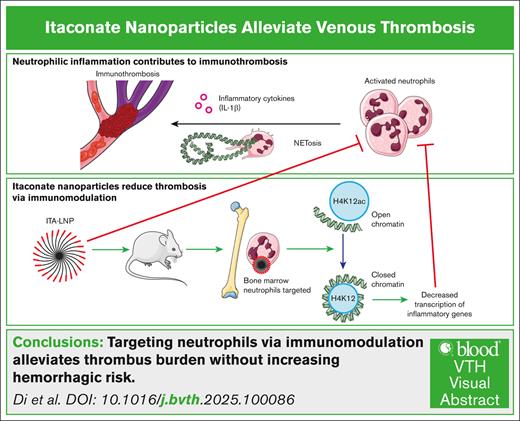
Key Points
ITA-LNPs are a hemostatically safe, anti-immunothrombotic therapy.
ITA-LNPs target neutrophils, a major component of immunothrombosis, and lower inflammation via H4K12ac deacetylation.
Visual Abstract
Targeting the coagulation cascade is a mainstay approach to therapy for thrombosis. One of the most prevalent complications of anticoagulation is a significant bleeding risk that can result in hospitalization. Recent studies on immunothrombosis (ie, inflammation-linked thrombosis) proposed myeloid cells, especially neutrophils, to actively participate in thrombus formation through neutrophil extracellular traps (NETosis) and activation of inflammatory pathways. Here, we developed lipid nanoparticles (LNPs) with coagulation cascade–independent action that target neutrophils to alleviate venous thrombosis. A single intravenous dose of immunomodulatory metabolite itaconate (ITA)-bearing LNPs (ITA-LNPs) targeted >90% of bone marrow–resident neutrophils, but not macrophages or T lymphocytes. ITA-LNPs significantly diminished inflammation in cultured neutrophils and macrophages and improved survival in the mouse model of endotoxemia. When tested in an inferior vena cava ligation model of deep vein thrombosis, ITA-LNPs decreased the size and weight of thrombi as compared with control LNPs. In addition, the thrombi from ITA-LNP–administered mice had significantly reduced neutrophil infiltration, decreased NETosis, and interleukin-1β expression. Intriguingly, one of the mechanisms through which this occurred was histone H4K12ac deacetylation, resulting in chromatin condensation and transcriptional repression of inflammatory genes and transcription factors involved in DNA damage response. Finally, we found that ITA-LNPs were hemostatically safe and neither targeted nor activated platelets in vivo. In sum, our work explores an LNP technology with an epigenetic mode of action serving as an anti-immunothrombotic therapy that could improve clinical outcomes without additional hemorrhagic risk.
Introduction
Vascular thrombosis confers devastating consequences in patients and is a leading source of morbidity and mortality worldwide. The Centers for Disease Control and Prevention estimates that venous thrombosis alone affects as many as 900 000 people each year in the United States,1 and thrombosis is responsible for at least 1 in 4 deaths worldwide.2 Current anticoagulation therapies for thrombosis target various aspects of the coagulation cascade and thus significantly disrupt hemostasis; hence, bleeding remains the primary side effect of anticoagulants and results in millions of emergency visits and hospitalizations every year, worldwide.3,4 Hence, antithrombotic therapies that do not affect hemostatic pathways while targeting inflammation are urgently needed.
Traditionally, studies of thrombosis have focused largely on platelets (arterial clots), red blood cells (venous clots), and the coagulation factors. However, a growing body of data gathered over the past decade provides evidence of an intimate link between inflammation and thrombosis (immunothrombosis).5-7 This link results in a vicious cycle in which inflammation begets thrombosis and thrombosis amplifies the inflammatory response. Specifically, myeloid cells (neutrophils and monocytes) are implicated as important cells in the generation of a prothrombotic milieu, sometimes termed as the “thromboinflammatory” process.8 The molecular basis underlying how myeloid cells contribute to the thrombotic process is complex, and recent work points to the importance of 2 critical events in myeloid cells: (1) generation of neutrophil extracellular traps (NETs) and (2) inflammatory cytokine release.5-7 NETs are web-like scaffolds composed of condensed chromatin and are abundant in both arterial and venous thrombi.5-7 NETs are normally released by activated neutrophils to entrap pathogens, but they are implicated in thrombosis as well.5-7 Although the process remains incompletely understood, what is known is that NET formation (NETosis) requires the production of reactive oxygen species (ROS).9,10 Published data also demonstrate that NETs prime some immune cells to release interleukin-1β (IL-1β), a critical proinflammatory cytokine that then activates various prothrombotic factors.11,12 This body of evidence identifies neutrophils as a key noncoagulation cascade target for an antithrombotic therapy.
We recently demonstrated that neutrophils are particularly important players in both venous and arterial thrombosis, and that the targeting of inflammatory neutrophils is a therapeutically sound strategy.13 This was accomplished through targeting activated, prothrombotic neutrophils via nanoparticle-based immunobiologics with specificity to P-selectin glycoprotein ligand 1 clusters.13,14 Although the use of antibody-conjugated nanoparticles proved to be effective, an alternative, nonantigenic strategy may confer significant benefits.15 As such, nonantigenic nanoparticles that bear no antibody or peptide conjugation are likely to be inexpensive, easier to manufacture, and do not present the challenge of antibody humanization. Additionally, an important attribute is their coagulation cascade–independent mode of action, which offers decreased chances of adverse bleeding effects either on its own or in conjunction with another anticoagulant. Collectively, if such a therapy is realized, it could significantly alter current paradigms of treatment and offer safer alternatives to traditional anti-inflammatory and anticoagulation agents.
Here, we describe a nonantigenic lipid nanoparticle approach that targets venous thrombosis. Our strategy builds upon our recent work on itaconate (ITA), a tricarboxylic acid cycle metabolite, that we, and others, have discovered to be implicated in inflammation resolution in аthеrоsсlеrоtic plaques of mice and humans.16-18 ITA is a dicarboxylic acid metabolite that exhibits inhibitory effects on ROS and IL-1β, broad anti-inflammatory actions (Figure 1A), and effects on histone acetylation (epigenetic regulation).16 An ITA derivative, octyl-itaconate (OI), has been shown recently to inhibit coagulopathy, although OI was administered systemically at high doses and did not specifically target neutrophils.19 Notably, OI or other derivatives of ITA have not been tested in bona fide venous thrombosis models.
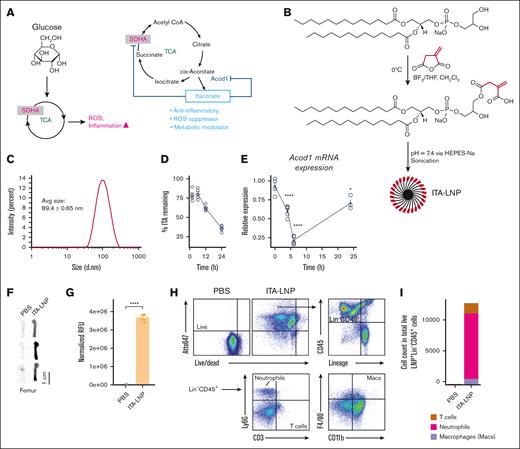
Physicochemical characteristics, itaconate release and bio/cell distribution of ITA-LNPs. (A) Schematic of ITA synthesis in biological systems. Selected biological actions of endogenous and exogenous ITA are depicted in blue. (B) Synthesis of ITA-LNPs using phosphatidyl glycerol as a starting material. (C) Dynamic light scattering analysis of ITA-LNP. (D) Time course of ITA release from ITA-LNPs incubated with liver microsomes ex vivo. (E) Quantitative polymerase chain reaction analysis of Acod1 mRNA expression over time in macrophages exposed to 1-μM ITA-LNP. (F) Biodistribution analysis in isolated bone femurs of C57BL/6 mice injected with a bolus IV with 50 mg/kg ITA-LNPs (Atto647 labeled) or PBS and euthanized 4 hours after injection. (G) Quantification of fluorescence from experiments in panel F. (H) FACS gating strategy and analysis of single cells from the bone marrow in panel F. (I) Quantification of cell-specific distribution of ITA-LNPs in the bone marrow; n = 3 animals per group or n = 4 for in vitro experiments. Statistics: pairwise t test with Holm post hoc test. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001. PBS, phosphate-buffered saline; SDHA, succinate dehydrogenase complex flavoprotein subunit A; TCA, tricarboxylic acid cycle.
Physicochemical characteristics, itaconate release and bio/cell distribution of ITA-LNPs. (A) Schematic of ITA synthesis in biological systems. Selected biological actions of endogenous and exogenous ITA are depicted in blue. (B) Synthesis of ITA-LNPs using phosphatidyl glycerol as a starting material. (C) Dynamic light scattering analysis of ITA-LNP. (D) Time course of ITA release from ITA-LNPs incubated with liver microsomes ex vivo. (E) Quantitative polymerase chain reaction analysis of Acod1 mRNA expression over time in macrophages exposed to 1-μM ITA-LNP. (F) Biodistribution analysis in isolated bone femurs of C57BL/6 mice injected with a bolus IV with 50 mg/kg ITA-LNPs (Atto647 labeled) or PBS and euthanized 4 hours after injection. (G) Quantification of fluorescence from experiments in panel F. (H) FACS gating strategy and analysis of single cells from the bone marrow in panel F. (I) Quantification of cell-specific distribution of ITA-LNPs in the bone marrow; n = 3 animals per group or n = 4 for in vitro experiments. Statistics: pairwise t test with Holm post hoc test. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001. PBS, phosphate-buffered saline; SDHA, succinate dehydrogenase complex flavoprotein subunit A; TCA, tricarboxylic acid cycle.
We show that ITA-based lipid nanoparticles (ITA-LNPs) are first-in-class nanomedicines that selectively and significantly reduce inflammation in vivo through targeting of bone marrow neutrophils. Because the bone marrow is the source of systemic prothrombotic neutrophils, ITA-LNPs allow for specific targeting at the origin of the thromboinflammation. ITA-LNPs taken up by neutrophils yield decreased acetylation status of major histones, especially histone mark H4K12ac. Such an approach epigenetically “reprograms” the source cells responsible for thromboinflammation, unlike existing biological drugs that target the consequences of the inflammation (eg, inflammatory cytokines such as IL-1β).
Methods
Animals
Male C57BL/6 mice, aged 8 to 12 weeks, were procured from The Jackson Laboratory and Charles River Laboratories and housed in American Association for Accreditation of Laboratory Animal Care-accredited quarters at Case Western Reserve University. The mice were housed in cages of 2 to 5 and allowed to acclimate for 1 to 2 weeks before beginning the experiments. Throughout the study, mice were maintained under a 12-hour light/dark cycle at a temperature of 22°C, and had free access to food and water.
LPS-induced endotoxemia
C57BL/6 male mice (10 weeks old, n = 15 per group) were IV injected with 50 mg/kg ITA- or control (Ctrl)-LNPs or 50 mg/kg anti-mouse IL-1β monoclonal antibody (clone 7E3, InvivoGen mil1b-mab9-10). Next, 15 mg/kg lipopolysaccharide (LPS) from Escherichia coli O55:B5 (Millipore Sigma L2880) was administered intraperitoneally 24 hours after the LNP/monoclonal antibody (mAb) injections. Mice were monitored for signs of sickness, and survival was recorded. In separate experiments (n = 7 to 11 per group), the LNPs and mAbs were injected as described earlier, followed by 8 kg/kg LPS, and body temperature measurements were monitored via a rectal probe (Braintree Scientific RET-3). The temperature measurements were repeated at 6 hours after the LPS injections. Blood was collected (20 μL) at 2 and 4 hours after LPS injection via tail tip bleeds and EDTA-coated capillary tubes. The mice were euthanized at the 6-hour time point, and blood was collected postmortem. The plasma was isolated by centrifugation at 300g for 25 minutes and analyzed as described hereafter.
IVC ligation venous thrombosis model
C57BL/6 male mice (8 weeks old, n = 3 per group) were IV injected with 50 mg/kg ITA- or Ctrl-LNPs. The inferior vena cava (IVC) ligation procedure was then carried out 24 hours after the injection, as outlined in a previous study.13,14 In brief, the mice were anesthetized with isoflurane. A sterile abdominal incision was made along the ventral midline. Cauterization was applied to all branches of the IVC, from the renal veins to the iliac bifurcation, and the side branches were ligated. The IVC was then isolated from the aorta just below the renal veins and securely ligated via 7-0 Prolene sutures. The laparotomy site was closed with sutures. After surgery, the mice were allowed to recover and were monitored for 2 hours before being returned to their housing. Thrombi from the IVC were then collected and weighed 48 hours after surgery at the time of euthanasia.
RNA sequencing and analysis
The RNA samples were adjusted to a concentration of 50 ng/μL and a total of 500 ng of RNA was then sent for single-end sequencing at the Beijing Genomics Institute, following standard protocols and the vendor’s recommendations. Library preparations were sequenced via the MISEQ-50 platform, and bioinformatics analysis was conducted as previously outlined using the Nextflow-based nf-core framework for community-curated bioinformatics pipelines.20 After the alignment to the mm10 genome and differential gene expression analysis using “nf-core/differentialabundance” bioinformatics pipeline, the gene lists were analyzed using the Appyters tool21 collection, which included Harmonizome ETL Gene Ontology (GO) (https://github.com/MaayanLab/Harmonizome-Data-Processing-Appyters) and ChIP-X enrichment analysis 3 (ChEA3) transcription factor overexpression analysis.22
All animal-related procedures adhered to the ethical guidelines set forth by the National Institutes of Health Guide for the Care and Use of Laboratory Animals (National Institutes of Health Publications no. 8523, revised 2011). These procedures were approved by the institutional animal care and use committee at Case Western Reserve University and conducted under protocol number 2016-0273.
This study involving human participants was conducted in compliance with applicable national regulations and institutional policies, which did not require ethical review and approval or written informed consent from participants.
Results
ITA-LNPs are vehicles for ITA delivery to neutrophils in the bone marrow
Using a previously developed synthetic strategy based on ITA anhydride ring opening under mild conditions (Figure 1B), we synthesized phosphatidylglycerol (PG)-ITA conjugate in quantitative yield (supplemental Figure 1). After pH adjustment and sonication, ITA-LNPs were successfully formed via self-assembly in HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid)-buffered saline. ITA-LNPs had a hydrodynamic diameter of 89.4 ± 0.65 nm (Figure 1C) with a polydispersity index of 0.194 ± 0.01 indicative of a narrow size distribution. ITA-LNPs had a negative charge of −56.0 ± 2.7 mV as determined by means of ζ-potential measurements. To confirm ITA release from the PG lipid, we incubated ITA-LNPs with rat liver microsomes known to have high esterase activity. The amount of ITA remaining in ITA-LNPs over a period of 24 hours was then quantified using high performance liquid chromatography. The data show that free ITA was formed under actions of esterases with >50% at 24 hours and >25% at 2 hours of ITA released after incubation (Figure 1D). To confirm ITA release intracellularly, we took advantage of a signaling feedback loop between ITA and the ITA-producing enzyme Acod1 (Figure 1A). In macrophages, when the concentration of intracellular ITA becomes high, the messenger RNA (mRNA) expression of Acod1 diminishes because there is no need to synthesize endogenous ITA. We incubated bone marrow–derived macrophages (BMDMs) with 1-μM ITA-LNPs, and Acod1 mRNA expression was analyzed using quantitative polymerase chain reaction over a time course of 24 hours (Figure 1E). The expression of Acod1 dropped precipitously over the first 6 hours of incubation, however, was restored over the next 18 hours, suggestive of ITA elimination.
To test in vivo biodistribution, we labeled ITA-LNPs with Atto647 fluorophore conjugated to a phosphatidylethanolamine. Upon bolus IV injection of Atto-647-ITA-LNPs at 50 mg/kg in C57BL/6 mice, we noted significant accumulation of the label in the bone 4 hours after injection, as shown in Figure 1F-G. ITA-LNPs also deposited to the liver, spleen, and lungs among other organs, as expected for any LNP injected IV (supplemental Figure 2A-C). Strikingly, fluorescence-activated cell sorting (FACS) analysis revealed that ITA-LNPs significantly targeted neutrophils in the bone marrow compared with macrophages and T cells (Figure 1H-I; supplemental Figure 2D), at least 90% of ITA-LNP+ cells in the bone marrow were neutrophils. Collectively, these data suggest that PG lipid–based ITA-LNPs are a suitable vehicle candidate for ITA delivery to neutrophils, given their bone marrow distribution and esterase-catalyzed ITA release.
ITA-LNPs exhibit anti-inflammatory effects in vitro and in vivo
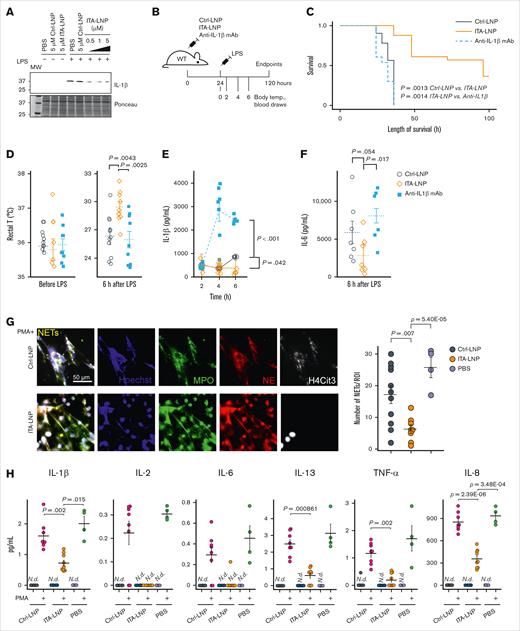
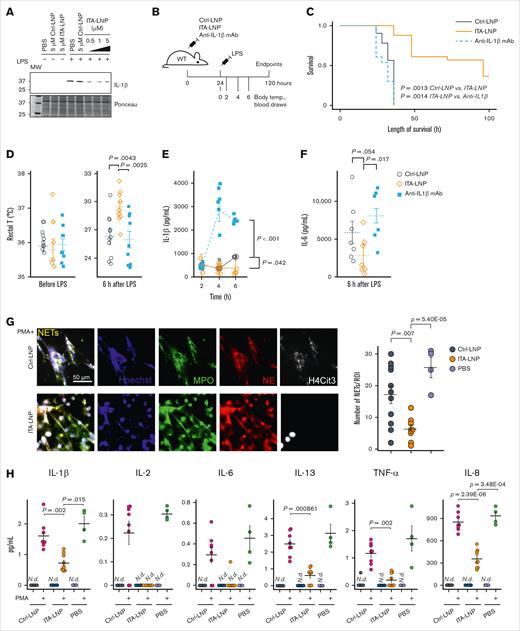
Given the potent in vitro anti-inflammatory effects of ITA observed by us and others,16,23-25 we hypothesized that ITA-LNPs synthesized from PG lipid would be an effective immunomodulator as well. We thus incubated BMDMs stimulated with 100 ng/mL E coli LPS with ITA-LNPs and Ctrl nanoparticles (Ctrl-LNPs, PG-lipid without ITA conjugation) dosed at different concentrations (0.5-5 μM) for 24 hours. The lysates from these BMDMs were then probed for IL-1β as a readout, because it has been previously demonstrated that ITA and its derivatives potently reduce the levels of this inflammatory cytokine (Figure 2A).26 ITA-LNPs but not Ctrl-LNPs effectively reduced the expression of IL-1β even at the lowest dose, thus demonstrating that ITA-LNPs were able to recapitulate the anti-inflammatory effect of ITA.
Immunomodulatory effects of ITA-LNP in vitro and in vivo. (A) Immunoblot probing for IL-1β in BMDMs treated with 100 ng/mL LPS in the presence of various doses of ITA-LNPs. (B) Schematic of the experiments in C57BL/6 mice subjected to LPS-induced endotoxemia. (C) Kaplan-Meier survival analysis after LPS-induced (15 mg/kg) endotoxemia in C57BL/6 mice pretreated with Ctrl/ITA-LNPs or anti–IL-1β mAb. (D) Rectal temperature measured in the mice from panel C before and 6 hours after LPS injections. (E-F) The levels of IL-1β and IL-6 in the serum from mice of a separate cohort injected as in panel B but with a sublethal dose of 8 mg/kg LPS; n = 10 to 15 mice per group. (G) NETs in primary human neutrophils incubated for 5 hours in vitro with 50 μM LNPs as indicated and in the presence of 100 nM PMA followed by immunofluorescence analysis; n = 6 for Ctrl-LNP and ITA-LNP without PMA; n = 8 for Ctrl-LNP with PMA; n = 9 for ITA-LNP with PMA; and n = 4 for PBS groups with or without PMA. (H) Cytokine analysis in supernatants from experiments in panel G. Statistical analysis is through pairwise t test with Holm post hoc test. N.d., not detected; PBS, phosphate-buffered saline; ROI, region of interest; T, temperature.
Immunomodulatory effects of ITA-LNP in vitro and in vivo. (A) Immunoblot probing for IL-1β in BMDMs treated with 100 ng/mL LPS in the presence of various doses of ITA-LNPs. (B) Schematic of the experiments in C57BL/6 mice subjected to LPS-induced endotoxemia. (C) Kaplan-Meier survival analysis after LPS-induced (15 mg/kg) endotoxemia in C57BL/6 mice pretreated with Ctrl/ITA-LNPs or anti–IL-1β mAb. (D) Rectal temperature measured in the mice from panel C before and 6 hours after LPS injections. (E-F) The levels of IL-1β and IL-6 in the serum from mice of a separate cohort injected as in panel B but with a sublethal dose of 8 mg/kg LPS; n = 10 to 15 mice per group. (G) NETs in primary human neutrophils incubated for 5 hours in vitro with 50 μM LNPs as indicated and in the presence of 100 nM PMA followed by immunofluorescence analysis; n = 6 for Ctrl-LNP and ITA-LNP without PMA; n = 8 for Ctrl-LNP with PMA; n = 9 for ITA-LNP with PMA; and n = 4 for PBS groups with or without PMA. (H) Cytokine analysis in supernatants from experiments in panel G. Statistical analysis is through pairwise t test with Holm post hoc test. N.d., not detected; PBS, phosphate-buffered saline; ROI, region of interest; T, temperature.
Next, we tested whether ITA-LNPs could be similarly effective in vivo, specifically in endotoxemia.25,27 C57BL/6 mice were bolus-injected IV with ITA- or Ctrl-LNPs at 50 mg/kg or 50 mg/kg anti-mouse IL-1β mAb (mAb 7E3).At 24 hours after injection, a lethal dose of 15 mg/kg E coli LPS (Figure 2B) was delivered intraperitoneally and survival was recorded. ITA-LNPs significantly improved survival compared with Ctrl-LNPs (P = .0013) and, strikingly, anti-IL-1β mAb (Figure 2C). In a separate cohort of animals, we administered LNPs and mAb in the same manner except that LPS was given at a sublethal dose of 8 mg/kg. We then monitored body temperature and performed blood draws over time as indicated in Figure 2B, followed by subsequent analysis for serum cytokines IL-1β and IL-6. As expected, ITA-LNPs protected mice from LPS-induced hypothermia (Figure 2D) and significantly lowered serum cytokines (IL-1β and IL-6 at 6 hours; Figure 2E-F).
Finally, to demonstrate biological activity of ITA-LNPs in human cells, we isolated primary human neutrophils from whole blood by immunomagnetic negative selection followed by incubation with 50 μM Ctrl- or ITA-LNPs for 5 hours at 37°C. Some cells were simultaneously stimulated with phorbol 12-myristate 13-acetate (PMA) at 100 nM to induce oxidative stress and inflammation. At the end of these experiments, we performed immunofluorescence analysis of formed NETs, a typical response to PMA stimulation (Figure 2G).28-30 NETs were identified as extracellular structures costained with antibodies against myeloperoxidase (MPO), neutrophil elastase, citrullinated histone H4 (H4Cit3), and DNA. NETs were readily detected in PMA-stimulated cells; however, they were absent in unstimulated neutrophils (supplemental Figure 2E). Interestingly, ITA- but not Ctrl-LNPs, significantly reduced NETs formation in PMA-stimulated human neutrophils (Figure 2G), in addition to demonstrating nucleus-restricted staining for H4Cit3, whereas Ctrl-LNP-treated cells showed H4Cit3 that was predominantly extracellular. Cytokine analysis of the supernatants revealed that ITA-LNPs achieve significant reduction in IL-1β, IL-8, IL-13, and tumor necrosis factor α (TNF-α). This is consistent with the previous finding that ITA-LNPs significantly reduce serum levels of proinflammatory cytokines in mice (Figure 2E-F).
Collectively, the aforementioned data showcase ITA-LNPs as powerful immunomodulatory nanoparticles that are able to resolve inflammation likely through a number of mechanisms distinct from IL-1β inhibition.31 Importantly, beneficial actions on inflammation suppression and NETs formation in human neutrophils suggest ITA-LNP effects are not restricted to mouse cells.
ITA-LNPs alleviate venous thrombosis
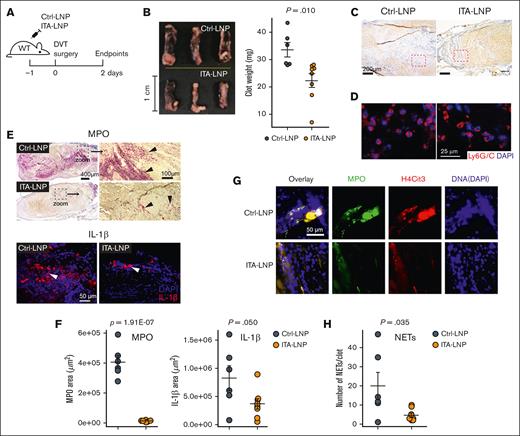
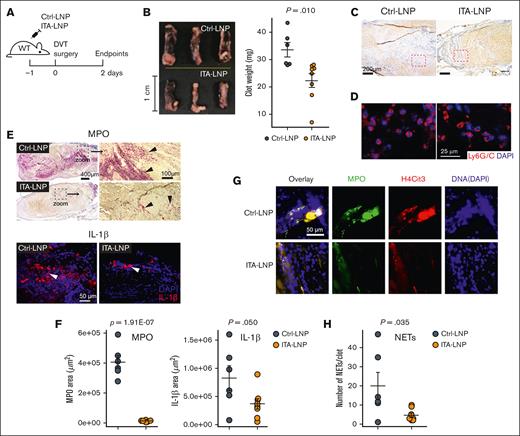
Having demonstrated the anti-inflammatory potential of ITA-LNPs in vitro and in vivo, with an advantage of neutrophil targeting, we reasoned that ITA-LNPs could be efficacious in thrombosis. We tested ITA-LNPs and corresponding Ctrl-LNPs in the model of deep vein thrombosis (DVT). C57BL/6 mice were bolus-injected with the indicated formulations, and DVT was induced 24 hours later by IVC ligation (Figure 3A). Figure 3B depicts gross pathology of the blood clot removed from these animals 48 hours after DVT and their measured weight. Strikingly, ITA-LNPs significantly decreased clot size and weight. Histologically, IVC thrombi from ITA-LNP–injected mice contained less myeloid cells (blue) vs Ctrl-LNPs (Figure 3C). We found that neutrophils (identified via Ly6G/C staining in Figure 3D) in Ctrl-LNP–treated mice were trapped within a dense fibrin network. In contrast, ITA-LNPs treatment resulted in less-occlusive thrombi, a significantly smaller number of infiltrated neutrophils, and a scarce fibrin mesh. Inflammation resolution associated with these neutrophils was further tested by immunostaining with antibodies against MPO (neutrophil marker) and IL-1β (Figure 3E-F). Indeed, both markers were significantly reduced in clots from ITA-LNP–injected mice by a magnitude of change that was especially drastic for MPO (54.6 ± 13.2-fold change vs Ctrl-LNPs; P = .0039). The result is a fully formed blood clot (Figure 3C-E), but that is less inflammatory and occlusive. These data suggest that ITA-LNPs targeted the myeloid components of DVT, especially neutrophils, but spared the coagulation cascade needed for normal (nonpathological) blood clotting.
ITA-LNPs alleviate venous thrombosis in IVC ligation model. (A) Schematic of the experiments. WT mice IV pretreated (50 mg/kg, bolus) with ITA- or Ctrl-LNPs were subjected to IVC ligation surgery followed by the isolation of the blood clots; n = 6 for Ctrl-LNP and n = 8 for ITA-LNP. (B) Gross pathology with clot weight quantification. (C) Hematoxylin and eosin staining of blood clot sections from these experiments. (D) Immunofluorescence staining of the same sections using Ly6G/C mAb (Red) and nuclei (blue). (E) MPO (top, alkaline phosphatase immunohistochemistry) and IL-1β (bottom, immunofluorescence) staining in the same clots. (F) Quantification of the staining in panel E. For each biological replicate (n = 6 for Ctrl-LNP and n = 8 for ITA = LNP), 3 to 4 histopathological locations at different depths (100 μm apart) were analyzed. (G) NET staining in the same sections using triple-positive identification through colocalization of the signals from MPO (green), H4Cit3 (red), and DNA (DAPI; blue). (H) Quantification of the staining in panel G. Quantification was accomplished using 2 to 3 different histopathological locations and the same aforementioned biological replicates. Statistical analysis is through a 2-tailed t test. DAPI, 4′,6-diamidino-2-phenylindole; H4Cit3, citrullinated histone H4; MPO, myeloperoxidase; WT, wild type.
ITA-LNPs alleviate venous thrombosis in IVC ligation model. (A) Schematic of the experiments. WT mice IV pretreated (50 mg/kg, bolus) with ITA- or Ctrl-LNPs were subjected to IVC ligation surgery followed by the isolation of the blood clots; n = 6 for Ctrl-LNP and n = 8 for ITA-LNP. (B) Gross pathology with clot weight quantification. (C) Hematoxylin and eosin staining of blood clot sections from these experiments. (D) Immunofluorescence staining of the same sections using Ly6G/C mAb (Red) and nuclei (blue). (E) MPO (top, alkaline phosphatase immunohistochemistry) and IL-1β (bottom, immunofluorescence) staining in the same clots. (F) Quantification of the staining in panel E. For each biological replicate (n = 6 for Ctrl-LNP and n = 8 for ITA = LNP), 3 to 4 histopathological locations at different depths (100 μm apart) were analyzed. (G) NET staining in the same sections using triple-positive identification through colocalization of the signals from MPO (green), H4Cit3 (red), and DNA (DAPI; blue). (H) Quantification of the staining in panel G. Quantification was accomplished using 2 to 3 different histopathological locations and the same aforementioned biological replicates. Statistical analysis is through a 2-tailed t test. DAPI, 4′,6-diamidino-2-phenylindole; H4Cit3, citrullinated histone H4; MPO, myeloperoxidase; WT, wild type.
One possible mechanism by which such drastic reduction in thrombosis was observed is through reduction in NETosis, NETs formation. Indeed, staining for MPO, citrullinated histone H3 (H3Cit) and DNA in these thrombi and their colocalization (collective markers for NETs) demonstrated that web-like NETs were only sporadically found in sections from ITA-LNP–treated mice unlike the abundantly observed NETs in thrombi from Ctrl-LNP–treated mice (Figure 3G-H). Collectively, these data indicate that ITA-LNPs are able to alleviate venous thrombosis without changes in homeostatic clotting, likely through targeting neutrophils in the bone marrow.
ITA-LNPs epigenetically modulate neutrophils in the bone marrow through histone H4 deacetylation
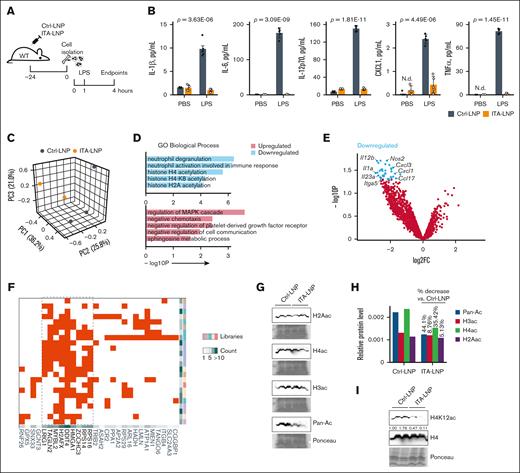
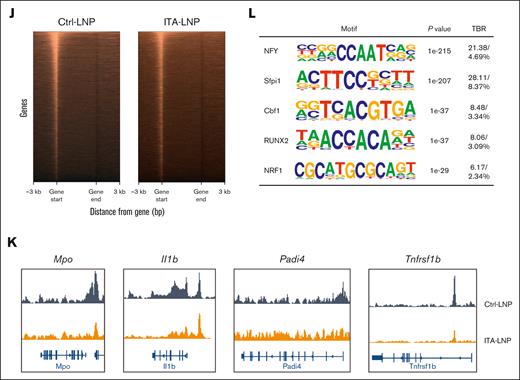
The time course of ITA-LNP effects in IVC thrombosis model 24 hours after injection (Figure 3A) suggested sustained long-term therapeutic efficacy. Given the deposition of ITA-LNP in the bone marrow after only a single injection (Figure 1F-I), and persistent effects over 48 hours could be explained through neutrophil “reprogramming” via epigenetic actions, similar to what we have reported with our previous ITA-LNP formulation.16 To test this, and because neutrophils are highly abundant within the bone marrow, we injected a single dose of 50 mg/kg ITA- or Ctrl-LNPs in C57BL/6 mice and collected the bone marrow 24 hours later. The neutrophils were then isolated via Histopaque density gradient centrifugation and rested in culture for 1 hour, after which 100 ng/mL LPS was added and the incubation continued for 3 more hours (Figure 4A). Notably, at the end of the experiment, the neutrophils from animals injected with ITA-LNPs secreted significantly lower levels of proinflammatory cytokines (IL-1β, IL-6, TNF-α, IL-12p70, and others) than Ctrl-LNPs (Figure 4B), paralleling the findings seen in human neutrophils (Figure 2H). To investigate these neutrophils at a transcriptional level, we performed bulk RNA sequencing. Principal component analysis in neutrophils stimulated with LPS demonstrated clear clustering of ITA- vs Ctrl-LNPs samples, indicative of vastly different transcriptomes between these 2 groups (Figure 4C). Furthermore, GO “biological process” terms analysis (Figure 4D) showed that ITA-LNP injection downregulated terms associated with neutrophil activation (neutrophil degranulation, and neutrophil activation involved in immune response) and histone acetylation (histone H4 acetylation, histone H4K8 acetylation, and others). In line with these findings, GO terms “molecular function” showed significant downregulation of a number of terms associated with histone acetyltransferase activity (supplemental Figure 3A). Major differentially expressed genes with downregulated expression (Figure 4E) included inflammatory cytokines (Il1a, Il12b, and Il23a) and chemokines (Cxcl3, Cxcl1, and Ccl17), as well as Nos2 (nitric oxide synthase), a prototypical marker of myeloid cell activation in response to LPS.32-34 The RNA sequencing data sets comprising top 50 most significantly downregulated genes were then analyzed using ChEA3,22 to understand the overrepresentation of transcription factors (TFs) in ITA-LNP vs Ctrl-LNP groups (Figure 4F). Interestingly, ChEA3 analysis demonstrated overenrichment of TFs involved DNA damage response (MYBL2, H2AFX, and DDIT4), chromatin organization (HMGA1), and protein synthesis (RPS14 and RPS16). These results are suggestive that ITA-LNPs protect neutrophils from excessive inflammation and LPS-induced cell death via epigenetic regulation and chromatin organization. To investigate this further and because GO terms were indicative of changes in histone acetylation (Figure 4D), we then screened for protein expression of all major pan-acetylated histones in primary bone marrow–derived neutrophils incubated with either 1-μM ITA- or Ctrl-LNPs for 3 hours. Immunoblotting of lysates from these neutrophils (Figure 4G-H) demonstrated that pan-protein acetylation was significantly reduced in ITA-LNPs vs Ctrl-LNPs and this effect was mostly driven by the reduction in histone H4 acetylation (35.42% of 44.1% in total reduction). Because H4 acetylation was shown to be altered with ITA-LNP treatment (Figure 4D), we probed the same lysates for all H4Kac variants for which antibodies were commercially available to assess whether ITA-LNPs were regulating the acetylation of specific lysine residues (Figure 4I; supplemental Figure 3B). Strikingly, only H4K12ac demonstrated significant and strong downregulation in response to ITA-LNPs, thus demonstrating an unusual specificity to a particular histone target and acetylation site. To understand whether such changes are impactful on a whole-genome level and accessible chromatin, we performed the assay for transposase-accessible chromatin with sequencing in neutrophils treated with either ITA- or Ctrl-LNPs under LPS stimulation as described earlier (Figure 4J). Notably, the accessible chromatin was decreased in ITA-LNP–treated neutrophils. The chromatin accessibility at Mpo and Il1b was greatly reduced in ITA-LNP–treated neutrophils as compared with that in Ctrl-LNP–treated cells (Figure 4K), thus corroborating the aforementioned results obtained in IVC thrombosis model (Figure 3). Finally, hypergeometric optimization of motif enrichment analysis identified overrepresented TFs such as nuclear transcription factor Y, a pioneer factor of chromatin accessibility, Sfpi1 (also known as PU.1), a TF that controls cell fate by decompacting heterochromatin, and Cbf1, a DNA binding factor that plays a role in transcriptional regulation (Figure 4L). In sum, these data strongly support epigenetic mode of action of ITA-LNPs in activated neutrophils and specific actions on H4K12 acetylation resulting in dampening inflammation and cell survival.
ITA-LNPs are hemostatically safe
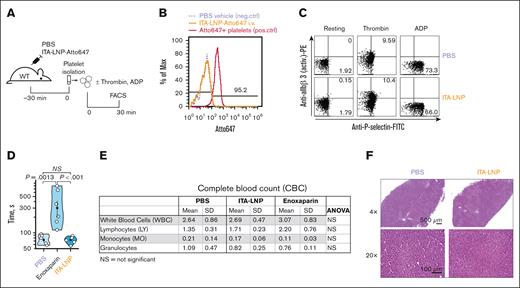
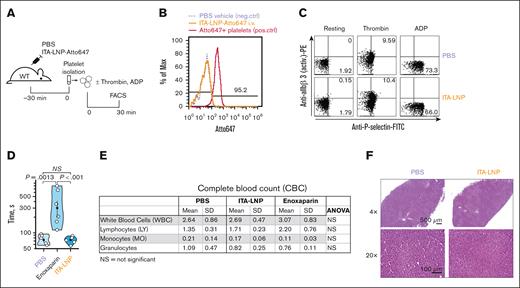
Because many anticoagulation therapies are fraught with hemostatic side effects such as bleeding and direct actions on platelets, we sought to investigate these effects in ITA-LNP–treated C57BL/6 mice. Importantly, ITA-LNPs did not target platelets in vivo, as demonstrated by FACS experiments after a bolus injection of Atto647-labeled ITA-LNP or vehicle (phosphate-buffered saline) in C57BL/6 mice (Figure 5A). Platelets were identified by side scatter and positivity for CD42b. There was virtually no Atto647 signal in platelets 30 minutes after ITA-LNP injection (Figure 5B). Platelets incubated ex vivo with Atto647 lipid (conjugated to a phosphatidylethanolamine) served as a positive Ctrl in these experiments (Figure 5B). In line with this data, ITA-LNPs had no effect on platelet activation (Figure 5C). Even under conditions of adenosine 5′-diphosphate activation (a weak agonist that reversibly stimulates integrin αIIbβ3 activation) and thrombin treatment (a very strong platelet agonist inducing irreversible integrin αIIbβ3 activation and P-selectin expression), ITA-LNP effects on platelets were indistinguishable from PBS vehicle Ctrl (Figure 5C). This additionally suggested that ITA-LNP does not exhibit any synergistic effect on platelet activation in the presence of agonists.
Hemostatic and liver safety of ITA-LNPs. (A) Schematic of experiments testing ITA-LNP actions on platelets. (B) FACS histograms examining accumulation of ITA-LNPs in platelets after bolus injection in panel A. (C) FACS-based platelet activation assays in isolated platelets from experiments in panel A. (D) Bleeding assays in C57BL/6 mice after daily injections of ITA-LNPs (50 mg/kg), 100 μL PBS, or enoxaparin (1 mg/kg) for a total duration of 3 days. (E) Complete blood counts from blood samples obtained in panel D. (F) Hematoxylin and eosin staining in liver sections from C57BL/6 mice used in panel D. The images were taken at different magnification powers as indicated. active. PE, phosphatidylethanolamine; ANOVA, analysis of variance; ADP, adenosine 5′-diphosphate; FITC, fluorescein isothiocyanate; Max, maximum; neg., negative; NS, not significant; PBS, phosphate-buffered saline; pos., positive; SD, standard deviation; WT, wild type.
Hemostatic and liver safety of ITA-LNPs. (A) Schematic of experiments testing ITA-LNP actions on platelets. (B) FACS histograms examining accumulation of ITA-LNPs in platelets after bolus injection in panel A. (C) FACS-based platelet activation assays in isolated platelets from experiments in panel A. (D) Bleeding assays in C57BL/6 mice after daily injections of ITA-LNPs (50 mg/kg), 100 μL PBS, or enoxaparin (1 mg/kg) for a total duration of 3 days. (E) Complete blood counts from blood samples obtained in panel D. (F) Hematoxylin and eosin staining in liver sections from C57BL/6 mice used in panel D. The images were taken at different magnification powers as indicated. active. PE, phosphatidylethanolamine; ANOVA, analysis of variance; ADP, adenosine 5′-diphosphate; FITC, fluorescein isothiocyanate; Max, maximum; neg., negative; NS, not significant; PBS, phosphate-buffered saline; pos., positive; SD, standard deviation; WT, wild type.
Importantly, ITA-LNPs did not prolong tail bleeding in mice after daily injections for 3 days at 50 mg/kg IV (Figure 5D). Conversely, the clinically used anticoagulant enoxaparin significantly increased bleeding times (Figure 5D) when administered according to the same injection schedule. Importantly, ITA-NPs had no effect on major blood counts, with no observed significant differences in the complete blood counts between ITA-LNP– vs ctrl-LNP–treated animals (Figure 5E). Finally, the liver samples from the same animals stained with hematoxylin and eosin did not show any noticeable abnormalities or cell infiltrations; samples from ITA-LNP–injected mice were virtually indistinguishable from Ctrl-LNP animals (Figure 5F). Collectively, these data suggest that ITA-LNPs are safe hemostatically (do not prolong bleeding) and are likely to be safe for thrombosis therapeutic applications in which platelet activation or bleeding is a major concern.
Discussion
This work demonstrates that targeting neutrophils, a major constituent of immunothrombosis, with nonantigenic particles significantly reduces thrombosis with no hemorrhagic risk.
We have previously been successful at identifying a lipid-ITA conjugate, phosphatidyl ethylene glycol-ITA (Ptd-EG-ITA), with biological actions mirroring those described in the literature for unconjugated ITA and ITA ester derivatives.16 However, Ptd-EG is an expensive, unnatural synthetic lipid with unclear long-term safety. Additionally, and in light of our quest to target neutrophils, the uptake of the Ptd-EG-ITA nanoparticles by neutrophils in plaque and then blood was <40% of all NP+ cells.16 We therefore sought to engineer ITA-LNPs with better neutrophil affinity and that are composed of a phospholipid with known safety profile.
Ptd-EG-ITA was successful in targeting the bone marrow likely because of its negative charge,35 so we postulated that a particle with increased negative charge could increase bone marrow targeting and, by proxy, neutrophil targeting. The ζ-potential (indicative of LNP’s charge) will be reduced if a highly negatively charged phospholipid is used in LNP’s formulation. The most commonly used phospholipids for in vivo nanoparticle applications, including COVID-19 mRNA-LNP vaccines, either do not possess a functional group available for ITA conjugation or are only able to form amide derivatives (eg, phosphatidylserine or phosphatidylethanolamine; supplemental Figure 1A). Amide conjugates are not conducive to ITA detachment from the lipid via actions of intracellular esterases (supplemental Figure 1B). We thus turned our attention to PG, a highly negatively charged phospholipid class present in human biological fluids and tissues with a synthetic functionality of forming esters through a primary hydroxyl group of the glycerol backbone. Indeed, an PG-ITA formulation produced a highly negatively charged ITA-LNPs (ζ = −56.0 ± 2.7), which achieved successful bone marrow deposition with increased neutrophil targeting (>90% of all targeted cells). Moreover, these ITA-LNPs released free ITA in response to esterases (Figure 1D-E). The fact that the change in phospholipid dramatically shifted the targeted subset in the bone marrow is intriguing and a subject to further investigation in future studies.
In the endotoxemic mouse models, anti–IL-1β mAb was observed to be ineffective at protecting against endotoxemia, possibly because other proinflammatory mediators such as TNFα and IL-6, play a significant and redundant role in inflammation.36-40 Our results are in line with clinical observations demonstrating that canakinumab therapy (anti–IL-1β mAb; CANTOS trial), had detrimental long-term effects in human patients, resulting in increased infections and sepsis.41 In contrast, we demonstrated that ITA-LNPs additionally lowered a number of inflammatory cytokines in PMA-stimulated human neutrophils (eg, IL-2, IL-8, TNF-α), in line with previous research on ITA and ITA-LNPs, that have been shown to act through multiple anti-inflammatory upstream pathways besides the IL-1β pathway.31 These multifaceted mechanisms are likely the reason that they are effective in vivo.31 In fact, the ability of ITA-LNPs to limit the consequences of endotoxemia and improve survival after a lethal dose of LPS hints at possible clinical scenarios in which thrombotic complications in endotoxemia and sepsis are of major concern.42,43
We observed that ITA-LNPs cause a significant decrease in NET formation in murine thrombi with ITA-LNP pretreatment and in human neutrophils stimulated with PMA. Interestingly, in bone marrow neutrophils of LNP-pretreated mice, the chromatin was equally accessible for ITA- and Ctrl-LNP groups at Padi4, a gene encoding for peptidyl arginine deiminase 4, that has been previously identified in activated neutrophils to be responsible for the formation of neutrophil extracellular traps.44 The decreased NETosis without decreased Padi4 is likely a result of a different mechanism that ITA targets, such as reduced ROS production.31
In previous work, we documented Ptd-EG-ITA’s ability to deacetylate H3K27ac,16 which differs from the H4K12ac deacetylation effects by ITA-LNP that are observed in this work. Notably, deacetylation of histone 4 and histone 3 can have differing effects on chromatin structure and transcription,45 although both instances of ITA-LNPs were found to be anti-inflammatory and yielded similar outcomes (eg, decreased Il1b).16 The observed difference could be due to a number of factors. First, the lipid used to form the nanoparticle was changed to PG for this body of work, although we attribute the therapeutic effects to ITA. Second, these particles largely target neutrophils instead of macrophages, and in a different disease model than in our previous work. Ultimately, this is an intriguing finding that inspires future investigation.
In summary, targeting immunothrombosis, specifically neutrophils, has been shown to reduce thrombosis without increasing hemorrhagic risk. This study shows that ITA-loaded LNPs target neutrophils and alleviate thrombus burden via decreased NETosis and immunomodulation in mice and in human neutrophils, including via a new epigenetic mechanism, H4K12ac deacetylation. This supports the use of ITA-LNPs as a nonantigenic, anti-immunothrombotic therapy and informs the development of novel targeted strategies with histone deacetylation mode of action.
Limitations
This study has several limitations. First, ITA-LNPs were unable to completely prevent thrombosis in DVT mouse models. However, their ability to reduce inflammation and clot burden suggests that ITA-LNPs could serve as an adjunct therapy to traditional anticoagulants without additional risk of bleeding. Additionally, it remains unclear exactly how NETs are reduced by ITA-LNPs, although this is likely because of ITA’s effects of reducing ROS and otherwise lowering inflammation in neutrophils including IL-1β.
Acknowledgments
This work was supported by the National Heart, Lung, and Blood Institute (NHLBI) grants HL130516 and HL155450 (A.M.). L.D. was supported by the American Heart Association Predoctoral Fellowship grant number 898722. N.E.H. was supported by the Case Western Reserve University Medical Scientist Training Program training program funded to Case Western Reserve University by the National Institutes of Health (NIH) grant numbers T32GM007250 and T32GM152319. L.N. was supported by the NIH NHLBI grant number R01HL142647.
Authorship
Contribution: L.N. and A.M. conceptualized the study; L.D., N.E.H., L.E.S., and A.M. performed formal analysis; L.D. and A.M. acquired funding; L.D., N.E.H., O.P., C.A., S.L., A.L.S.A., and A.M. carried out the investigation; L.D., L.N., and A.M. performed the methodology; A.M. was responsible for project administration; L.D., O.P., and A.M. acquired the resources; A.M. supervised the study; L.D., N.E.H., O.P., L.N., and A.M. validated the study; L.D., N.E.H., L.N., and A.M. were responsible for visualization; L.D., N.E.H., L.N., A.L.S.A., and A.M. wrote the original draft; and L.D., N.E.H., L.E.S., L.N., A.L.S.A., and A.M. curated the data and reviewed and edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Andrei Maiseyeu, Case Western Reserve University, 2103 Cornell Rd, Room 4401A, Cleveland, OH 44106; email: axm1079@case.edu.
References
Author notes
The RNA sequencing data have been deposited in the Gene Expression Omnibus database (accession number GSE283897). The full gene lists are included in the data supplement.
Original data are available on request from the corresponding author, Andrei Maiseyeu (axm1079@case.edu).
The full-text version of this article contains a data supplement.