Key Points
Nascent reticulocytes exhibit distinctly unique proteomes compared with mature reticulocytes.
Deformation-based studies show a correlation between the membrane linkage and decreased expression of surface proteins during R1 maturation.
Visual Abstract
Reticulocyte maturation, the final stage of erythropoiesis after enucleation, is a dynamic 48- to 72-hour process remodeling multilobular, motile, and fragile nascent reticulocytes into deformable, biconcave red blood cells. This maturation involves organelle clearance and extensive membrane remodeling. A significant knowledge gap in our understanding is a lack of knowledge of the proteins involved in the earliest stages of reticulocyte maturation, the nascent bone marrow reticulocyte. To address this, we isolated and characterized the proteomic profiles of distinct reticulocyte populations from healthy human bone marrow. Density gradient separation, fluorescence-activated cell sorting analyses, and electron microscopy revealed a continuum of different stages of reticulocyte maturation in the marrow. Deformation-induced micropipette aspiration analyses combined with imaging of membrane proteins labeled with specific fluorescent probes, performed to better understand mechanisms of decreased expression of surface proteins during reticulocyte maturation, revealed a correlation between the extent of linkage and the decreased expression of surface proteins during reticulocyte maturation. There was a marked decrease in the total protein content between nascent and mature reticulocytes (from 56 to 39 pg per cell), with a modest decrease between mature reticulocytes and mature erythrocytes (from 39 to 37 pg per cell). Comparison of proteomes from nascent reticulocytes (2532 unique proteins), mature reticulocytes, and mature erythrocytes revealed only 867 proteins were shared among the 3 populations, with significant changes in many classes of proteins. These studies provide comprehensive insights into maturation of the human reticulocyte proteome, advancing our understanding of the molecular mechanisms governing this process, and offer potential implications for acquired and inherited red blood cell disorders.
Introduction
Mammalian erythropoiesis is a dynamic process responsible for the production of 2.5 million red blood cells per second.1 Numerous studies have focused on terminal erythroid differentiation, culminating in the enucleation at the orthochromatic erythroblast stage, resulting in the generation of the nascent reticulocyte and the pyrenocyte, which is rapidly phagocytosed by the macrophage in the erythroblastic niche.2-5 Nascent reticulocytes contain mitochondria, endoplasmic reticulum, endosomes, a centriole, and ribosomes that are lost during maturation.6-9 In addition, during reticulocyte maturation, a very large but specific set of proteins are lost, accompanied by an extensive membrane remodeling, leading to membrane surface area loss, decreased cell volume, and increased membrane mechanical stability.10-16
Reticulocyte maturation occurs over an ∼72-hour period, two-thirds of which occurs in the bone marrow and the last 24 hours in the circulation. Early microscopic and biophysical studies have documented that nascent reticulocytes (R1 reticulocytes) are multilobulated and motile,11,14 and express high levels of cell adhesion molecules. While absent from the circulation of healthy adults, R1 reticulocytes can be observed under conditions of erythropoietic stress, such as birth, when they are prematurely released from the bone marrow into circulation.12 Because R1 reticulocytes reside exclusively in the bone marrow of healthy adults, current knowledge of reticulocyte biology primarily focuses on the later stages of development of mature human reticulocytes. Consequently, information about nascent, immature reticulocytes remains limited.
In the present study, we explored quantitative changes in the protein expression levels during maturation of nascent reticulocytes into mature reticulocytes, and subsequently to mature red blood cells, to define the mechanistic basis for distinct morphological and biophysical properties of reticulocytes and red blood cells. Using mass spectrometry–based proteomics on sorted reticulocytes from human bone marrow, we quantified the expression levels of >2000 proteins in the R1 population. Comparison of R1 proteome with that of mature reticulocytes and erythrocytes showed a marked decrease in the total protein content in conjunction with the disappearance of mitochondrial and ribosomal proteins. Further analyses demonstrate that most of the proteomic changes occur between the R1 and mature reticulocyte stages, and offer comprehensive insights into the proteome of human reticulocytes. These findings have implications for our understanding of the reticulocyte biology, and into the potential contribution of defective reticulocyte maturation to the pathophysiology of red blood cells in various red blood cell disorders, including sickle cell disease and parasitic infections, such as those caused by Plasmodium.
Materials and methods
Isolation and purification of human nascent reticulocytes
Reticulocytes were enriched by density gradient centrifugation of bone marrow obtained from deidentified healthy adults undergoing hip replacement surgery provided by the Tissue Donation Protocol (Northwell), and diluted to 15 mL with room temperature (RT) fluorescence-activated cell sorting (FACS) buffer (phosphate-buffered saline [PBS], 0.5% bovine serum albumin, 2 mM EDTA) by density gradient centrifugation. Needle aspiration was not involved in the collection of the bone marrow specimens. A 2-layer Percoll (GE HealthCare) gradient (15 mL each) was prepared with densities of 1.078 g/mL (62% volume-to-volume ratio [v/v]) and 1.087 g/mL (70% v/v) using a 1.1215 g/mL (100% v/v) Percoll stock solution in PBS with 0.1 M HEPES (N-2-hydroxyethylpiperazine-Nʹ-2-ethanesulfonic acid), and further diluted in Iscove modified Dulbecco medium (Gibco) at RT. For improved visualization, the top layer (1.078 g/mL) was prepared using phenol-red–free Iscove modified Dulbecco medium or PBS. The 45 mL gradient was centrifuged in a swinging bucket rotor (30 minutes, 400g, RT, low acceleration, no brake). The supernatant was removed, and the enriched reticulocyte fraction above the 1.087 g/mL Percoll layer was collected. After resuspension and washing with FACS buffer, the fraction was pelleted (300g, RT, 10 minutes). Cells were resuspended in 1 mL cold FACS buffer and counted (Countess II FL; Thermo Scientific).
Fluorescence-imaged micropipette deformation
The density distribution of proteins within the red blood cell membrane during deformation was measured using the combination of fluorescence imaging and micropipette aspiration, as previously described by Discher et al.17 Different membrane proteins on the red blood cell surface were labeled with fluorescently tagged monoclonal antibodies to assess their density distribution in the deformed state.
Analyses of tandem mass spectrometry data
Obtained mass spectrometry data and raw data of mature reticulocytes and erythrocytes from PXD009258 were analyzed using MaxQuant software, version 1.6.1.0.18 Details on the analyses are available in the supplemental Methods. The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium via the PRIDE19 partner repository with the data set identifier PXD062723 reviewer_pxd062723@ebi.ac.uk.
Additional methods and study approval
Additional methods can be found in the supplemental Methods.
All studies were conducted in accordance with the Declaration of Helsinki, and under institutional review board approval of Northwell Health.
Results
Resolving the continuum of human reticulocytes
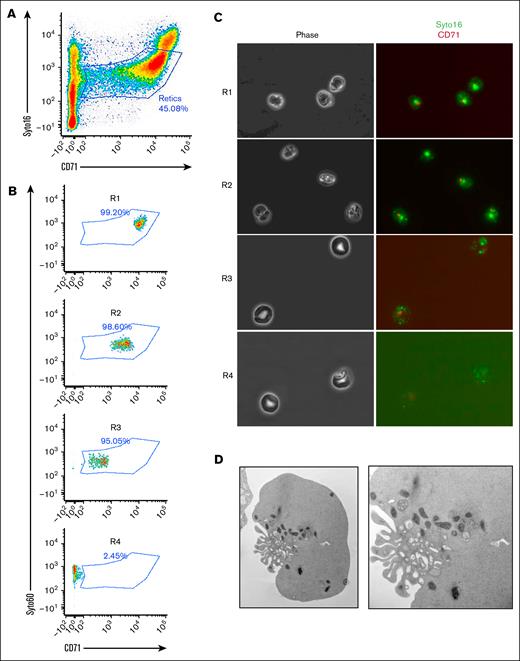
Numerous studies on reticulocyte maturation have relied primarily on animal models or anemic human blood, potentially overlooking the potential heterogeneity of the reticulocyte populations within the bone marrow. In the present study, human reticulocytes were obtained directly from the bone marrow of healthy individuals undergoing hip replacement surgery, but who were otherwise healthy. After density gradient separation, the cells were stained for the transferrin receptor (CD71) and RNA (SYTO16; Figure 1A). As expected, both markers progressively decreased during maturation, highlighting a continuum of reticulocyte maturation in the bone marrow. To resolve this continuum, we sorted the different populations of reticulocytes based on the expression levels of the 2 markers, and isolated 4 different populations of reticulocytes, R1 to R4 (Figure 1B-C). Based on morphology, the multilobulated shape of the nascent reticulocyte was the dominant feature of the R1 population, while the stomatocytic shape of the mature reticulocytes was the main characteristic of R3 and R4 populations. Transmission electron microscopy pictures of the R1 fraction demonstrated the multilobulated shape with numerous mitochondria and vesicles at the region of nuclear extrusion (Figure 1D). These data imply that the human bone marrow contains a continuum of reticulocytes at different stages of maturation that can be resolved based on the RNA and CD71 expression levels.
Resolving the heterogeneity of human nascent reticulocytes. (A) Gating strategy using CD71 and SYTO16 as surface markers. (B) Four populations of reticulocytes (R1-R4) can be isolated and sorted based on the 2 surface markers. (C) Morphology of sorted reticulocyte populations by phase contrast microscopy (left panels), and immunofluorescence (right panels). (D) Transmission electron microscopy of a nascent reticulocyte highlighting numerous ruffles and organelles.
Resolving the heterogeneity of human nascent reticulocytes. (A) Gating strategy using CD71 and SYTO16 as surface markers. (B) Four populations of reticulocytes (R1-R4) can be isolated and sorted based on the 2 surface markers. (C) Morphology of sorted reticulocyte populations by phase contrast microscopy (left panels), and immunofluorescence (right panels). (D) Transmission electron microscopy of a nascent reticulocyte highlighting numerous ruffles and organelles.
Decreased surface expression of membrane protein expression during reticulocyte maturation
Next, we evaluated the surface expression of several membrane proteins known to decrease during reticulocyte maturation (Figure 2A). Indeed, it was previously reported that several membrane proteins, especially the transferrin receptor and several proteins involved in cell adhesion, present a decrease in their expression levels during the maturation of mature reticulocytes (R2 reticulocytes). As expected, the most dramatic decrease in cell surface expression was noted for transferrin receptor (CD71), with the highest level of expression in R1 reticulocytes, followed by gradual loss during maturation, leading to undetectable levels of expression in mature red blood cells. Marked reductions in cell surface expression were also noted for several membrane proteins involved in cell adhesion events, such as α4, α5, and β1-integrin, Lu, and CD36. More modest reductions were observed for proteins carrying blood group antigens, such as band 3, glycophorin A (GPA) and glycophorin C (GPC). RhD, RhAG, Kell, and Duffy were also noted during nascent reticulocyte maturation.
Decreased expression of surface markers and biophysical characteristics of nascent reticulocytes. (A) Flow cytometry of nascent reticulocytes (red histograms) vs mature red blood cells (gray histograms) for the different surface markers indicated. (B) Fluorescence-imaged micropipette deformation experiments performed on GPC (phycoerythrin-conjugated), CD59 (fluorescein isothiocyanate [FITC]-conjugated), CD44 (FITC-conjugated), and CD71 (FITC-conjugated). CD44 and CD71 were dual-stained with band 3 (phycoerythrin-conjugated).
Decreased expression of surface markers and biophysical characteristics of nascent reticulocytes. (A) Flow cytometry of nascent reticulocytes (red histograms) vs mature red blood cells (gray histograms) for the different surface markers indicated. (B) Fluorescence-imaged micropipette deformation experiments performed on GPC (phycoerythrin-conjugated), CD59 (fluorescein isothiocyanate [FITC]-conjugated), CD44 (FITC-conjugated), and CD71 (FITC-conjugated). CD44 and CD71 were dual-stained with band 3 (phycoerythrin-conjugated).
Prior research identified several mechanisms contributing to decreased membrane protein expression, including exosome release (CD71),20 endocytosis (GPA),21 and membrane fragmentation, leading to reduced membrane surface area.22,23 A common feature of these mechanisms involves the loss of linkage between membrane proteins and the underlying spectrin-based membrane skeleton, resulting in the loss of unlinked proteins. To investigate the linkage between membrane proteins and the spectrin-based membrane skeleton in nascent reticulocytes, we employed deformation-induced micropipette aspiration combined with fluorescent imaging of specifically labeled membrane proteins. This technique allowed us to assess the extent of protein anchoring to the membrane skeleton; as previously described,17 we used this experimental strategy to study this linkage in nascent reticulocytes. GPC is tightly linked to the membrane skeleton in R1 reticulocytes as in mature red blood cells,24 and did not partition with vesicle generated by deformation-induced membrane fragmentation (Figure 2B). In marked contrast, glycosylphosphatidylinositol-linked protein CD59, unlinked to the membrane skeleton, freely diffuses to regions of the membrane depleted of the skeletal components, and partitions with the vesicle generated. CD44 exhibits an intermediate profile with both skeletal linkage and a freely diffusible component, and is also associated with the vesicular compartment, while CD71 appears to be predominantly associated with the intracellular vesicular compartment (Figure 2B). Together, these data suggest a correlation between the extent of linkage and the decreased expression of surface proteins during reticulocyte maturation.
Analyses of sorted reticulocyte populations reveal major changes at the proteome level
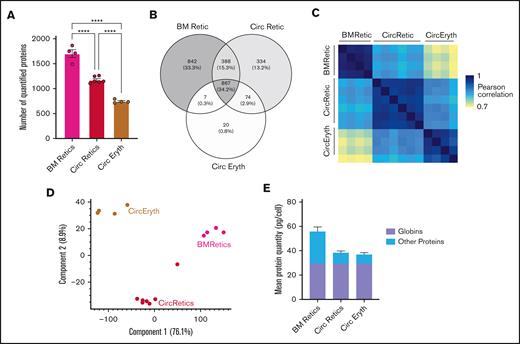
To further characterize nascent reticulocytes, we used an unbiased proteomic approach using mass spectrometry on sorted R1 reticulocyte cells from the bone marrow of 4 healthy donors (supplemental Figure 1A), and compared them with published proteomes of mature reticulocytes and erythrocytes.25 This led to the identification of 2541 proteins (supplemental Table 1). The mean number of expressed proteins changed dramatically from nascent reticulocytes to mature red blood cells (Figure 3A), with only 867 proteins (34.2%) shared across all 3 populations (Figure 3B). We calculated the copy number of each protein using a modified version of Perseus proteomic ruler plugin that allows to use the mean corpuscular hemoglobin as a reference for whole-cell lysates, and the mean corpuscular hemoglobin was set at 30 pg per cell hemoglobin. As quality control, Pearson correlations between samples of the same population were very high for the different biological replicates (mean of 0.97 for R1 reticulocytes, 0.95 for mature reticulocytes, and 0.94 for mature red blood cells; Figure 3C), demonstrating high reproducibility among samples of the same population. Pearson correlations between the 3 populations are lower, showing that the proteomes are more different between 2 populations than for samples inside the same population. Moreover, within the different ribosomal proteins, the mean copy numbers for the 3 populations were close together, ∼1 log2, as expected (supplemental Figure 1B). We then used the protein expression profile for principal component analysis (Figure 3D). We observed that the 3 populations separate on both the x- and y-axes, contrary to the erythroid progenitors and precursors, which separate specifically on the y- and x-axes, respectively.26,27 These data suggest that in addition to changes in total protein content, specific changes occur strictly depending on the extent of reticulocyte maturation. As quality control, correlations between each biological replicate for the 4 bone marrow donors remain high (supplemental Figure 1C). Based on copy numbers and molecular weights of proteins, we assessed the absolute protein content of the 3 populations of cells. There was a marked decrease in the total protein content between nascent reticulocytes and mature reticulocytes (from 56 to 39 pg per cell), while this decrease between mature reticulocytes and mature red blood cells was more modest (from 39 to 37 pg per cell; Figure 3E).
Comparison of nascent reticulocytes proteome (BMRetic) with Gautier et al25data of mature reticulocytes (CircRetic) and purified circulating erythrocytes (CircEryth). (A) Mean number of quantified proteins in each population. Statistics: 1-way analysis of variance; ∗∗∗∗P < 0001. (B) Venn diagram of quantified proteins in each population (proteins quantified in at least 1 sample). (C) Pearson correlations of log2(protein copy numbers per cell) between each sample. (D) Mean quantity (in picograms per cell) of globins and other proteins in each population. (E) Principal component analysis of log2(protein copy numbers per cell) of every sample (proteins with at least 70% of valid values in at least 1 condition were selected). BM, bone marrow.
Comparison of nascent reticulocytes proteome (BMRetic) with Gautier et al25data of mature reticulocytes (CircRetic) and purified circulating erythrocytes (CircEryth). (A) Mean number of quantified proteins in each population. Statistics: 1-way analysis of variance; ∗∗∗∗P < 0001. (B) Venn diagram of quantified proteins in each population (proteins quantified in at least 1 sample). (C) Pearson correlations of log2(protein copy numbers per cell) between each sample. (D) Mean quantity (in picograms per cell) of globins and other proteins in each population. (E) Principal component analysis of log2(protein copy numbers per cell) of every sample (proteins with at least 70% of valid values in at least 1 condition were selected). BM, bone marrow.
Retention of a specific set of ribosomal and mitochondrial proteins during reticulocyte maturation
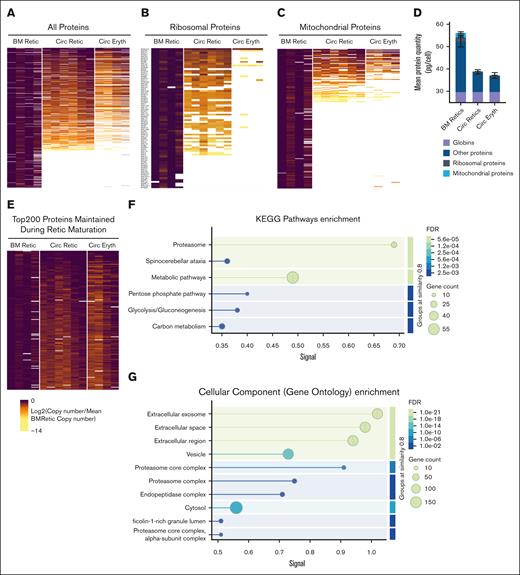
Having established that the total protein content is mostly decreased during the maturation of nascent reticulocytes from the bone marrow to the mature stage, we undertook a deeper analysis into the changes in global protein expression patterns. Overall, we observed that about one-third of the total number of proteins was lost by reticulocytes at the mature stage, with more granular changes between the mature stage and the mature red blood cells (Figure 4A, left panel). Further detailed analysis showed, as expected, a dramatic decrease in ribosomal protein expression during reticulocyte maturation, with a near-complete absence in mature red blood cells, except for RPS27A and a few other ribosomal proteins (Figure 4B). A similar pattern was noted for mitochondrial proteins, with the exception of residual mitochondrial proteins present in mature red blood cells (Figure 4C). Based on the same quantification method used in Figure 3, we assessed the absolute protein content of the different subclasses of proteins, both ribosomal and mitochondrial (Figure 4D). We observed that while the few ribosomal proteins remaining at the red blood cell stage did not account for a significant amount of the total amount of proteins per cell, the residual presence of 48 mitochondrial proteins in the mature red blood cells still accounted for 0.1 pg per cell. Based on these data, we compiled the top 200 proteins maintained during reticulocyte maturation, which included globins (supplemental Figure 2), and both membrane and other cytosolic proteins. These proteins could be classified into membrane proteins, cytoskeletal proteins, adhesion molecules, kinases, phosphatases, and proteins belonging to the proteasome pathway (Figure 4E; supplemental Table 2). An over-representation analysis performed on these 200 maintained proteins using String db (version 12.0) showed an enrichment of proteasomal proteins and proteins involved in metabolic pathways, including the pentose phosphate pathway, glycolysis, and carbon metabolism, as well as proteins located in exosomes or involved in vesicular trafficking (Figure 4F-G).
Heat map representations of protein copy numbers normalized by mean copy number of nascent reticulocytes during reticulocyte maturation. (A) All proteins. (B) Ribosomal proteins. (C) Mitochondrial proteins. (D) Mean protein quantity (in picograms per cell) of ribosomal and mitochondrial proteins, with globins and other proteins during reticulocyte maturation. (E) Top 200 proteins the most maintained during reticulocyte maturation. (F-G) String db graphical representation of pathways (F) and cellular components (G) enriched in the top 200 proteins maintained during maturation. BM, bone marrow.
Heat map representations of protein copy numbers normalized by mean copy number of nascent reticulocytes during reticulocyte maturation. (A) All proteins. (B) Ribosomal proteins. (C) Mitochondrial proteins. (D) Mean protein quantity (in picograms per cell) of ribosomal and mitochondrial proteins, with globins and other proteins during reticulocyte maturation. (E) Top 200 proteins the most maintained during reticulocyte maturation. (F-G) String db graphical representation of pathways (F) and cellular components (G) enriched in the top 200 proteins maintained during maturation. BM, bone marrow.
Detailed composition of the human reticulocyte proteome
Having shown the overall changes in the proteome during reticulocyte maturation, we undertook a comprehensive analysis of the different subclasses (Figure 5).
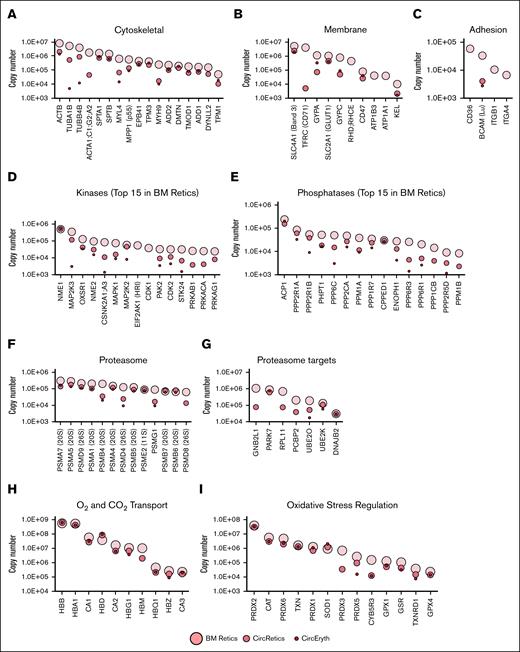
Mean protein copy numbers in nascent reticulocytes (BM Retics), mature reticulocytes (CircRetics), and circulating erythrocytes (CircEryth) (logarithmic scale). Only means quantified with at least 3 values are shown. (A) Cytoskeletal proteins. (B) Membrane proteins. (C) Cell adhesion proteins. (D-G) Top 15 in nascent reticulocytes of protein kinases (D) and protein phosphatases (E) and examples of proteins associated to the GOCC “proteasome complex” (F) and GOBP “proteasome-mediated ubiquitin-dependent protein catabolic process” (G). (H) Examples of proteins associated to O2 and CO2 transport, and (I) oxidative stress regulation. BM, bone marrow.
Mean protein copy numbers in nascent reticulocytes (BM Retics), mature reticulocytes (CircRetics), and circulating erythrocytes (CircEryth) (logarithmic scale). Only means quantified with at least 3 values are shown. (A) Cytoskeletal proteins. (B) Membrane proteins. (C) Cell adhesion proteins. (D-G) Top 15 in nascent reticulocytes of protein kinases (D) and protein phosphatases (E) and examples of proteins associated to the GOCC “proteasome complex” (F) and GOBP “proteasome-mediated ubiquitin-dependent protein catabolic process” (G). (H) Examples of proteins associated to O2 and CO2 transport, and (I) oxidative stress regulation. BM, bone marrow.
In terms of cytoskeletal proteins (Figure 5A), β-actin (ACTB) was highly expressed in nascent reticulocytes, and decreased 10-fold in mature reticulocytes without further changes in expression during their maturation into red blood cells. Tubulins A1B and B4B were also highly expressed in nascent reticulocytes, and decreased >100-fold to 1000-fold during maturation to red blood cells. One million copies of myosin light chain 4 (MYL4) were present in nascent reticulocytes, and decreased by 100-fold during maturation to red blood cells, while heavy chain of nonmuscle myosin IIa (MYH9) also decreased over 50-fold during reticulocyte maturation. Dynein light chain LC8-type 2 (DYNLL2), expressed in nascent reticulocytes, was absent from mature red blood cells. In contrast, expression levels of the red blood cell membrane skeletal proteins a- and b-spectrins, protein 4.1, tropomodulin, adducins, and dematin changed slightly during reticulocyte maturation, while there was a small decrease in tropomyosin.
In terms of membrane proteins (Figure 5B), SCL4A1 (band 3) was the highest expressed membrane protein in nascent reticulocytes, and there was a small decrease during maturation into red blood cells. In marked contrast, transferrin receptor (CD71), the second highest expressed membrane protein in nascent reticulocytes, was rapidly lost during maturation into mature reticulocytes, and was undetectable in red blood cells. Similarly, subunits of the Na+/K+ adenosine triphosphatase (ATP1B3 and ATP1A1) were expressed in nascent reticulocytes, but were below detectable levels on mature reticulocytes and red blood cells. SLC2A1 (GLUT1) was highly expressed in nascent reticulocytes, with a slight decrease during maturation of the R1 reticulocytes, and levels remaining constant for the rest of the maturation. Other membrane proteins, GPA, GPC, and CD47, showed a peculiar pattern during maturation from R1 reticulocytes to red blood cells. Indeed, while their expression decreased from nascent reticulocytes to mature reticulocytes, the expression increased from mature reticulocytes to red blood cells. Among the blood group antigens, RHD;RHCE was quantified in only 2 samples, but appeared to be decreased during maturation from R1 reticulocytes to mature reticulocytes. In contrast, a fivefold decrease in Kell was noted from nascent reticulocytes to mature reticulocytes, but its expression then remained unchanged.
The proteins involved in cell adhesion were the ones presenting the most dramatic changes in their expression during reticulocyte maturation (Figure 5C). CD36, α4- and β1-integrins (ITGA4 and ITGB1) were highly expressed in nascent reticulocytes, but were undetectable in both mature reticulocytes and red blood cells. BCAM (Lu) is one of the rare adhesion proteins that remained expressed in red blood cells.
Among the protein kinases expressed in nascent reticulocytes, the one with the highest level of expression (500 000 copies) is the histidine kinase NME/NM23 nucleoside diphosphate kinase 1 (NME1; Figure 5D). The expression level of this kinase remained unchanged during reticulocyte maturation into red blood cells. The second most expressed protein kinase in nascent reticulocytes was the MEK3 (MAP2K3) kinase, the activating kinase of p38 MAPK, responsible for responding to osmotic shock,28 and oxidative stress in erythrocytes in hemolytic diseases.29 MEK3 dramatically decreased during reticulocyte differentiation, but remained quantifiable in mature erythrocytes. Other kinases, such as OXSR1, NME2, CSKN2A1;A3, and MAPK1 presented a similar pattern of decrease during differentiation, while remaining expressed at the red blood cell stage. Despite reticulocytes lacking a nucleus, the cyclin-dependent kinases, CDK1 and CDK2, were quantified at several 10 000 copies in nascent reticulocytes, and while CDK1 was totally lost at the mature reticulocyte stage, several thousands of copies of CDK2 remained at the red blood cell stage.
Protein phosphatases were also detected in nascent reticulocytes (Figure 5E). The most highly expressed protein phosphatase in nascent reticulocytes was the ACP1 phosphotyrosine phosphatase, also known as the red blood cell acid phosphatase 1 that has been shown to dephosphorylate band 3.30 PHPT1, 1 of the 3 known histidine phosphatases in mammals, and responsible for dephosphorylating proteins phosphorylated by NME1,31 was one of the most highly abundant protein phosphatases expressed in reticulocytes. Other phosphatases followed a pattern of decreased expression during maturation, but remained expressed at the red blood cell stage, except for PPM1B, involved in dephosphorylating CDK2 and CDK6 among others.32 The calcineurin-like phosphoesterase domain-containing 1 serine/threonine-protein phosphatase (CPPED1) remained highly expressed throughout maturation. Interestingly, very little is known about its activity apart from its ability to dephosphorylate AKT and PAK4,33,34 and its function in red blood cells is currently unknown.
With regard to the proteasome signaling pathway, we observed that the stoichiometry of proteasome in the nascent reticulocytes was different from that from mature erythrocytes, with many of the proteins of the 20S subunit remaining unchanged during maturation, while those of the 26S subunit showing markedly decreased expression (Figure 5F). While the proteasome target proteins PARK7, UBE2K, and DNAJB2 were unchanged during reticulocyte maturation, no detectable expression of GNB2l1, RPL11, and PCBP2 was noted in red blood cells (Figure 5G).
Finally, expression of proteins involved in hemoglobin-mediated O2 and CO2 transport, and proteins that mitigate the redox effect remained unchanged during maturation (Figure 5H-I).
Discussion
Reticulocyte maturation is the last step of erythropoiesis and involves loss of cell motility, extensive membrane remodeling with significant changes in protein composition resulting in morphological changes, surface area loss, decreased cell volume, and increased membrane mechanical stability.
In contrast to significant advances made during the last few decades in our understanding of the structural basis for the function of the mature red blood cell, there are continued significant gaps in our understanding of reticulocyte biology. The present study represents an effort to begin to fill this gap by exploring quantitative changes in the proteome during maturation of nascent reticulocytes into mature red blood cells using mass spectrometry–based quantitative proteomics in conjunction with biophysical approaches. Indeed, very few studies used proteomics to study reticulocyte maturation, and none of those used nascent reticulocytes from the human bone marrow.25,35 Indeed, previous studies used cellulose columns to isolate pure populations of mature red blood cells, and have shown that use of these columns not only removes white blood cells, but also removes nucleated red blood cells and populations of nascent reticulocytes.21,25,36,37 Because the flow-through fractions of these filters contain primarily mature reticulocytes, we used Percoll gradients to enrich for and select nascent R1 reticulocytes, while at the same time reducing the total number of cells for FACS using SYTO16 and CD71. We validated our isolation technique with purity of isolated R1 reticulocytes reaching 99%, as shown in Figure 1A, and morphology of the cells exhibiting a multilobular shape characteristic of R1 reticulocytes, as shown in Figure 1B.
We quantified the expression levels of 2104 proteins in the R1 population compared with 968 in mature red blood cells. Comparison of R1 proteome with that of mature reticulocytes and erythrocytes showed a marked decrease in the total protein content in conjunction with the disappearance of mitochondrial and ribosomal proteins. Further analyses demonstrated that most of the proteomic changes occur between the R1 and mature reticulocyte stage. This massive and selective loss of proteins occurs in <48 hours of reticulocyte maturation in the bone marrow, and is a unique feature of erythroid biology following nuclear extrusion. It needs to be emphasized that ATP-dependent protein degradation was first documented in reticulocytes, and subsequent studies defined the role of the proteasome in the degradation of ubiquitinated proteins.38 In our proteomic analysis we identified many specific ubiquitinating enzymes, including those extensively characterized by Finley et al.39 We anticipate that the comprehensive list of enzymes we identified will enable future work on deciphering the specific role of these enzymes in degradation of different classes of proteins during reticulocyte maturation.
With regard to the globin chains, we did not observe an increase in globin expression from the R1 reticulocyte to the mature reticulocyte stage. While we observed expression of ribosomal proteins, and an increase in globin synthesis has been reported during reticulocyte maturation in rabbits in vitro,40,41 responsible for increased hemoglobin content from the reticulocyte to the red blood cell stage, this was not documented in humans,42,43 suggesting species differences.
Cytoskeletal proteins such as tubulins, actins, myosins, and dynein identified in nascent reticulocytes are likely to play a key role in reticulocyte motility, and they are entirely lost or markedly decreased in red blood cells. Similarly, proteins involved in cell adhesion are entirely lost during reticulocyte maturation, thereby eliminating the ability of mature red blood cells to participate in cell-cell interactions that might impair their circulation and compromise their integrity.
The present study also enabled the identification of many kinases and phosphatases. Differences in phosphorylation of numerous proteins have been observed in the maturation of reticulocytes to mature red blood cells induced by sheer stress and deformation in the circulation.44 Most of skeletal and membrane proteins are phosphorylated and subsequently dephosphorylated, generating great interest in defining the role of kinases and phosphatases in erythroid biology. For example, NME1, also known as NME/NM23 nucleoside diphosphate kinase 1, can form heterohexameric complexes with the histidine kinase NME2 that is the fourth most expressed kinase in nascent reticulocytes. While the double knockout of these 2 known histidine kinases in mammals induces a perinatal death associated with anemia, suggesting a role for these kinases in erythropoiesis,45 the function of these 2 histidine kinases in red blood cells is currently unknown. Interestingly, among the small intermediate potassium channels, KCa3.1, known as the Gardos channel in erythrocytes, absolutely requires histidine phosphorylation by nucleoside diphosphate kinase B for function in T lymphocytes.46 It appears likely that this is required for channel function in erythrocytes.
Similarly, the presence of CDK1/2, despite the fact that no cyclins could be quantified, suggests that CDK1 and CDK2 could have another role besides their well-known function in promoting cell cycle progression, such as in myoblasts where they phosphorylate MyoD on serine 200, and modulate its half-life and myogenic activity.47 Further studies, using phosphoproteomic approaches such as the one used in an in vitro culture system,48 will be needed to address these questions.
One limitation to consider in the context of this study lies in the fact that mass spectrometry–applied proteomics is not exhaustive in the identification of the proteins within a given sample. Consequently, one must consider that a protein can be present in a sample without being quantified by our approach.
Despite significant efforts, the field still lacks significant biological and functional insights. What has been established by in vitro biochemical studies is that phosphorylation of the red blood cell skeletal proteins as well as membrane proteins reduce the binding affinity to their binding partners.49,50 It should also be noted that, to date, only the role of a subclass of classical kinases such as protein kinase A, cyclic adenosine monophosphate-dependent kinase and the protein kinase C family, has been studied in regulating red blood cell function. Some previous studies have shown that some of the skeletal proteins are hyper-phosphorylated in reticulocytes, and this could in part account for their decreased membrane mechanical stability. It will be important for future studies to define the role of the large number of kinases and phosphatases identified in the present study, in regulating the function of reticulocytes and red blood cells.
The significant remodeling of the proteome during reticulocyte maturation and selective loss of specific proteins is impressive, and continued exploration of the mechanistic basis for this remodeling is likely to provide significant new biological insights. We anticipate the findings from the present study have the potential to contribute to furthering our understanding of the reticulocyte biology, and into the potential contribution of defective reticulocyte maturation to the pathophysiology of parasite invasion (eg, Plasmodium vivax) and various red blood cells disorders, including sickle cell disease.
Acknowledgments
The authors thank John Hale for his efforts with the micropipette aspiration experiments.
This work was supported, in part, by the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health grant DK32094 (P.G.G. and N.M.) and National Heart, Lung, and Blood Institute, National Institutes of Health grant HL171168 (L.B.), and by the France 2030 program through the Idex Université Paris Cité (ANR-18-IDEX-001).
Authorship
Contribution: M.L., J.P., H.Y., E.-F.G., N.M., and L.B. designed the research; J.P., H.Y., and M.L. performed the experiments; M.L., M.L.G., and E.-F.G. analyzed the proteomics data; J.P., H.Y., M.L., M.L.G., W.E.N., C.H., P.G.G., E.-F.G., N.M., and L.B. analyzed and interpreted the data; N.M. and L.B. cowrote the manuscript; M.L., J.P., H.Y., W.E.N., C.H., P.G.G., and E.-F.G. edited the manuscript; and all authors read and commented on the final manuscript.
Patrick G. Gallagher is the Editor-in-Chief of Blood Red Cells & Iron. As an author of this article, he was recused by journal policy from any involvement in its peer review, and he had no access to information regarding its peer review process.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Lionel Blanc, Northwell, 2000 Marcus Ave, Suite 300, New Hyde Park, NY 11042-1069; email: lblanc@northwell.edu.
References
Author notes
M.L., J.P., and H.Y. contributed equally to this work.
E.F.G., N.M., and L.B. are joint senior authors.
Mass spectrometry proteomics data have been deposited with the ProteomeXchange Consortium via the PRIDE19 partner repository (data set identifier PXD062723, reviewer_pxd062723@ebi.ac.uk).
Other data and reagents are available from the corresponding author, Lionel Blanc (lblanc@northwell.edu), on request.
The full-text version of this article contains a data supplement.

![Decreased expression of surface markers and biophysical characteristics of nascent reticulocytes. (A) Flow cytometry of nascent reticulocytes (red histograms) vs mature red blood cells (gray histograms) for the different surface markers indicated. (B) Fluorescence-imaged micropipette deformation experiments performed on GPC (phycoerythrin-conjugated), CD59 (fluorescein isothiocyanate [FITC]-conjugated), CD44 (FITC-conjugated), and CD71 (FITC-conjugated). CD44 and CD71 were dual-stained with band 3 (phycoerythrin-conjugated).](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodrci/1/2/10.1016_j.brci.2025.100012/4/m_brci_rci-2025-000156-gr2.jpeg?Expires=1767724673&Signature=4GiC7gnBCgXa7AqQmKJoHRjqM8lj0Lun396XP5qVzIMSjc9iB0lhUFDkKmZrefXo0-OSENbJvIKFmrIccl4YJgHWMTR80P1m-8WTPtF4wGQBjDW~oaFA50dOBDXXqpKJo170hEKZYHiTZ3-GDKyMMJgZ7HqaUXTxMitk50dHqWXmXGqmbHjnFzVxormNkJGCSCGAXMMS1ovT5XJle05~Thol3ndtSA2cBsSlOEL0a0z58URphczqz3roQmA5ZvbmPspUeWpgwDt~TICGmDo95uWuI6eJkns7kd7FA4NbGO2zNLyf03GtdZeE5iJGPscYFfjUEFFarWBN5vrwzSLoog__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)