TO THE EDITOR:
In sickle cell disease (SCD), chronic intravascular hemolysis causes release of excessive levels of cell-free heme and iron, resulting in increased production of reactive oxygen species (ROS).1 The hemolysis-driven increased oxidative stress contributes to inflammation, increased neutrophil activation, adhesion and aging, endothelial activation, and hypercoagulability.1-3 Furthermore, heme induces toll-like receptor-4 signaling as an erythrocytic danger-associated molecular pattern, activating the immune system and endothelial cells.4 These downstream processes have been shown to contribute to vaso-occlusion.3,5,6
Voxelotor is a first-in-class sickle hemoglobin (HbS) polymerization inhibitor. Voxelotor reversibly binds to HbS, shifting it from the T-state to the R-state, thereby increasing its oxygen binding, and resulting in decreased HbS polymerization and hemolysis.7
The primary aim of this study was to evaluate whether voxelotor-induced reduction in hemolysis results in changes in phenotype (markers of activation, adhesion, and aging) and oxidative activity of neutrophils. Secondary aims were to determine the effects of voxelotor on erythrocyte deformability, and markers of endothelial and coagulation activation.
Patients with SCD with the HbSS and HbSβ0-thalassemia genotypes, with hemoglobin (Hb) levels ≤10.5 g/dL were eligible for inclusion in this phase 2 open-label single-arm study. Participants were treated with voxelotor 1500 mg once daily. Blood samples were collected at baseline and after 3 months of treatment. This study was approved by the institutional review board of Amsterdam UMC, was registered in the EudraCT database (2019-003766-41), and was performed in accordance with the Declaration of Helsinki 2013. Ethnicity-matched volunteers were included as healthy controls for neutrophil counts, and markers of endothelial activation and coagulation.
Erythrocyte assays were performed within 24 hours after sampling of venous blood in EDTA-coated vacutainers stored at 4°C.8 Erythrocyte deformability was assessed as previously described.8 Erythrocyte deformability is interpreted with the maximum elongation index (EImax) and minimum elongation index (EImin) at normal (47 mm Hg) and low (10 mm Hg) oxygen pressure, respectively, and the point of sickling, the oxygen tension at which 5% reduction of the EImax is observed. Measurements were performed with a laser optical rotational red cell analyzer (Lorrca; RR Mechatronics, Zwaag, The Netherlands). Neutrophil assays were performed within 24 hours of collection in EDTA blood samples stored at room temperature. Neutrophil surface antigen expression was measured, using flow cytometry, as described previously.9-11 Flow cytometry data were measured using a Canto II flow cytometer (BD Biosciences), and analyzed with FacsDiva software (version 9). Results were depicted as mean fluorescent intensity. Neutrophil markers were divided in subgroups of activation, adhesion, and maturation markers, based on previous reports.12 For the assessment of neutrophil function, oxidative activity and burst capacity were measured.9 Von Willebrand factor antigen (VWF:Ag) was measured in citrated plasma (Dako, Glostrup, Denmark). Serum soluble vascular cell adhesion molecule 1 (VCAM-1) levels (R&D Systems, Minneapolis, MN), plasma prothrombin fragment 1+2 (F1+2; Enzygnost; Dade Behring, Marburg, Germany), D-dimer (Assera-chrom D-Di; Roche), and thrombin-antithrombin complex (Enzygnost) levels were measured using an enzyme-linked immunosorbent assay.
Data were analyzed using SPSS (version 26) and GraphPad Prism (version 9). Data are presented as medians with interquartile range. Paired data were analyzed using paired t tests and Wilcoxon-signed rank tests. Post hoc analyses were performed with Bonferroni correction for multiple testing. P value < .05 was considered significant.
Out of the 17 included participants, 15 patients (11 with HbSS, 4 with HbSβ0-thalassemia, median age 35 years [interquartile range, 27-46], 10 female) completed the 3-month follow-up period (supplemental Table 1). No vaso-occlusive episodes were reported between at least 2 weeks prior to the baseline and 3 months visit.
After voxelotor treatment, Hb levels increased from 7.4 g/dL (6.9-8.1 g/dL) to 9.7 g/dL (7.9-11 g/dL), P = .002, whereas reticulocyte counts, lactate dehydrogenase, and total bilirubin levels showed significant decreases (Table 1). In addition, point of sickling decreased significantly (from 20.1 mm Hg [17.5-26.1 mm Hg] to 16.1 mm Hg [10.3-18.1 mm Hg], P = .006), corresponding to significant increases in values of EImax (from 0.33 [0.24-0.42] to 0.38 [0.28-0.48], P = .029) and EImin (from 0.13 [0.07-0.26] to 0.21 [0.16-0.41], P = .007), potentially leading to improved rheological properties of sickle erythrocytes. These findings confirm earlier observations.13 Fetal hemoglobin (HbF) percentages decreased following voxelotor treatment (Table 1). This could be due to a longer life span of the erythrocytes treated with voxelotor, also those containing lower fractions of HbF, resulting in a net increase in HbS%.14
Effect of voxelotor on Hb levels and markers of hemolysis and erythrocyte deformability (n = 15)
| . | Baseline . | Follow-up at 3 months . | P value . |
|---|---|---|---|
| Hemoglobin, g/dL | 7.4 (6.9-8.1) | 9.7 (7.9-11) | .002 |
| Reticulocyte count, 109 per L | 289 (230-331) | 185 (126-257) | .006 |
| Total bilirubin, μmol/L | 59 (38-113) | 37 (19-61) | .001 |
| LDH, U/L | 504 (365-678) | 318 (272-590) | .003 |
| HbF, % | 6.8 (3.1-8.3) | 3.3 (1.0-6.3) | <.001 |
| Point of sickling, mm Hg | 20.1 (17.5-26.1) | 16.1 (10.3-18.1) | .006 |
| EImax | 0.33 (0.24-0.42) | 0.38 (0.28-0.48) | .029 |
| EImin | 0.13 (0.07-0.26) | 0.21 (0.16-0.41) | .007 |
| . | Baseline . | Follow-up at 3 months . | P value . |
|---|---|---|---|
| Hemoglobin, g/dL | 7.4 (6.9-8.1) | 9.7 (7.9-11) | .002 |
| Reticulocyte count, 109 per L | 289 (230-331) | 185 (126-257) | .006 |
| Total bilirubin, μmol/L | 59 (38-113) | 37 (19-61) | .001 |
| LDH, U/L | 504 (365-678) | 318 (272-590) | .003 |
| HbF, % | 6.8 (3.1-8.3) | 3.3 (1.0-6.3) | <.001 |
| Point of sickling, mm Hg | 20.1 (17.5-26.1) | 16.1 (10.3-18.1) | .006 |
| EImax | 0.33 (0.24-0.42) | 0.38 (0.28-0.48) | .029 |
| EImin | 0.13 (0.07-0.26) | 0.21 (0.16-0.41) | .007 |
Data are median (interquartile range).
LDH, lactate dehydrogenase.
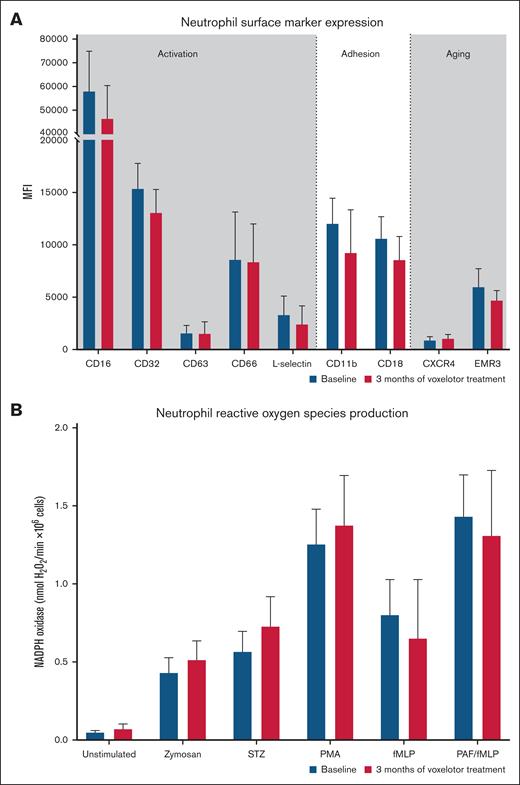
Neutrophil counts were significantly higher in patients with SCD at baseline as compared with matched healthy controls, and seemed to decrease after voxelotor treatment, although the difference was not statistically significant (Table 2). Markers of neutrophil activation, adhesion, and aging did not change significantly (Figure 1A). Neutrophil ROS production remained unchanged, both at steady state (unstimulated) and under stimulated conditions (Figure 1B). Changes in neutrophil phenotype and ROS production did not correlate with changes in Hb or markers of hemolysis following voxelotor treatment (data not shown). Also, when divided into responders (n = 11) vs nonresponders (n = 4), with responder being defined by an increase of >1 g/dL in Hb level, changes in markers of neutrophil phenotype and ROS production did not differ between the responders and nonresponders.
Effect of voxelotor on neutrophil counts, markers of endothelial activation, and coagulation
| . | Matched controls (n = 19) . | Patients with SCD (n = 15) . | P value∗ . | |
|---|---|---|---|---|
| Baseline . | Follow-up at 3 mo . | |||
| Neutrophils, 109/L | 2.9 (2.6-4.8) | 4.6 (3.5-5.7)† | 3.5 (2.5-5.0) | .057 |
| VWF-Ag, % | 112 (77-143) | 270 (214-257)† | 178 (140-219) | .005 |
| F1+2, pmol/L | 130 (104-209) | 365 (238-590)† | 279 (210-360) | .510 |
| D-dimer, mg/L FEU | 0.33 (0.21-0.55) | 2.4 (1.1-3.8)† | 1.5 (0.8-4.4) | .683 |
| sVCAM-1, pg/mL | N.A. | 1179 (887-1650) | 975 (662-1510) | .221 |
| TAT, μg/L | N.A. | 5.2 (3.6-8.3) | 4.3 (3.0-6.4) | .638 |
| . | Matched controls (n = 19) . | Patients with SCD (n = 15) . | P value∗ . | |
|---|---|---|---|---|
| Baseline . | Follow-up at 3 mo . | |||
| Neutrophils, 109/L | 2.9 (2.6-4.8) | 4.6 (3.5-5.7)† | 3.5 (2.5-5.0) | .057 |
| VWF-Ag, % | 112 (77-143) | 270 (214-257)† | 178 (140-219) | .005 |
| F1+2, pmol/L | 130 (104-209) | 365 (238-590)† | 279 (210-360) | .510 |
| D-dimer, mg/L FEU | 0.33 (0.21-0.55) | 2.4 (1.1-3.8)† | 1.5 (0.8-4.4) | .683 |
| sVCAM-1, pg/mL | N.A. | 1179 (887-1650) | 975 (662-1510) | .221 |
| TAT, μg/L | N.A. | 5.2 (3.6-8.3) | 4.3 (3.0-6.4) | .638 |
FEU, fibrinogen equivalent units; N.A., not available; sVCAM-1, soluble vascular cell adhesion molecule 1; TAT, thrombin-antithrombin complexes.
Data are medians, with interquartile ranges in parentheses.
P value for difference between baseline and 3-months follow-up within the treated patients.
Significantly different (P < .001) compared with ethnicity-matched healthy controls.
Effect of voxelotor on markers of neutrophil activation, adhesion and aging, and ROS production. (A) Neutrophil markers of activation, adhesion, aging at baseline, and after 3 months of voxelotor treatment. (B) Neutrophil ROS production as measured by Amplex red assay at steady state and when using zymosan, serum-treated zymosan (STZ), phorbol myristate acetate (PMA), N-formylmethionyl leucyl phenylalanine (fMLP), and platelet-activating factor (PAF)–fMLP. Data are presented as medians, with error bars indicating interquartile ranges. NADPH, reduced NAD phosphate.
Effect of voxelotor on markers of neutrophil activation, adhesion and aging, and ROS production. (A) Neutrophil markers of activation, adhesion, aging at baseline, and after 3 months of voxelotor treatment. (B) Neutrophil ROS production as measured by Amplex red assay at steady state and when using zymosan, serum-treated zymosan (STZ), phorbol myristate acetate (PMA), N-formylmethionyl leucyl phenylalanine (fMLP), and platelet-activating factor (PAF)–fMLP. Data are presented as medians, with error bars indicating interquartile ranges. NADPH, reduced NAD phosphate.
Levels of VWF:Ag, F1+2, and D-dimer were significantly higher in patients with SCD at baseline as compared with matched healthy controls (Table 2). Significant reduction was observed in levels of VWF:Ag, as a marker of endothelial activity, after voxelotor treatment as compared with baseline (from 270% [214%-257%] to 178% [140%-219%], P = .005), whereas no changes were observed in levels of sVCAM-1, F1+2, D-dimer, and thrombin-antithrombin complexes (Table 2).
Although voxelotor has recently been retracted from the market, clinical trials with osivelotor, a drug with a similar mechanism of action, are currently recruiting. Therefore, the results of this study may contribute to a greater insight in the effect of this class of polymerization inhibitors on erythrocyte rheology, neutrophil phenotype, and endothelial activation.
Despite the improved erythrocyte deformability, no significant improvements in neutrophil proinflammatory phenotype and oxidative activity were observed. Although hemolysis is postulated to cause neutrophil activation, adhesion, and aging, the decrease in hemolysis due to voxelotor might not be significant enough to cause alterations in neutrophil phenotype and function. Alternatively, elevated Hb levels might increase blood viscosity, potentially resulting in a vaso-occlusive phenotype, which in turn can cause ischemia-reperfusion injury. Oxidative stress due to ischemia-reperfusion injury might abrogate the effects of reduced hemolysis in patients treated with voxelotor.15 We recently observed that some markers of inflammation (L-18, NCR1, CD4, CD40, PD-L1, GZMH, LGALS1, and TRAIL) as part of a large inflammatory protein panel decreased in patients treated with voxelotor (n = 10).16 Although these decreases were not statistically significant after correction for multiple testing, these results suggest an ameliorating effect of antihemolysis interventions on the proinflammatory condition in SCD. Plasma levels of VWF:Ag decreased significantly following voxelotor treatment, probably reflecting decreased endothelial activation as a result of reduced hemolysis. Increased endothelial activation as reflected by elevated levels of VWF:Ag has been previously shown to be associated with the degree of hemolysis in SCD.17 Due to their proximity to the site of intravascular hemolysis, endothelial cells endure the bulk of oxidative damage induced by cell-free heme.5 However, in contrast to the decreased VWF:Ag levels, plasma levels of sVCAM-1 and markers of coagulation did not decrease after voxelotor treatment. The sample size might be too small to observe changes in these parameters with relatively large variability. However, as endothelial activation and hypercoagulation in SCD are mediated by several pathways, including ischemia-reperfusion and chronic inflammation, the degree of reduction in hemolysis as a result of voxelotor might not be sufficient for these abnormalities to be ameliorated.
This study is limited by its relatively small study population of 15 patients. Furthermore, variation in neutrophil antigen expression between the patients is large, rendering it difficult to detect significant changes.
In conclusion, although voxelotor significantly improved erythrocyte deformability and decreased hemolysis, no significant improvements were seen in neutrophil proinflammatory phenotype and activity. Decreases in plasma levels of VWF:Ag after voxelotor treatment might reflect reduced endothelial activation due to reduction in hemolysis as a result of voxelotor treatment.
Acknowledgments: The authors extend their gratitude to all the patients who participated in the study.
This study was funded by Global Blood Therapeutics/Pfizer.
Contribution: K.K. performed data acquisition, analysis, and interpretation, and drafted the manuscript; A.E.G. performed data acquisition, analysis, and interpretation, and critically reviewed the manuscript; B.M.B., M.V., and R.v.Z. performed the data acquisition and analysis, and critically reviewed the manuscript; R.v.B. interpreted the data, and critically reviewed the manuscript; B.J.B. recruited study participants, performed data interpretation, and critically reviewed the manuscript; and E.N. studied conception and design, recruited study participants, performed data analysis and interpretation, and drafted the manuscript.
Conflict-of-interest disclosure: E.N. has received research funding from Novartis, and serves on a speakers bureau for Vertex Pharmaceuticals. B.J.B. serves on advisory boards for CSL Behring, Novo Nordisk, Celgene/Bristol Myers Squibb (BMS), Pfizer and Novartis; and has received research funding from Novartis, BMS, Global Blood Therapeutics/Pfizer, and Sanquin. The remaining authors declare no competing financial interests.
Correspondence: Erfan Nur, Amsterdam UMC, University of Amsterdam, De Boelelaan 1117, 1081 HV Amsterdam, The Netherlands; email: e.nur@amsterdamumc.nl.
References
Author notes
K.K. and A.E.G. contributed equally to this study.
Data are available from the corresponding author, Erfan Nur (e.nur@amsterdamumc.nl), on request.
The full-text version of this article contains a data supplement.