Key Points
Patients with iTTP exhibit various degrees of renal insufficiency.
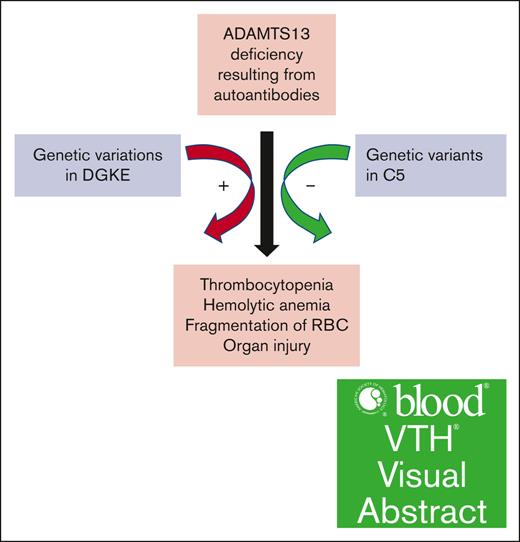
We found that genetic variations in C5 and DGKE modify the renal presentation in iTTP.
Visual Abstract
Immune thrombotic thrombocytopenic purpura (iTTP) is a potentially fatal blood disorder, resulting from autoantibodies against ADAMTS13. However, the contribution of genetic variations that may modulate its clinical presentations remains unknown. This study aimed to determine the potential contribution of variants in the genes associated with coagulation, complement activation or regulation, and platelet activation to pathophysiology of iTTP. Multicenter case series, whole-exome sequencing, and bioinformatic approaches were used. We focused on analysis of 20 genes that are involved in regulation of coagulation (eg, ADAMTS13, THBD, MMACHC, INF2, and PLG), complement activation (eg, C3, C3AR1, C5, CFB, CFH, CFI, C4BPA, CD46 [MCP], CD59, and CFHR1-CFHR5), and platelet activation (eg, DGKE) from 40 adult patients with iTTP. Multiple genetic variations were identified in 12 of 20 genes of interest. More than 80% of patients harbored genetic variants in CFI, CFH, C5, and ADAMTS13; 15% to 55% of patients had variants in C3, INF2, CFHR5, and PLG; and <10% of patients had variants in CD46, C3AR1, DGKE, and THBD. Of these, the variants in C5 are associated with a more favorable renal function, whereas the variants in DGKE are associated with more persistently elevated creatinine levels. These results demonstrate that variants in the genes involved in coagulation, complement, and platelet activation are common in patients with iTTP, which may contribute to phenotypical modulations of or predispose to iTTP resulting from severe ADAMTS13 deficiency.
Introduction
Thrombotic thrombocytopenic purpura (TTP) is the prototype of a potentially fatal thrombotic microangiopathy (TMA). The disease is primarily caused by 2 different mechanisms: (1) immune-mediated TTP (iTTP), resulting from acquired autoantibody-mediated inhibition of plasma ADAMTS13 activity1,2; and (2) congenital or hereditary TTP, resulting from mutations of ADAMTS13, leading to congenital deficiency of plasma ADAMTS13 activity.3-6 However, another prototype of TMA, known as atypical hemolytic uremic syndrome (aHUS), may be caused by other mechanisms, such as overwhelming activation of complement resulting from gain-of-function mutations in complement factor B (CFB)7 and complement 3 (C3),8 or loss-of-function mutations in the complement regulatory genes, including complement factor H (CFH),9-11 complement factor I (CFI),12 membrane cofactor protein (MCP),13,14 thrombomodulin (THBD),15 and platelet diacylglycerol kinase epsilon (DGKE),16 etc. TTP can be differentiated from aHUS based on clinical assessment scores such as the French score17 or PLASMIC score,18 plus other laboratory results including plasma ADAMTS13 activity and inhibitors. The degree of thrombocytopenia and renal dysfunction are the key parameters for differentiating iTTP from aHUS. For instance, patients with iTTP usually present with more severe thrombocytopenia (eg, platelet count <30 × 109/L) but less severe renal insufficiency (eg, serum creatinine <2.0 mg/dL).19 However, renal functions may vary from being normal to severely impaired among patients with iTTP on admission,20-22 and such a variability of renal dysfunction in patients with acute iTTP is not fully understood.
We hypothesize that genetic variants in genes associated with complement, coagulation, and platelets may contribute to the pathogenesis of iTTP when plasma ADAMTS13 activity is severely deficient. Studies have demonstrated that a lack of plasma ADAMTS13 activity alone is not sufficient to cause TTP in human23 and in mice.24 For instance, a heterozygous mutation in cfh (W1206R) in mice does not result in TMA,25 but it does so when plasma ADAMTS13 is severely deficient. These mice exhibited an increased level of blood urea nitrogen (BUN) and creatinine in addition to thrombocytopenia.26 Also, serum or plasma markers of complement and coagulation activation are significantly elevated in patients with iTTP.20,27-29 Some patients with refractory TTP respond to treatment with eculizumab,30-32 a monoclonal antibody against C5, which blocks the formation of terminal membrane attack complexes. These findings suggest that the complement activation pathway may participate in phenotypic modulations of iTTP.
To gain insight into the contribution of genetic variants in the genes associated with coagulation, platelet activation, and complement activation to phenotypic modifications of iTTP, we performed whole-exome sequencing on 40 patients with acute iTTP.33 We selected 20 putative candidate genes involved in the renal pathways, including ADAMTS13, C3, C3AR1, C5, CFB, CFH, CFI, C4BPA, MCP (CD46), CD59, CFHR1 to CFHR5, DGKE, THBD, MMACHC, INF2, and PLG for further analysis. Our results indicate that genetic variants, particularly in CFHR5, THBD, and DGKE, may be associated with renal function in patients with acute iTTP.
Methods
Patients
The institutional review boards of The University of Alabama at Birmingham and The University of Kansas Medical Center approved the study protocol. All data and samples were collected after an informed consent was obtained from each participant. The clinical and laboratory features of all 40 patients enrolled in the study were described in our previous study.33 The diagnosis of iTTP was confirmed with plasma ADAMTS13 activity <10 U/mL (or 10% of normal) and the presence of ADAMTS13 inhibitors or anti-ADAMTS13 immunoglobulin G (IgG), in the presence of appropriate clinical setting according to the International Society of Thrombosis and Haemostasis (ISTH) guidelines.19 Twenty local heathy individuals who do not have a history of iTTP and any other hematologic disorders were included as the control.
DNA sample preparation, sequencing, and variant findings
Genomic DNA was isolated from peripheral blood samples of patients. The quality was checked with the Agilent DNA/RNA analysis platform. The exome capture was performed using a Roche SeqCap EZ Human Exome Kit, version 3.0, and sequenced on the Illumina Hiseq3000 platform.33 All nonsynonymous and stop-gain/loss variants were extracted corresponding to all the significant genes found using the sequence kernel association test and further filtered based on the frequency of the variant in the 1000 Genomes Project.33 Only “rare” variants with a 1000 Genomes frequency of <0.05 were used in the analysis. The detailed workflow and data analysis of the exome sequence were described previously.33 Variant effects were cited from National Center for Biotechnology Information ClinVar, which was determined based on the guidelines published in 2015 by the American College of Medical Genetics and Genomics and the Association for Molecular Pathology.34
Results
Clinical and laboratory characteristics of patients
The demographic, clinical, and laboratory parameters of 40 iTTP patients are summarized in our previous publication.33 Of these, 14 were males (35%) and 26 were females (65%), with a median age of 44 years. African Americans accounted for 75% of patients. On admission, the median platelet count and hematocrit were 13 × 109/L and 24%, respectively. The median serum level of lactate dehydrogenase (LDH) was 839 U/L, and the median creatine level was 1.3 mg/dL. All patients had serum haptoglobin <30 mg/dL and plasma ADAMTS13 activity <5% of normal on admission. The median level of plasma ADAMTS13 inhibitor was 1.1 U/mL. Therapeutic plasma exchange (TPE) was performed in all 40 patients, with the median number of procedures of 15. Corticosteroids were prescribed to 92.5% of patients. Only one-third of patients received rituximab for therapy. Forty percent of patients achieved clinical remission, but exacerbation and relapse occurred in 22.5% and 27.5% of patients before caplacizumab era. The mortality rate of this cohort selected for genetic study was only 5%. Patients who passed away within a week in the hospital were not enrolled in the genetic study with the intention of a long-term follow-up.
Nonsynonymous variants in the selected genes of interest
We chose to investigate 20 genes associated with coagulation, complement, and platelet activation that are implicated in the pathogenesis of TMA. Our results revealed that 12 genes including ADAMTS13, C3, C3AR1, C5, CFHR5, CFH, CFI, MCP, PLG, INF2, THBD, and DGKE carry nonsynonymous variants or stop-gain/loss functional mutations. The number of patients and percentage of patients carrying nonsynonymous polymorphisms in the coding region of these 12 genes and their potential effects on protein function and disease pathology are summarized in Table 1.
Nonsynonymous variants in CFI, CFH, C5, ADAMTS13, and PLG were identified in >50% of patients with iTTP; C3, INF2, THBD, and CFHR5 in 10% to 50% patients; and C3AR1, MCP, and DGKE in 10% patients. We did not identify any exonic variants in CFB, MMACHC, C4BPA, and CFHR1 to CFHR4.
The exact site of genetic variants, amino acid changes, the combined annotation dependent depletion (CADD) score, and allele frequency of C3, C3AR1, C5, CFH, CFHR5, CFI, and MCP in iTTP and controls are shown in supplemental Table 1. Most variants are heterozygous, and ∼65% (42/65) are predicted to be “benign or likely benign” according to National Center for Biotechnology Information ClinVar database.
Additionally, we identified 15 variants (3 novel) in ADAMTS13. The exact sites, amino acid changes, the domains of ADAMTS13 that may be affected, the CADD score, the percentage of patients with iTTP carrying such a variant, and the allele frequency in both patients and controls are shown in supplemental Table 2. Most of these variants appear to be “most likely benign,” or “unknown” with respect to ADAMTS13 activity, although several were reported to potentially affect ADAMTS13 activity.
Variants found in genes associated with cHUS or aHUS
Mutations in C3, CFH, and CFI, as well as CFHR5, are implicated in the pathogenesis of complement-associated HUS (cHUS) or aHUS. We demonstrated the association of renal dysfunction in patients with iTTP with nonsynonymous polymorphisms in the coding region of complement or complement-related genes (eg, C3, C3AR1, C5, CFH, CFHR5, and CFI) (Figure 1; Table 2). Additionally, we found nonsynonymous variants in 4 other noncomplement-related genes (eg, PLG, INF2, THBD, and DGKE) (supplemental Table 3).
Dynamic changes of serum creatinine in patients with iTTP from admission to discharge in association with genetic variants in genes encoding complements and complement-related proteins. Serum creatinine levels are shown on the y-axis. The numbers 1 to 5 on the x-axis indicate serum creatinine levels on admission, at peak, at nadir, at discharge, and during follow-up visit, respectively.
Dynamic changes of serum creatinine in patients with iTTP from admission to discharge in association with genetic variants in genes encoding complements and complement-related proteins. Serum creatinine levels are shown on the y-axis. The numbers 1 to 5 on the x-axis indicate serum creatinine levels on admission, at peak, at nadir, at discharge, and during follow-up visit, respectively.
One novel variant in C3AR1 (p.S357N), PLG (p.P162T), CFHR5 (p.S78X), and DGKE (p.D379N) and 2 novel variants in C5 (p.G344S/G350S and p.S851T/S857T) were identified, although their biological significances remain unknown. Nearly all variants in CFH appear to be “benign” or “likely benign.” Some variants in C3, CFI, and CFHR5 have conflicting interpretations in terms of their pathogenicity, whereas a few variants in CFH, CFHR5, and INF2 have “uncertain” or “not determined for their significance” in the general population (supplemental Table 1). However, this may not be benign in those with severe deficiency of plasma ADAMTS13 activity.
Three variants in C5 were detected in 3 African American patients with a CADD score >30. One nonsense (Q19X) and 2 missense variants (A252T/A258T and R928Q/R934Q) are present in exons 1, 7, and 21 of the C5. The nonsense variant occurring in residue 19 with c.55C>T results in a change of Gln19 to a stop codon. A single nucleotide variant from G to A at nucleotides 754 and 2783 results in 2 missense variants (eg, A252T/A258T and R928Q/R934Q). Both Q19X and A252T were shown to be the detrimental changes, resulting in C5 deficiency.35,36 R928Q in C5 showed normal expression and lytic activity.37 Q19X and A252T variants were detected in 7% of African American patients who had a recurrent meningococcal disease.36 R928Q is only reported in the single-nucleotide polymorphism (SNP) databases but has not been shown to be associated with any disease yet. In our case series, patients carrying C5 variants appeared to have normal renal function on admission and throughout hospitalization resulting from acute iTTP (Table 2). These results suggest that C5 deficiency may be protective for renal function in acute iTTP.
Rare variants in CFHR5, THBD, and DGKE may be associated with renal dysfunction in iTTP. We performed global genetic classification and analysis of each variant in these selected genes of interest and determined the association between specific variants and serum creatinine levels. Of 40 patients with iTTP, 18 (45%) had normal creatinine, and 22 (55%) had elevated creatinine levels on admission or at any time point during the disease course. Of 7 patients with iTTP with creatinine levels >2.0 mg/dL on admission, 5 had 1 variant in 3 different genes (eg, CFHR5, DGKE, and THBD) with a CADD score >20, and 2 had no detrimental variants associated with the selected genes (1 had comorbidity of diabetes mellitus and hypertension; and the other 1 had end stage renal disease). Four variants were found in CFHR5 (c.233C>A; p.S78X, c.1172C>T; p.P391L, c.1541T>G; p.M514R; and c.1704T>A; p.C568X) in 4 different patients. Three of 4 variants were associated with persistent severe renal insufficiency (eg, remarkably increased creatinine level on admission and remained elevated at discharge and during follow-up). The patient carried the novel DGKE variant (c.1135G>A; p. D379N) had a good response after treatment with TPE and corticosteroids, but the patient’s creatinine level remained elevated (>2 mg/dL) during follow-up. One patient had a missense variant in THBD (c.1504G>C; p.G502R) and showed a delayed recovery of renal function despite normal platelet counts and serum lactate dehydrogenase levels (Table 2). These results indicate that variants in CFHR5, DGKE, and THBD may be associated with abnormal renal function in iTTP.
Discussion
This study demonstrates using whole-exome sequencing and patient subclassification that genetic variants in complement activation, regulatory, or cHUS- or aHUS-associated genes may modify the phenotype of iTTP resulting from acquired deficiency of plasma ADAMTS13. This is particularly the case in terms of renal dysfunction. Rare genetic variants in CHFR5, THBD, and DGKE appear to be associated with the persistently elevated serum creatinine levels and/or the end stage renal disease after acute iTTP.
Variants in THBD in a heterozygous form were reported in cases of aHUS. Mutations in THBD may result in a diminished complement regulatory activity of THBD, leading to excessive complement activation.15,38 The loss-of-function mutations in DGKE are also found in patients with aHUS.16,39-41 The DGKE protein is synthesized in endothelium, platelets, and podocytes. In endothelial cells, arachidonic acid–containing diacylglycerol (DAG) activates protein kinase C42 and promotes thrombosis by increasing endothelial cell production of several hemostatic factors including von Willebrand factor (VWF), plasminogen activator inhibitor 1 (PAI-1), platelet-activating factor, and tissue factor, as well as tissue-type plasminogen activator (t-PA). The DGKE normally inactivates the DAG signaling,16 thus inhibiting endothelial and platelet activation. It is still not clear whether mutations in DGKE affect the complement activation pathway. Some studies show a small contribution DGKE to the expression of MCP on endothelial cell surfaces, but other studies show more dramatic effect on complement activation.42,43
Interestingly, of 3 variants in C5 with CADD score >30, two (Q19X and A252T/A258T) were shown to cause C5 deficiency with R928Q being expressed normally. As expected, patients carrying variants in C5 appeared to have more favorable renal function. For instance, a patient with iTTP with Q19X in C5 exhibited normal creatinine during the entire disease course, but other patients with A252T and Q928R in C5 exhibited only a transient mild elevation of serum creatinine, although 1 patient died of disease exacerbation.
Elevated serum or plasma markers of complement activation in the alternative pathway are reported in patients with acute iTTP.20,28,44-46 Unlike the classic pathway, the alternative pathway is constitutively activated, thus requiring tight control by its regulatory proteins, including CFH, CFI, MCP, and THBD, etc. Mutations in complement activation components (eg, C3 or CFB) confer predisposition to aHUS,7,8 although we did not identify any detrimental variant in C3 and CFB in our cohort of patients with iTTP. However, we do find detrimental variants in C5 that may dampen the formation of membrane attack complex that would otherwise damage vascular endothelium and activate platelets.47 Patients with refractory iTTP who do not respond to the standard of care therapy (eg, corticosteroids, TPE, and rituximab) do benefit from adjunctive therapy with eculizumab, an anti-C5 monoclonal antibody.30,32,48
Renal failure is a prominent feature of cHUS or aHUS.49-52 Serum creatinine >2.0 or 2.2 mg/dL has been used to differentiate TTP from HUS.18,19,53,54 However, this distinction may not be so absolute because abnormalities in complements or its regulatory proteins that may be found in patients with iTTP predisposes them to renal deterioration. However, a delayed presentation to the hospital or a delayed treatment for iTTP and other comorbidities including hypertension and diabetes mellitus may also contribute to renal dysfunction. More recently, caplacizumab, an anti-VWF nanobody,55-57 which can stop platelet-VWF interaction and inhibit thrombosis, may improve renal perfusion and function in iTTP.
Our study has some limitations. First, it remains observational and descriptive. Only a small subset of genes related to coagulation and complement activation or its regulation have been analyzed. In our previous data, we did find variants in genes encoding enzymes or proteins in glycosylation and inflammation or immune response that may contribute to the pathogenesis of iTTP33; second, additional expression and animal studies may be needed to validate the clinical relevance of these genetic variants in iTTP; third, our study was carried out in a small cohort of patients with iTTP, and a larger multicenter study involving more patients ought to be conducted to confirm these findings.
Nevertheless, our results demonstrate that complement dysregulation in the context of acquired ADAMTS13 deficiency may contribute to the phenotypic modification, particularly the renal dysfunction in iTTP. Whether an early intervention with anticomplement, such as eculizumab, or antiplatelet agent in a subset of patients with iTTP who present with severe and persistent renal injury may be beneficial to prevent end stage renal disease is yet to be further investigated.
Acknowledgments
The study was supported in part by grants from the National Institutes of Health, National Heart, Lung, and Blood Institute (HL144552, HL157975-01A1, and HL164016-01A1 [X.L.Z.]), and the Answering Thrombotic Thrombocytopenic Purpura (TTP) Foundation (AWD10000126 [X.L.Z.]).
Authorship
Contribution: All authors designed the study, analyzed the results, wrote the manuscript, and read and approved the final version of the manuscript for submission.
Conflict-of-interest disclosure: X.L.Z. is a speaker and/or consultant for Alexion, Apollo, GC Biopharma, Sanofi, Stago, and Takeda and is also the cofounder of Clotsolution. The remaining authors declare no competing financial interests.
Correspondence: X. Long Zheng, Department of Pathology and Laboratory Medicine, The University of Kansas Medical Center, 3901 Rainbow Blvd, Kansas City, KS 66160; email: xzheng2@kumc.edu.
References
Author notes
Data are available on request from the corresponding author, X. Long Zheng (xzheng2@kumc.edu).
The full-text version of this article contains a data supplement.