALL and non-Hodgkin lymphoma (NHL).8-12 However, the properties of the new humanized scFvs such as affinity, stability, the spectrum of targeting, and toxicity in comparison with the most successful FMC63 CAR have not been fully studied. Our laboratory at City of Hope previously developed an FMC63-based, CD19-CAR T cell platform. Across several clinical trials, this CD19-CAR T cell platform is safe and effective against NHL and ALL.13-15 However, the durability of remission remains a challenge. As part of further translational efforts with this CD19-CAR T cell platform, we undertook humanization of the FMC63 scFv to create a clinically viable humanized CAR. This CAR is anticipated to perform on par with the FMC63 CAR while mitigating potential anti-mouse immunogenic responses and enhancing persistence in patients with NHL or ALL. After humanization, we characterized the stability and binding affinity of our humanized scFv. We performed a preclinical evaluation of 2 huCD19 CAR candidates and found that the lead candidate, huCD19R (VH4vk1), had equivalent in vitro effector function and in vivo antitumor activity to that of the parental CD19-CAR against tumors with CD19 expression varying on a clinically relevant range.
Materials and methods
Cell lines
Raji wild-type (WT) cells (CCL-86), Nalm6 (CRL-3273), and KG-1a (CCL-246.1) were purchased from American Type Culture Collection. The CD19 gene was edited by CRISPR–associated protein 9 technology on Raji-WT cells. Raji CD19 knockout (KO) cells were established by fluorescence-activated cell sorting the CD19− population. The low-antigen cell line was produced by expressing CD19 using a weak 3-phosphoglycerate kinase (PGK) promoter (PGK100p) on Raji KO cells by lentiviral transduction. To achieve clonal similarity, these low-antigen–expressing cells were seeded at a density of 1 cell per well. Four single-cell clones were examined for CD19 expression by flow cytometry and clone 4 was used as the Raji CD19-low cell line for the follow-up experiments. Raji/Nalm6 cells were transduced with a lentiviral vector encoding an enhanced green fluorescent protein and firefly luciferase. OKT3-expressing LCL (OKT3-LCL) cells were generated as previously described.16,17
Flow cytometry and antibodies
CD3 (BD, Clone UCHT1), epidermal growth factor receptor (EGFR; Biolegend, Clone AY13), CD8 (BD, Clone SK1), CD4 (BD, Clone SK3), CD19 (Beckman Coulter, Clone J3-119) and (BD, Clone SJ25C1), CD27 (BD, Clone M-T271), CD80 (BD, Clone L307.4), CD86 (BD, Clone 2331), CD107a (BD, Clone H4A3), interferon gamma (IFN-γ; BD, Clone B27), tumor necrosis factor α (BD, Clone MAb11), CD45RA (BD, Clone HI100), CCR7 (Biolegend, Clone G043H7), CD28 (Biolegend, Clone CD28.2), programmed cell death protein 1 (Invitrogen, Clone MIH4), T-cell immunoglobulin and mucin-domain containing-3 (TIM3; Invitrogen, Clone F38-2E2), lymphocyte-activation gene 3 (LAG-3; Invitrogen, Clone 3DS223H), and programmed death ligand 1 (PD-L1; Invitrogen, Clone MIH1). A Quantum Simply Cellular kit (Bangs Laboratories, 815) was used to determine CD19 expression using an anti-CD19 antibody (Beckman Coulter, Clone J3-119) following the manufacturer’s protocol. Briefly, beads with 4 different levels of immobilized anti-mouse antibody and negative control beads were labeled using anti-CD19 antibody. Fluorescence was assessed using flow cytometry, and median fluorescence intensity for each bead population was used to generate a standard curve using a lot-specific quantification spreadsheet from the manufacturer. Cell lines were labeled using the same anti-CD19 antibody and analyzed by flow cytometry. Median fluorescence was correlated to CD19 expression through the generated standard curve. A similar protocol was followed for measuring CD80/86, and PD-L1 density using appropriate antibodies. Data acquisition was performed on a MACSquant (Miltenyi) and analyzed using FCS Express version 7 software.
Humanization of the FMC63 scFv
The FMC63 scFv was humanized using 2 separate complementary determining region (CDR) grafting approaches: (1) CDR grafting onto a stable variable region framework,18 and (2) structure-guided homology modeling onto a closely related human variable region framework.19 For the first approach, CDRs H1, H3, L1, L2, and L3 were grafted onto the 4D5 framework, with CDR H2 containing the first 10 residues from FMC63 and the final 6 residues left as the sequence native to that framework. In the second approach, a homology model for the FMC63 variable heavy and variable light chains was built using the PIGS server for later structural comparison. Next, the closest human homologs to the heavy and light chains were identified using the IMGT DomainGapAlign tool, yielding IGHVR-30-4∗01 and IGKV1-33∗01 frameworks with the highest homology to the parental murine domains. The FMC63 CDRs were then grafted onto these human frameworks using TabHu,20 with 2 back mutations made in the light chain based on the probability of contacting antigen. The resulting homology model of huCD19R(VH4vκ1) was then subjected to energy minimization using the GROMOS96 force field in Swiss PDB Viewer, demonstrating that no further back mutations were necessary to correct steric clashes or voids in the humanized framework.
Yeast surface display
Yeast culture and display were performed using previously described methods.21 Plasmids encoding for the FMC63 scFv or the humanized variants fused N-terminally to agglutinin 2 and a polypeptide linker with flanking HA (amino acid sequence of N-Tyr-Pro-Tyr-Asp-Val-Pro-Asp-Tyr-Ala-C) and c-Myc epitope tags were transformed into EBY100 Saccharomyces cerevisiae yeast using a Frozen EZ-Yeast Transformation II kit following the manufacturer’s protocol (Zymo Research). Yeast harboring plasmids of interest were grown to an optical density between 0.5 and 1 in SD-CAA medium (synthetic defined medium that is used to grow yeast cells) at 30°C with shaking. Protein expression was induced by media change to SRG-CAA medium followed by overnight growth (8-24 hours) at 30°C with shaking.
Affinity titration of yeast-displayed scFvs
Yeast displaying scFvs of interest was resuspended in PBSA (phosphate-buffered saline containing 0.1% bovine serum albumin) containing defined concentrations of biotinylated recombinant CD19 extracellular domain. Yeast displaying the FMC63 scFv was incubated with 0, 0.56, 1.67, 5, 15, 45, and 135 nM CD19. Yeast displaying humanized variants were incubated with 0, 27, 81, 243, and 500 nM CD19. After incubation, yeast was resuspended with goat anti–c-Myc fluorescein isothiocyanate antibody (Bethyl Laboratories, Inc) and streptavidin Alexa Fluor-647 conjugate. Fluorescence was analyzed with a flow cytometer. Median fluorescence in the streptavidin Alexa Fluor-647 channel was fit to a sigmoidal curve with fluorescence minimum and maximum calculated by the fit.
Thermal denaturation analysis of yeast-displayed scFvs
Yeast displaying scFvs of interest were heated to defined temperatures (room temperature [RT] or 40°C-90°C) for 30 minutes, followed by 10-minute incubation on ice. Yeast cells were then resuspended in 20 μL PBSA containing a fold-specific binding reagent (50 nM recombinant biotinylated CD19 for FMC63, biotinylated protein L for humanized variants) for 20 minutes at RT. Yeast were then incubated with goat anti–c-Myc fluorescein isothiocyanate antibody and streptavidin Alexa Fluor-647 for 20 minutes at RT and analyzed with a flow cytometer. Median fluorescence in the streptavidin Alexa Fluor-647 channel was fit to a 2-state unfolding model with maximum fluorescence calculated from the RT sample and minimum fluorescence calculated using a secondary reagent only control.
CAR design and transduction
The variable fragment heavy chain (VH) and variable fragment light chain (VL) gene segments of the CD19-specific FMC63 or humanized CD19 scFvs (huCD19R VH4vκ1 or 4D5) were cloned into a second-generation CAR lentiviral vector consisting of immunoglobulin G4 hinge with CH3 connected by a 10-amino-acid glycine and serine linker (ch2Δ), CD4 transmembrane domain, cytoplasmic 4-1BB costimulatory domain, and cytoplasmic CD3ζ stimulatory domain. A non-signaling truncated epidermal growth factor receptor (EGFRt) is separated from the CAR by a T2A ribosome skip sequence for tracking transduction efficiency. T-naïve and -memory (Tn/mem) cells defined as CD62L+CD14−CD25− were used for CAR engineering as in our clinical platform.13
In vitro functional assays
Murine and humanized CD19 CAR T cells were cocultured with tumor targets at an effector-to-target (E:T) ratio of 2:1 for 5 hours followed by CD107a flow cytometry analysis. CAR T cells at an E:T ratio of 1:1 were cocultured overnight followed by IFN-γ cytokine staining. Raji-WT tumor rechallenge assay was performed using an E:T ratio of 2:1. Fold expansion of effector CAR T cells followed by each rechallenge was analyzed compared with day 0. At the end of the assay on day 12, exhaustion markers were analyzed by flow cytometry.
In vivo animal studies
All animal experiments were performed under protocols approved by the City of Hope institutional animal care and use committee. Six- to 10-week-old immunodeficient NOD/Scid IL2RᵞCnull (NSG)mice were used for in vivo experiments. Mice were given 0.5 × 106 Raji/Nalm6 WT or Raji CD19-low tumor cells intravenously. For initial sorting, mice were imaged in random groups using the same scale for luminescence followed by sorting based on average flux signal to limit the standard deviation between each group. A total of 1 × 106 CAR T cells were intravenously injected 5 days after tumor engraftment. Untreated and mock groups were used as controls. Firefly luciferase–positive tumor growth was monitored by bioluminescence imaging using Lago X-spectral Instruments once every week. Mice were monitored daily for survival analysis.
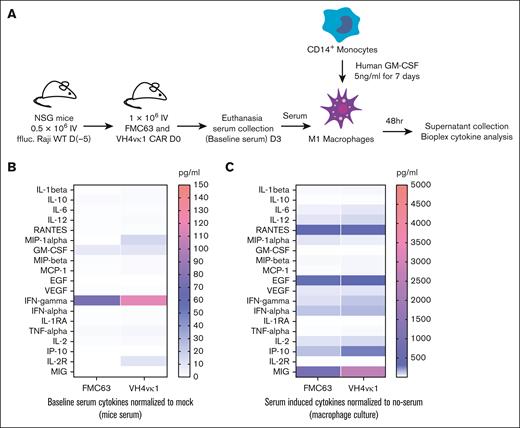
In vitro macrophage activation and cytokine analysis
Cytokine analysis was carried out for macrophage activation as previously described.22 Briefly, CD14+/CD16+ monocytes were enriched from healthy donor peripheral blood mononuclear cells using a STEMCELL enrichment kit (19058) and differentiated to macrophages by treating with 5 ng/mL human granulocyte-macrophage colony-stimulating factor (GM-CSF; Biolegend, 572903) for 7 days. Meanwhile, NSG mice were engrafted with 0.5 × 106 Raji WT followed by treatment with 1 × 106 CAR T cells (n = 4). Three days later, serum was collected from these mice and either frozen for baseline cytokines or directly added to the donor-matched activated macrophages in culture; 48 hours after serum addition, the supernatant was collected from macrophages and cytokine analysis was carried out by Luminex cytokine assay.
Immunogenicity analysis
Major histocompatibility complex 1 (MHC-1) binding predictions for FMC63, huCD19R(VH4Vκ1), and huCD19R(4D5) were made on 26 January 2024 using the Immune Epitope Database (IEDB) analysis resource Consensus tool,23 which combines predictions from ANN, also known as NetMHC (4.0);24-26 SMM;27 and Comblib.28 The analyses involved inputting the complete scFv sequences (including linkers) individually and predicting peptide epitopes of any length likely to be presented in HLA-A∗02:01, HLA-A∗01:01, HLA-A∗03:01, and HLA-A∗24:02, 4 abundant MHC alleles.29 From these predictions, the number of peptides with positive total prediction scores, MHC 50% inhibitory concentration (IC50) of <10 nM, and MHC IC50 of <100 nM were quantified. The top 10 peptide epitopes for each scFv, as determined by total prediction scores, were mapped in each scFv to determine which regions were likely to be the most immunogenic.
Statistical analysis
Data are presented as mean ± standard error of the mean. The number of samples in figure legends represent biological replicates of different healthy donor-derived CAR T cells. All in vitro assays were performed using 2 technical replicates for each sample. In vitro, functional assays were analyzed using the nonparametric Kruskal-Wallis test followed by the Mann-Whitney U test. In vivo assays were carried out using 5 to 10 technical replicates per group. Kaplan-Meier survival analysis for in vivo experiments was carried out using the log-rank (Mantel-Cox) test. All data are analyzed using GraphPad Prism 8. ns, P > .05; ∗P < .05; ∗∗P < .01; and ∗∗∗P < .001.
Ethics approval statement
Ethics approval and consent to participate: all human tissues and blood samples were obtained under protocols approved by the institutional review board (University Health Network Research Ethics Board). Surgical specimens were obtained from the UHN (University Health Network)Biospecimen Program. Written informed consent was obtained from all donors.
Results
Generation and characterization of humanized CD19 scFvs
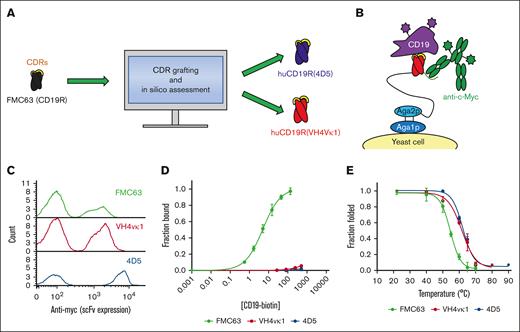
Antibody humanization often involves grafting CDRs from an antibody of nonhuman origin onto human variable region frameworks, with design iteration needed to preserve CDR geometries that yield functional binding. We used 2 established protein engineering approaches to humanize the FMC63 variable regions (Figure 1A). In 1 approach, we used CDR grafting onto a stable antibody framework known to be capable of accepting a variety of nonnative CDRs,18 yielding the huCD19R(4D5) clone. In a second approach, we used structure-guided homology modeling to graft the FMC63 CDRs onto the closest related human framework19,20 in an attempt to keep the CDRs in their most native-like molecular environment, yielding the huCD19R(VH4vκ1) clone. The parental murine FMC63 and the humanized variants were displayed on the surface of the yeast (Figure 1B). scFv expression was assessed by flow cytometry using an anti–c-Myc antibody, demonstrating robust display (Figure 1C). Affinity titration of yeast displayed scFvs with recombinant CD19 extracellular domain demonstrated remarkable affinity reduction (∼2000-fold and 10 000-fold for huCD19R(VH4vκ1) [KD at least 8.3 μM] and huCD19R(4D5) [KD at least 45 μM], respectively) of the humanized variants relative to parental (KD = 4.8 ± 0.7 nM; Figure 1D). Thermal denaturation of yeast that displayed scFvs demonstrated improved thermal stability of both huCD19R(VH4Vκ1) (61 ± 0.9°C) and huCD19R(4D5) (62 ± 0.6°C) relative to the WT (55 ± 0.7°C; Figure 1E). Immunogenicity analysis of the humanized scFvs predicts lower immunogenicity through MHC-I presentation relative to FMC63 (supplemental Tables 1 and 2). FMC63 contains 22 peptide epitopes with positive prediction scores for MHC-I presentation and 3 epitopes with MHC-I IC50 of <10 nM, which is predictive of eradication of the cells presenting such epitopes.30 In contract, huCD19R(VH4Vκ1) and huCD19R(4D5) contain 20 and 15 peptide epitopes with positive prediction scores, respectively. Importantly, neither humanized scFv showed any epitopes predicted to have MHC-I IC50 of <10 nM, suggesting that these scFvs are less likely to elicit an ablative immune response.
Engineering and characterization of humanized CD19-targeted scFvs. (A) The murine anti-CD19 scFv FMC63 (black) was subjected to CDR grafting and in silico assessment, resulting in the generation of 2 humanized variants, huCD19R(4D5) (blue) and huCD19R(VH4vκ1) (red). (B) CD19-targeted scFvs (red with yellow CDRs) were displayed on the surface of yeast cells as N-terminal fusions to a flexible polypeptide linker (black) and agglutinin 2 (Aga2p; light blue). Fusions were anchored to the yeast cell wall via disulfide linkage to Aga1p (dark blue). scFv expression is detected by antibody labeling of a C-terminal c-Myc epitope (green) and antigen binding is detected using fluorescently tagged CD19 extracellular domain (purple). (C) Successful expression of FMC63 or humanized variants scFvs on the yeast surface was detected by c-Myc epitope tag labeling. One representative trial is shown. (D) The binding affinity of the indicated scFvs was tested using flow cytometry with titration of biotinylated recombinant CD19 extracellular domain and fit to a 1:1 equilibrium binding model. Data are presented as mean ± standard deviation of 3 trials. Titration curve fits are calculated based on the average estimated Kd for each clone. Concentrations on the x-axis are in nanomolar (nM). (E) The thermal stabilities of the indicated scFvs were determined by heating yeast displaying the indicated scFvs followed by labeling with either 50 nM biotinylated recombinant CD19 extracellular domain (FMC63) or conformation-specific binder biotinylated protein L (humanized variants). The fluorescence proportion of foldedness was determined by flow cytometry. Data are fit to a 2-state unfolding model. Thermal stabilities are presented as mean ± standard deviation of 3 to 4 trials.
Engineering and characterization of humanized CD19-targeted scFvs. (A) The murine anti-CD19 scFv FMC63 (black) was subjected to CDR grafting and in silico assessment, resulting in the generation of 2 humanized variants, huCD19R(4D5) (blue) and huCD19R(VH4vκ1) (red). (B) CD19-targeted scFvs (red with yellow CDRs) were displayed on the surface of yeast cells as N-terminal fusions to a flexible polypeptide linker (black) and agglutinin 2 (Aga2p; light blue). Fusions were anchored to the yeast cell wall via disulfide linkage to Aga1p (dark blue). scFv expression is detected by antibody labeling of a C-terminal c-Myc epitope (green) and antigen binding is detected using fluorescently tagged CD19 extracellular domain (purple). (C) Successful expression of FMC63 or humanized variants scFvs on the yeast surface was detected by c-Myc epitope tag labeling. One representative trial is shown. (D) The binding affinity of the indicated scFvs was tested using flow cytometry with titration of biotinylated recombinant CD19 extracellular domain and fit to a 1:1 equilibrium binding model. Data are presented as mean ± standard deviation of 3 trials. Titration curve fits are calculated based on the average estimated Kd for each clone. Concentrations on the x-axis are in nanomolar (nM). (E) The thermal stabilities of the indicated scFvs were determined by heating yeast displaying the indicated scFvs followed by labeling with either 50 nM biotinylated recombinant CD19 extracellular domain (FMC63) or conformation-specific binder biotinylated protein L (humanized variants). The fluorescence proportion of foldedness was determined by flow cytometry. Data are fit to a 2-state unfolding model. Thermal stabilities are presented as mean ± standard deviation of 3 to 4 trials.
Humanized CD19 CAR T cells exhibit similar transduction efficiency and phenotype to FMC63 CAR T cells
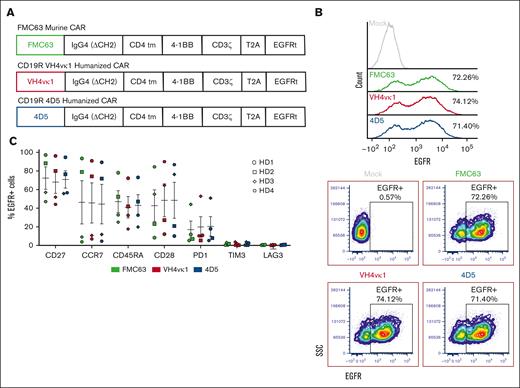
Tn/mem cells isolated from healthy donors were activated and lentivirally transduced31 with huCD19R(VH4vκ1), huCD19R(4D5), and FMC63 CAR with the same backbone as a control (Figure 2A) and expanded for 14 days. We were able to successfully transduce both humanized CARs with similar transduction efficiency to FMC63 CAR as assessed by EGFR staining (Figure 2B). Phenotypic analysis of the same donor FMC63 and humanized CARs show similar expression of memory and stem memory markers such as CD27 and CCR7 and CD45RA and CD28, respectively, and low exhaustion markers such as PD1, TIM3, and LAG3 (Figure 2C).
CAR T-cell design and characterization. (A) Diagram of lentiviral cassette design for CD19-targeted CARs including CD19 scFv (FMC63, VH4vκ1, and 4D5), immunoglobulin G4 hinge, and CH3 connected by a 10-amino-acid glycine and serine (GS) linker (ch2Δ), CD4 transmembrane domain, cytoplasmic 4-1BB costimulatory domain, and cytoplasmic CD3ζ stimulatory domain. A non-signaling truncated EGFR is separated from the CAR by a T2A ribosome skip sequence for tracking transduction efficiency. (B) CD19 CARs were expressed in the healthy donor (HD) Tn/mem cells by lentiviral transduction. CAR expression is measured as % EGFR+. Representative histograms (top) and dot plots (bottom) are shown (n = 3). (C) Murine and humanized CAR T cells were characterized by analyzing the expressions of CD27, CCR7, CD45RA, CD28, programmed cell death protein 1 (PD1), TIM3, and LAG3 (n = 4). Data are shown as mean ± standard error of the mean (SEM).
CAR T-cell design and characterization. (A) Diagram of lentiviral cassette design for CD19-targeted CARs including CD19 scFv (FMC63, VH4vκ1, and 4D5), immunoglobulin G4 hinge, and CH3 connected by a 10-amino-acid glycine and serine (GS) linker (ch2Δ), CD4 transmembrane domain, cytoplasmic 4-1BB costimulatory domain, and cytoplasmic CD3ζ stimulatory domain. A non-signaling truncated EGFR is separated from the CAR by a T2A ribosome skip sequence for tracking transduction efficiency. (B) CD19 CARs were expressed in the healthy donor (HD) Tn/mem cells by lentiviral transduction. CAR expression is measured as % EGFR+. Representative histograms (top) and dot plots (bottom) are shown (n = 3). (C) Murine and humanized CAR T cells were characterized by analyzing the expressions of CD27, CCR7, CD45RA, CD28, programmed cell death protein 1 (PD1), TIM3, and LAG3 (n = 4). Data are shown as mean ± standard error of the mean (SEM).
Humanized CD19 CAR T cells exhibit antigen-specific effector function
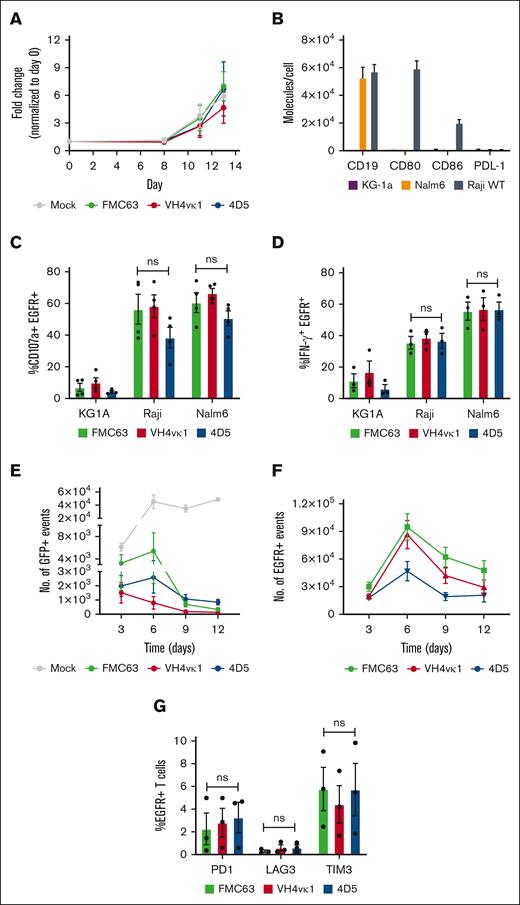
The expansion of T cells harboring the humanized CARs was similar in comparison with those harboring the FMC63 CAR (Figure 3A). To evaluate the function of the humanized CD19 CARs, B-cell lines that represent different B-cell tumor types were used. To note, CD19 expression based on molecules per cell was similar on both ALL Nalm6 and Burkitt lymphoma Raji cells whereas CD80/86 expression was only found on the Raji cell line (Figure 3B), which results in different responses to CAR T cell therapy.32 CD19− acute myeloid leukemia KG-1a was used as a negative control. Stimulation of huCD19R(VH4vκ1) and huCD19R(4D5) CARs produced an antigen-dependent expression of CD107a and production of IFN-γ that was equivalent to levels expressed by FMC63 CAR T cells (Figure 3C-D).
Humanized CAR T cells exhibited antigen-specific effector function. (A) The growth curve for Mock, FMC63, VH4vκ1, and 4D5 is shown as fold change normalized to day 0. (B) Surface expression of CD19 antigen, costimulatory molecules CD80, CD86, and inhibitory ligand PD-L1 on tumor lines KG1a, Nalm6, and Raji. Tn/mem cells expressing the specified CAR constructs were analyzed for their ability to (C) degranulate (107a), (D) produce IFN-γ and in response to KG1a, CD19+ Raji, and CD19+ Nalm6 by flow cytometry. Data are shown as mean ± SEM for CAR+ CD107a+ (n = 4) and CAR+ IFN-γ (n = 3). Tn/mem cells expressing the specified CAR constructs were challenged with CD19+ green fluorescent protein-positive (GFP+) Raji cells at an E:T ratio of 2:1 on day 0. CAR T cells were subsequently rechallenged with the same number of Raji cells every 3 days. (E) The remaining Raji tumor is presented as the number of GFP+ events on days 3, 6, 9, and 12 in the control mock group and the 3 CAR groups. (day 6, 9, and 12: P < .01 Mock vs FMC63, VH4vκ1, and 4D5; day 9: P < .05 FMC63 vs VH4vκ1 and P < .01 VH4vκ1 vs 4D5; day 12: P < .01 VH4vκ1 vs 4D5). (F) Proliferation of CAR T cells is presented as the number of EGFR+ events on days 3, 6, 9, and 12 (day 9: P < .05 FMC63 vs 4D5 and VH4vκ1 vs 4D5). (G) Exhaustion markers on EGFR+ cells such as PD1, LAG3, and TIM3 are analyzed on day 12 (ns). For panels E-G, data are presented as mean ± SEM (n = 3).
Humanized CAR T cells exhibited antigen-specific effector function. (A) The growth curve for Mock, FMC63, VH4vκ1, and 4D5 is shown as fold change normalized to day 0. (B) Surface expression of CD19 antigen, costimulatory molecules CD80, CD86, and inhibitory ligand PD-L1 on tumor lines KG1a, Nalm6, and Raji. Tn/mem cells expressing the specified CAR constructs were analyzed for their ability to (C) degranulate (107a), (D) produce IFN-γ and in response to KG1a, CD19+ Raji, and CD19+ Nalm6 by flow cytometry. Data are shown as mean ± SEM for CAR+ CD107a+ (n = 4) and CAR+ IFN-γ (n = 3). Tn/mem cells expressing the specified CAR constructs were challenged with CD19+ green fluorescent protein-positive (GFP+) Raji cells at an E:T ratio of 2:1 on day 0. CAR T cells were subsequently rechallenged with the same number of Raji cells every 3 days. (E) The remaining Raji tumor is presented as the number of GFP+ events on days 3, 6, 9, and 12 in the control mock group and the 3 CAR groups. (day 6, 9, and 12: P < .01 Mock vs FMC63, VH4vκ1, and 4D5; day 9: P < .05 FMC63 vs VH4vκ1 and P < .01 VH4vκ1 vs 4D5; day 12: P < .01 VH4vκ1 vs 4D5). (F) Proliferation of CAR T cells is presented as the number of EGFR+ events on days 3, 6, 9, and 12 (day 9: P < .05 FMC63 vs 4D5 and VH4vκ1 vs 4D5). (G) Exhaustion markers on EGFR+ cells such as PD1, LAG3, and TIM3 are analyzed on day 12 (ns). For panels E-G, data are presented as mean ± SEM (n = 3).
We further tested the long-term effector function of humanized CAR T cells by performing a tumor rechallenge assay in which huCD19R(VH4vκ1), huCD19R(4D5), and FMC63 CAR T cells were cocultured with Raji WT cells at an E:T ratio of 2:1. CAR T cells were repetitively challenged with Raji WT cells on days 3, 6, and 9 (supplemental Figure 1A). We observed significant killing of Raji and Nalm-6 tumor by huCD19R(VH4vκ1) CAR T cells on days 9 and 12 (Figure 3E; supplemental Figure 1B) as with FMC63 CAR T cells. However, huCD19R(4D5) CAR T cells essentially failed to proliferate upon tumor rechallenge (Figure 3F; supplemental Figure 1C). At the end of the 12-day assay, we found comparable levels of PD1 and LAG3 on huCD19R(VH4vκ1), huCD19R(4D5), and FMC63 CAR T cells (Figure 3G, supplemental Figure 1D). Together, these data suggest that both humanized CAR T cells mediate similar in vitro effector functions. However, huCD19R(VH4vκ1) but not huCD19R(4D5) CAR T cells were able to kill and proliferate upon repetitive antigen rechallenge.
huCD19R(VH4vκ1) CAR efficiently controls CD19+ lymphoma tumor in vivo
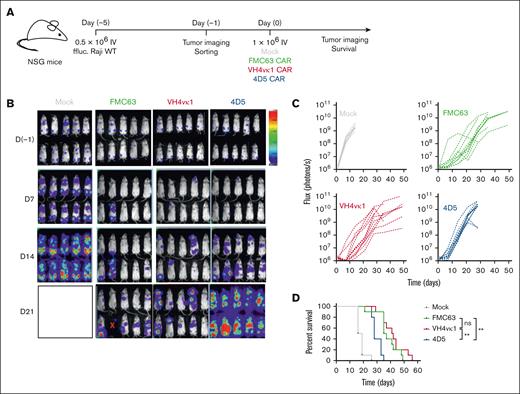
To determine the antitumor efficacy of the 2 humanized CARs in vivo in comparison with FMC63 CAR, we tested CAR T cells against the established CD19+ Raji model (Raji-WT; Figure 4A). We observed that huCD19R(VH4vκ1) CAR T cells and FMC63 CAR T cells mediated significantly better antitumor activity compared with huCD19R(4D5) CAR T cells (P < .001; Figure 4B-D). Furthermore, huCD19R(VH4vκ1) CAR T cells significantly improved the survival of mice in Nalm6 model compared with huCD19R(4D5) CAR T cells (P < .05; supplemental Figure 2).
Humanized VH4vκ1 CAR T cells exhibited efficient antitumor activity against B-cell lymphoma tumor in vivo. (A) Schema showing NOD/Scid IL2RᵞCnull (NSG) mice engrafted IV with enhanced GFP + firefly luciferase (ffluc) + Raji-WT cells followed by murine and humanized CAR T-cell treatments. Mock was used as the control group for FMC63 and humanized VH4vκ1- and 4D5-treated groups. (B-C) Tumor burden was determined using bioluminescent imaging (BLI; 11 technical replicates per group) and log-transformed flux is shown as linear mixed models for each treatment over time (day 21 and 28: P < .001 FMC63 vs 4D5; and P < .001 VH4vκ1 vs 4D5). (D) Survival of mice treated with murine FMC63 compared with the 2 humanized CARs was analyzed using a log-rank test, ∗∗P < .01. Representative data from n = 2 are presented.
Humanized VH4vκ1 CAR T cells exhibited efficient antitumor activity against B-cell lymphoma tumor in vivo. (A) Schema showing NOD/Scid IL2RᵞCnull (NSG) mice engrafted IV with enhanced GFP + firefly luciferase (ffluc) + Raji-WT cells followed by murine and humanized CAR T-cell treatments. Mock was used as the control group for FMC63 and humanized VH4vκ1- and 4D5-treated groups. (B-C) Tumor burden was determined using bioluminescent imaging (BLI; 11 technical replicates per group) and log-transformed flux is shown as linear mixed models for each treatment over time (day 21 and 28: P < .001 FMC63 vs 4D5; and P < .001 VH4vκ1 vs 4D5). (D) Survival of mice treated with murine FMC63 compared with the 2 humanized CARs was analyzed using a log-rank test, ∗∗P < .01. Representative data from n = 2 are presented.
Humanized CD19 CAR T cells exhibit ability to target CD19-low-expressing tumors
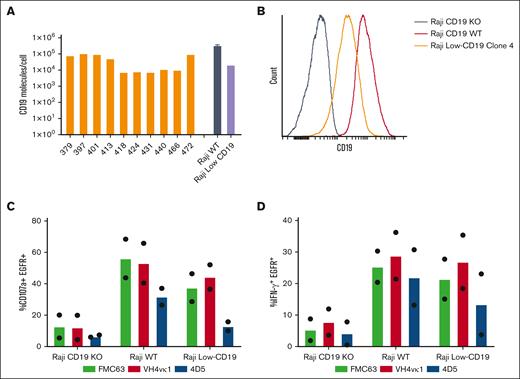
CD19 antigen expression is heterogenous because of disease type, stage, or antigen downregulation.33 As we expected, CD19 expression in primary ALL samples ranged from 105 to 104 molecules per cell measured by Quantum Simply Cellular assay (Figure 5A). To determine the efficacy of humanized CD19 CARs against low antigens, we created a Raji cell line with low CD19 density. First, CD19 was CRISPR-edited out of Raji-WT cells to produce Raji-KO cells. CD19 was then reintroduced into these Raji-KO cells by lentiviral transduction under the regulation of a PGK100 promoter (mutant PGK promoter driving weak expression),34 to produce Raji CD19-low cells. Raji CD19-low clones were generated by limiting dilution33 and expanded for functional assays (supplemental Figure 3). Flow cytometry analysis was used to determine the levels of CD19 expression on Raji-WT, Raji-KO, and 4 Raji CD19-low clones, with Raji CD19-low clone 4 matching the level of lower CD19 expression in certain patient samples (Figure 5A-B). Hence, Raji WT and Raji CD19-low clone 4, representing the range of high to low antigen expression in primary tumor cells from patients, were used as targets to assess CAR functionality. Notably, with Raji CD19-low tumor in vitro, huCD19R(4D5) CAR-T cells showed slightly lower levels of degranulation and IFN-γ production compared with huCD19R(VH4vκ1) and FMC63 CAR T cells (Figure 5C-D).
Humanized VH4vκ1 CAR is effective against the Raji lymphoma tumor line expressing low CD19 antigen in vitro. (A) Variability of CD19 antigen expression is analyzed on the surface of ALL patient peripheral blood mononuclear cells (PBMCs; n = 10) as well as Raji WT and Raji CD19-low cell lines. (B) Histogram showing CD19 expression on Raji-WT, Raji CD19-KO, and Raji CD19-low clone. Raji CD19-low cell line was used for the following experiments. Tn/mem expressing the specified CAR constructs were analyzed for their ability to (C) degranulate and (D) produce IFN-γ in response to Raji CD19-KO, Raji WT, and Raji CD19-low by flow cytometry. Data are shown as mean of two experiments.
Humanized VH4vκ1 CAR is effective against the Raji lymphoma tumor line expressing low CD19 antigen in vitro. (A) Variability of CD19 antigen expression is analyzed on the surface of ALL patient peripheral blood mononuclear cells (PBMCs; n = 10) as well as Raji WT and Raji CD19-low cell lines. (B) Histogram showing CD19 expression on Raji-WT, Raji CD19-KO, and Raji CD19-low clone. Raji CD19-low cell line was used for the following experiments. Tn/mem expressing the specified CAR constructs were analyzed for their ability to (C) degranulate and (D) produce IFN-γ in response to Raji CD19-KO, Raji WT, and Raji CD19-low by flow cytometry. Data are shown as mean of two experiments.
huCD19R(VH4vκ1) CAR T cells efficiently control CD19-low lymphoma tumor in vivo
When testing humanized CARs against Raji CD19-low tumor (Figure 6A; supplemental Figure 4A), we found that huCD19R(VH4vκ1) CAR T cells effectively controlled the tumor growth (Figure 6B-C). Importantly, treatment with huCD19R(4D5) CAR failed to improve survival of mice compared with the huCD19R(VH4vκ1) treated group (Figure 6D; P < .001), despite the similar CAR T cell expansion/persistence at the end points (Figure 6E), suggesting that the affinity of huCD19R(4D5) CAR is below the threshold required for CAR function in this context.35 Antitumor efficacy of huCD19R(VH4vκ1) CAR T cells was similar when compared with the FMC63 CAR T cell group. Tumor antigen expression (CD19 surface density) was assessed at the end of the experiments and found to have maintained low CD19 expression (supplemental Figure 4B), demonstrating that antigen loss is not the mechanism for continued tumor growth. Together these results imply that the huCD19R(4D5) CAR is not efficacious for in vivo tumor treatment with low levels of antigen. Therefore, huCD19R(VH4vκ1) CAR was the selected candidate for further study and clinical translation.
Humanized VH4vκ1 exhibited efficient antitumor activity against CD19-low tumor in vivo. (A) Schema showing NSG mice engrafted IV with eGFP+ ffluc+ Raji CD19-low cells followed by murine and humanized CAR T cell treatments. Mock was used as the control group for FMC63 and humanized VH4vκ1- and 4D5-treated groups. (B-D) Representative figure of tumor progression in different groups is shown over time. Tumor progression was monitored using BLI (5-9 technical replicates per group) and log-transformed flux is shown for each treatment over time. Tumor progression was effectively controlled by FMC63 and VH4vκ1 compared with 4D5 (day 6, 13, and 20: P < .001 FMC63 vs 4D5; and P < .001 VH4vκ1 vs 4D5). (D) Survival of mice treated with murine FMC63 compared with the 2 humanized CARs was analyzed using the log-rank test: ∗∗∗P < .001 (n = 5-9 mice per group). Representative data from 2 separate experiments are presented. (E) Percent of CAR T cells in mice blood was analyzed at the end of the experiment (P < .01, untransduced T cells [Mock] vs FMC63, VH4vκ1, and 4D5).
Humanized VH4vκ1 exhibited efficient antitumor activity against CD19-low tumor in vivo. (A) Schema showing NSG mice engrafted IV with eGFP+ ffluc+ Raji CD19-low cells followed by murine and humanized CAR T cell treatments. Mock was used as the control group for FMC63 and humanized VH4vκ1- and 4D5-treated groups. (B-D) Representative figure of tumor progression in different groups is shown over time. Tumor progression was monitored using BLI (5-9 technical replicates per group) and log-transformed flux is shown for each treatment over time. Tumor progression was effectively controlled by FMC63 and VH4vκ1 compared with 4D5 (day 6, 13, and 20: P < .001 FMC63 vs 4D5; and P < .001 VH4vκ1 vs 4D5). (D) Survival of mice treated with murine FMC63 compared with the 2 humanized CARs was analyzed using the log-rank test: ∗∗∗P < .001 (n = 5-9 mice per group). Representative data from 2 separate experiments are presented. (E) Percent of CAR T cells in mice blood was analyzed at the end of the experiment (P < .01, untransduced T cells [Mock] vs FMC63, VH4vκ1, and 4D5).
huCD19R(VH4vκ1) and FMC63 CAR T cells exhibited a similar cytokine production profile
Cytokine release syndrome (CRS) is a common adverse event in CAR T cell therapies. However, there is a lack of an optimal model for evaluating or predicting the risk of CRS. To mimic the sequence of events leading to CRS, we compared cytokine profiles of the lead candidate huCD19R(VH4vκ1) CAR and FMC63 CAR by adopting a previously reported hybrid in vitro/in vivo model.22 Briefly, serum from mice engrafted with Raji-WT and treated with huCD19R(VH4vκ1) CAR T cells, FMC63 CAR T cells, or mock T cells were added to donor-matched GM-CSF–activated macrophages. The supernatant was collected after 48 hours and analyzed using Luminex (Figure 7A). This model allows us to analyze the trigger cytokines from CAR T cell–tumor engagement as well as secondary inflammatory cytokines derived from bystander immune cells such as macrophages. We found that baseline serum collected from huCD19R(VH4vκ1) CAR–treated mice had higher INF-γ, compared with the FMC63 CAR group (Figure 7B). Importantly, when activated macrophages were exposed to this serum, no statistically significant differences in hallmark cytokines of CRS such as IL-6 and GM-CSF were observed between huCD19R(VH4vκ1) and FMC63 CAR groups (P > .05). We did, however, observe higher levels of monokine induced by IFN-γ (MIG) in the huCD19R(VH4vκ1) CAR group (Figure 7C). These data are consistent with higher levels of IFN-γ in serum that, in turn, upregulate MIG as an IFN-γ–induced cytokine.36 These data demonstrate that huCD19R(VH4vk1) CAR and FMC63 CAR exhibit similar cytokine safety profiles, making this humanized CAR ideal to translate to the clinic.
Cytokine analysis of murine FMC63 and humanized VH4vκ1 CAR T cells after macrophage activation. (A) Schema showing macrophage activation experiment. NSG mice were engrafted with Raji tumor followed by murine and humanized CAR treatment. Serum was collected 3 days after CAR treatment and added to GM-CSF–activated donor-matched macrophages. The supernatant was collected 48 hours later and Luminex cytokine analysis was carried out. (B) Serum collected from mice before adding to the macrophages was analyzed for baseline cytokine levels. (C) Macrophage-derived cytokines. Data are shown as mean ± SEM; n = 4 mice per group and respective macrophage conditions; P > .05.
Cytokine analysis of murine FMC63 and humanized VH4vκ1 CAR T cells after macrophage activation. (A) Schema showing macrophage activation experiment. NSG mice were engrafted with Raji tumor followed by murine and humanized CAR treatment. Serum was collected 3 days after CAR treatment and added to GM-CSF–activated donor-matched macrophages. The supernatant was collected 48 hours later and Luminex cytokine analysis was carried out. (B) Serum collected from mice before adding to the macrophages was analyzed for baseline cytokine levels. (C) Macrophage-derived cytokines. Data are shown as mean ± SEM; n = 4 mice per group and respective macrophage conditions; P > .05.
Translating to clinical trial
Built on the preclinical characterization and evaluation, we conducted studies with guanosine 3′,5′-cyclic monophosphate–grade lentivirus encoding huCD19R(VH4vκ1) CAR to conduct Investigational New Drug application (IND) enabling studies with T cells from 3 different healthy donors following the manufacturing standard operating procedures. We observed consistent transduction efficiency (supplemental Figure 5A) and expansion (supplemental Figure 5B). The CAR T cell products exhibited consistent memory phenotypical characteristics (supplemental Figure 5C) and effector function upon stimulation with CD19+ target (supplemental Figure 5D). Lastly, the CAR T cell products met all release criteria for infusion (supplemental Table 3).
Discussion
In this study, 2 candidate humanized CD19 scFvs were derived from the murine scFv FMC63, readily displayed on the yeast surface but demonstrated differences in binding affinity and thermal stability relative to the parental murine scFv. Both humanized variants had a drastic reduction in binding affinity, which is a relatively common outcome from the initial humanization process.37 Depending on the CAR design and the antigens in tumors, different binding affinities are required for the proper function of CAR T cells. Some CAR T cells with T cell receptor–level affinities (weak, μM) can work efficiently in certain conditions but not in all.38-41 Therefore, each CAR and target combination must be carefully validated. The ability of weakly binding CARs to mediate T cell activation is likely because of the high avidity between CAR T cells and antigen-expressing tumor cells, which can overcome the weak individual interactions (ie, low affinity) because of the strength of combined interaction (ie, high avidity). In contrast, the humanized scFvs both demonstrated improved thermal stability relative to the parental scFv. It has been shown previously that the stability of a folded state influences the immunogenicity of proteins of interest, with improving fold stability leading to diminished T cell epitope availability.42 This is because of well-folded proteins exposing fewer linear epitopes to proteolytic degradation, which protects from MHC presentation and subsequent immunogenicity. An improvement in fold stability alongside diminished quality of T cell epitope presentation in MHC-I, which was demonstrated through computational efforts, could improve the safety and efficacy of CD19-targeted CAR T cells through diminished prevalence and availability of T cell epitopes. This is especially important because a previous CD19-CAR T cell trial noted treatment rejection due to MHC-centric eradication of therapeutic cells.43
Despite the differing biophysical properties of the humanized scFv variants, all CAR T cell groups demonstrated comparable growth potential and phenotypic characteristics as compared with FMC63 CAR T cells. Both humanized CAR T cells were capable of effector functions such as degranulation and IFN-γ production while targeting different CD19-expressing tumors. Interestingly, the tumor rechallenge assay revealed differential proliferation dependent on binding affinity. huCD19R(VH4vκ1) but not huCD19R(4D5) CAR was able to mediate robust proliferation after repetitive antigen stimulation without increased exhaustion, indicating that there is a threshold of affinity for proper CAR function, an observation that has been made in prior work as well.39,44 Consistently, we observed significantly better antitumor activity and prolonged mouse survival when treated with huCD19R(VH4vκ1) as compared with huCD19R(4D5) CAR. The in vivo results using huCD19R(VH4vκ1) CAR T cells were equivalent to FMC63 CAR T cells.
CD19 antigen expression levels on tumor cells could be heterogeneous because of disease type, stage, and targeted treatment.45,46 The density of antigen expression can affect all components of an antitumor CAR T cell response.47,48 Although our humanized CD19 CARs have a low affinity, we observed a more efficient effector function of huCD19R(VH4vκ1) and FMC63 CD19 CAR T cells against Raji CD19-low tumor than huCD19R(4D5) CAR T cells. When Raji CD19-low tumor-bearing mice were treated with humanized CAR T cells, huCD19R(VH4vκ1) outperformed huCD19R(4D5) CAR T cells, resulting in significantly prolonged mouse survival. Again, a similar ability to target CD19-low tumors was observed between huCD19R(VH4vκ1) and FMC63 CAR T cells. Data also suggested that differences in the antitumor activity are indeed the result of the unfavorable function of low-affinity huCD19R(4D5) CAR rather than CAR T-cell persistence.35
To mimic the sequence of events leading to CRS, we compared cytokine profiles of huCD19R(VH4vκ1) CAR T cells and FMC63 CAR T cells by adopting a hybrid in vitro/in vivo model.22 There was higher IFN-γ secreted by huCD19R(VH4vκ1) CAR T cells than FMC63 CAR T cells. However, the increased IFN-γ did not induce higher levels of IL-6, GM-CSF, IL-1, and Macrophage Inflammatory Protein (MIP), which are the hallmarks of CRS.49
Based on preclinical studies, the huCD19R(VH4vκ1) T cells have received US Food and Drug Administration approval for a phase 1 clinical trial (IND 30031, IRB 23328). This trial aims to investigate the safety and feasibility of using huCD19 CAR T cells for the treatment of relapsed/refractory ALL.
Acknowledgments
This study was financially supported by Lymphoma SPORE (P50CA107399; S.J.F. and X.W.), the Borstein Family Foundation (X.W), and a postdoctoral fellowship award from the Tobacco-Related Disease Research Program of the Universities of California, 28FT-0072 (L.A.S.)
Authorship
Contribution: X.W., C.E.B., L.A.S., J.C.W., and S.J.F. contributed to the study concept and design, and data interpretation; V.V., L.A.S., L.L., C.H., R.U., R.E., Z.W., and M.S.T. performed experiments and generated figures; and X.W., V.V., and L.A.S. wrote the manuscript.
Conflict-of-interest disclosure: L.A.S., J.C.W., S.J.F. X.W., C.E.B. are the inventors of the huCD19CAR patent application. The remaining authors declare no competing financial interests.
The current affiliation for L.A.S. is the Department of Chemical, Biological, and Materials Engineering, University of South Florida, Tampa, FL
Correspondence: Xiuli Wang, Department of Hematology and Hematopoietic Cell Transplantation, City of Hope National Medical Center, 1500 E Duarte Rd, Duarte, CA 91010; email: xiuwang@coh.org.
References
Author notes
L.A.S. and V.V and joint first authors.
C.E.B. and X.W. are joint senior authors.
Data are available on request from the corresponding author, Xiuli Wang (xiuwang@coh.org).
The full-text version of this article contains a data supplement.

![Humanized VH4vκ1 exhibited efficient antitumor activity against CD19-low tumor in vivo. (A) Schema showing NSG mice engrafted IV with eGFP+ ffluc+ Raji CD19-low cells followed by murine and humanized CAR T cell treatments. Mock was used as the control group for FMC63 and humanized VH4vκ1- and 4D5-treated groups. (B-D) Representative figure of tumor progression in different groups is shown over time. Tumor progression was monitored using BLI (5-9 technical replicates per group) and log-transformed flux is shown for each treatment over time. Tumor progression was effectively controlled by FMC63 and VH4vκ1 compared with 4D5 (day 6, 13, and 20: P < .001 FMC63 vs 4D5; and P < .001 VH4vκ1 vs 4D5). (D) Survival of mice treated with murine FMC63 compared with the 2 humanized CARs was analyzed using the log-rank test: ∗∗∗P < .001 (n = 5-9 mice per group). Representative data from 2 separate experiments are presented. (E) Percent of CAR T cells in mice blood was analyzed at the end of the experiment (P < .01, untransduced T cells [Mock] vs FMC63, VH4vκ1, and 4D5).](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodneoplasia/1/4/10.1016_j.bneo.2024.100048/4/m_bneo_neo-2024-000237-gr6.jpeg?Expires=1764965685&Signature=Te-~T0kZZu4z1zNouPopvzhaT3ktIhMxUq9T~nKzB2mORmoY4ZpWHhDSO26VtpCcrmFa0AuL441Q2suG3LMI2HeaaX-bgI9PyB0sDQ4HiOxqOb6Aw3JzhuFnNpRce6cujkurKovdkDaH1U4zSk-GB593cF26dWhgTVAkjuu9-WaJ-9YUZTxX~iQ2aQ7aXgsFHKsaekBNQH~8twejA2MaMtozu3DYAQnzOBv2aKtAJYBphPiii1XOIL-86M8XYWyiWhZAG7KizWhhbeB7cLJYGrbOQXCZJy3qCCR0P2cHMlGSBEENfToZ1jezOkaE3go7Wqrg3BYNAyH8Q-h54ihQIw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)