Key Points
RADA16 hydrogel reduced ESS and improved anemia-related laboratory parameters.
RADA16 hydrogel may be a safe and effective conservative option for epistaxis in HHT.
Visual Abstract
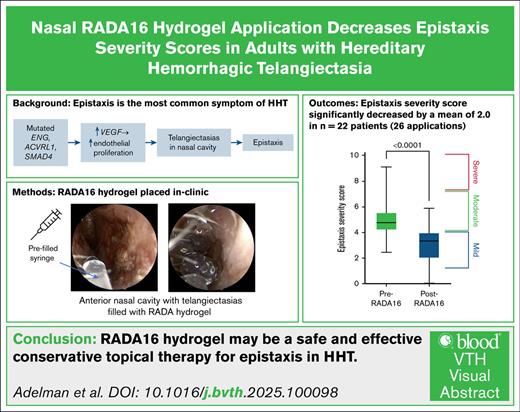
More than 90% of adults with hereditary hemorrhagic telangiectasia (HHT) experience epistaxis, which can be recurrent and cause significant morbidity. RADA16 is a self-assembling peptide hydrogel approved by the US Food and Drug Administration for hemostasis and wound healing. In this cohort study, we assessed the effectiveness of nasal RADA16 application in controlling HHT-related epistaxis. A retrospective chart review was performed on a cohort of adult patients who received nasal RADA16 without any other changes in treatment. Pre- and post-Epistaxis Severity Scores (ESS), hemoglobin, and hematocrit were collected to assess treatment response. Of the included patients (n = 22), there were 26 applications of RADA16. The cohort was 54.5% male and 86.4% White, had a mean age of 55.5 years, and had a predominantly ACVRL1 genotype (40.9%). Baseline mean ESS was 5.0 and decreased by an average of 2.0 at an average of 46.9 days after treatment, which is 2.8 times more than the minimal clinically important difference for ESS in HHT (0.71). The mean baseline hemoglobin (n = 13) and hematocrit (n = 10), were 11.3 g/dL and 37.5%, respectively, and did not exhibit significant changes after RADA16 application. No bleeding, pain, or allergic reactions occurred due to the application. Several patients reported mild nasal congestion. Our experience demonstrates that RADA16 can be considered as a safe epistaxis treatment modality for HHT that is easy to apply and does not require the operating suite.
Introduction
Hereditary hemorrhagic telangiectasia (HHT or Osler-Weber-Rendu syndrome) is the second most common hereditary bleeding disorder worldwide, affecting up to 1 in 5000 individuals.1-7 It is an autosomal dominant disorder primarily caused by loss-of-function mutations in genes that are involved in the signaling pathways of transforming growth factor beta and vascular endothelial growth factor; the most commonly mutated genes are ENG, ACVRL1, or SMAD4.1,5-8 The resulting defects in the vascular endothelium ultimately lead to the formation of visceral arteriovenous malformations and fragile mucocutaneous telangiectasias.4,5 The most commonly affected cutaneous sites include the fingers, hands, nailbeds, face, ears, and lips, whereas mucosal involvement mainly occurs in the nasal, buccal, and gastrointestinal mucosa.4-7 Clinical manifestations of HHT can range from mild skin telangiectasias to life-threatening complications such as gastrointestinal bleeding, iron deficiency anemia, portal hypertension, pulmonary hypertension, high-output cardiac failure, and intracranial hemorrhage.2,8,9
Over 90% of patients with HHT present with spontaneous and recurrent epistaxis, occasionally requiring emergent blood transfusions, and experience significantly diminished overall quality of life.8-10 Treatment of epistaxis in HHT remains a challenge; although several procedural and topical treatment options exist, each has its own shortcomings and are often inconsistently effective.4,8,9,11 The most recent International HHT Guidelines recommend escalating treatment as needed for refractory epistaxis from moisturizing topical therapies to oral tranexamic acid, ablative therapies, systemic angiogenic agents, septodermoplasty, or even nasal closure.4 A few of the many possible adverse effects of these treatments, particularly with procedural interventions, include further telangiectasia formation, thermal injury, septal perforation, intraoperative bleeding, and impairment of breathing.9,11,12
RADA16 is a synthetic peptide hydrogel that spontaneously self-assembles into a nanofiber scaffold upon exposure to biological fluids.13-18 This bioresorbable agent has been used in gastrointestinal and cardiovascular surgeries for years to enhance hemostasis, promote wound healing, and prevent adhesion formation.19,20 RADA16 received approval from the US Food and Drug Administration for sinus surgery in 2019, and has been successfully used for hemostasis and adhesion prevention in several types of otolaryngologic surgeries, with notable reports of its transparent application allowing for the visualization of incisional sites after placement.13,15,18 In this cohort study, we investigated the novel use of RADA16 for managing HHT-related epistaxis.
Methods
We performed a retrospective chart review of HHT patients 18 years or older that were treated for epistaxis within the University of Florida health system (Gainesville, FL) between January 2022 and December 2024. According to the Curacao criteria, the diagnosis of HHT was defined as meeting at least 3 of 4 criteria or positive genetic testing.21 We further refined this cohort to include those who received topical RADA16 hydrogel (3-D Matrix, Newton, MA) to reduce epistaxis. To isolate its treatment effects, we only included patients who were either not undergoing any systemic treatments (eg, doxycycline or tranexamic acid) or those who had been on stable doses of systemic treatments without any recent changes. RADA16 is manufactured in a prefilled syringe and ready for use. RADA16 was provided by 3-D Matrix at no cost. In all patients, RADA16 was applied to the anterior nasal cavity with endoscopic guidance in the outpatient setting. As is the case for all patients undergoing endoscopic examination or procedures, the nasal cavity was anesthetized locally with aerosolized 4% lidocaine and 0.05% oxymetazoline. Areas applied to included the bilateral septum, inferior turbinates, and lateral sidewalls, with a focus on regions with telangiectasias.
We collected demographic and clinical data, including genetic mutations, baseline epistaxis severity scores (ESS), and diagnostic laboratory results (hemoglobin and hematocrit). ESS, a validated epistaxis scoring index (0-10), quantifies the severity of epistaxis using the frequency, duration, severity, and medical impacts (ie, anemia and need for blood transfusions) of symptoms, which are stratified into the following categories: mild (1-4), moderate (4-7), and severe (7-10).22
We collected all ESS scores and labs that were available before and following RADA16 applications. In cases where there were multiple follow-up ESS scores, we only included these if there were no additional concomitant treatments outside of nasal moisturization. Changes in ESS scores and laboratory parameters were analyzed using paired t tests in GraphPad Prism 10.0 (La Jolla, CA). The significance level was set at 0.05. The minimal clinically important difference in the change in ESS in HHT is defined as 0.71.23
Results
Of the included patients (n = 22), there were 26 instances of RADA16 application, as 4 patients received 2 separate treatments. The cohort was 54.5% male, 86.4% White and had a mean age of 55.5 (standard deviation [SD], 17.2) years. The predominant genotype was ACVRL1 (n = 9) and the remainder were ENG (n = 7) or unknown (n = 6) due to lack of genetic testing. Baseline mean ESS from all patients was 5.0 (“moderate” severity), ranging from 2.4 to 9.1. The mean baseline hemoglobin (n = 13) and hematocrit (n = 10) based on available data, were 11.3 (SD, 2.8) g/dL and 37.5% (SD, 7.0), respectively. Baseline data and demographics are outlined in Table 1.
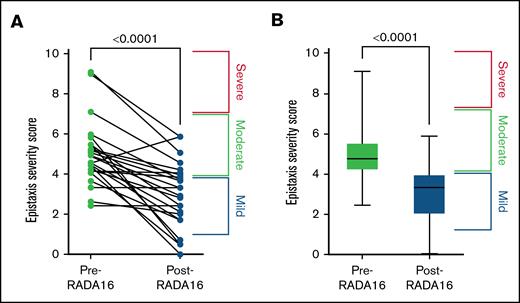
The mean change in ESS (P < .0001) was −2.0 (SD, 1.6), at a mean duration of 46.9 days post-treatment, to a mean ESS of 3.0 (SD, 1.6) (Figure 1A-B). This is 2.8 times the minimal clinically important difference. Hemoglobin and hematocrit changed as follows: +0.6 (P > .05) and −0.5 (P > .05). There was no bleeding or pain associated with the gel application. Adverse effects included short-term but self-resolving nasal congestion. Following application, the RADA16 hydrogel did not drip out of the nose, however some did extend down the back of the patients’ throat. Patients also did not report the gel coming out of their nose at any point at the follow-up visit. Change in the nasal mucosa following RADA16 application from a representative patient can be seen in Figure 2A-C.
Decreased ESS score post-RADA16. Change in ESS, represented on the individual patient (A) and cohort (B) level. Baseline ESS of 5.0 (SD, 1.6) decreased by a mean of 2.0 (SD, 1.6) to an ESS of 3.0 (SD, 1.6) at a mean duration of 46.9 days after treatment.
Decreased ESS score post-RADA16. Change in ESS, represented on the individual patient (A) and cohort (B) level. Baseline ESS of 5.0 (SD, 1.6) decreased by a mean of 2.0 (SD, 1.6) to an ESS of 3.0 (SD, 1.6) at a mean duration of 46.9 days after treatment.
Nasal mucosa before and after RADA16 application. Right nasal cavity of patient with HHT revealing (A) crusting and inflammation associated with HHT-related epistaxis, (B) Application of RADA16 in its hydrogel form combined with methylene blue for visualization (arrow). (C) Follow-up examination revealing well-moisturized nasal mucosa without inflammation, crusting, or bleeding.
Nasal mucosa before and after RADA16 application. Right nasal cavity of patient with HHT revealing (A) crusting and inflammation associated with HHT-related epistaxis, (B) Application of RADA16 in its hydrogel form combined with methylene blue for visualization (arrow). (C) Follow-up examination revealing well-moisturized nasal mucosa without inflammation, crusting, or bleeding.
Discussion
Epistaxis is the most common symptom of HHT, with consequences ranging from mild reductions in quality of life to life-threatening complications.10,12 There are a wide variety of treatment options available, each with varying degrees of efficacy and treatment-specific limitations. In this study, we observed that RADA16 hydrogel appears to improve short-term epistaxis control in patients with HHT, as evidenced by significant improvements in ESS scores. The proposed mechanism of action may be multifactorial, including maintaining moisture and forming a protective barrier over telangiectasias to reduce crusting and air-induced stress that can precipitate hemorrhage.24
RADA16 use in this context offers several advantages over other treatment options. Like other topical agents, it lacks systemic effects, eliminating concerns associated with systemic medications. This includes the need caution when prescribing antifibrinolytics in patients with atrial fibrillation or the thromboembolic risk and endocrinologic side effects of hormonal or antihormonal therapy.25,26
The delivery system and properties of RADA16 offer distinct advantages. The hydrogel is manufactured in a prefilled syringe, allowing for convenient and simple application requiring minimal training. Clinicians can apply it to specific telangiectasias or use it to cover larger areas. This versatility is particularly useful in cases where telangiectasias are diffusely spread, in which targeted therapies, such as sclerotherapy, are not designed to achieve.9 For anterior nasal lesions, RADA16 can be applied without endoscopic guidance, potentially allowing nonotolaryngology clinicians to offer this treatment.
Regarding safety, although there are no published studies on the use of RADA16 for HHT-related epistaxis, it is US Food and Drug Administration–approved for nasal and sinus surgery.13 In the limited studies on this application, the product appears to be safe, and furthermore promotes improved recovery following surgery.13,27,28 For instance, in a study by Lee et al,28 60 patients received RADA16 hydrogel following turbinate reduction, and they reported no postoperative bleeding or adhesion formation. This safety profile is an important consideration compared to surgical interventions like cautery or laser treatments, which can be effective, but carry risks of tissue damage.29,30 Conversely, RADA16 may instead be protective of tissue, as suggested by its current use in surgeries to improve wound healing.13-15
Finally, patient comfort and tolerability are important considerations when selecting a treatment modality. RADA16 can be applied in the outpatient setting and without any need for local anesthesia. In our cohort, it was common for patients to report temporary nasal congestion, which was resolved within a few hours. Concerns about nasal congestion did not prevent any patients from attempting this treatment. Importantly, no other adverse effects related to the hydrogel were reported.
Despite these promising findings, as RADA16 has not been rigorously investigated in this context previously, there are unknowns that need to be considered. For instance, although we observed significant improvements in epistaxis control, the precise duration of RADA16’s presence on the nasal mucosa remains unclear, especially considering the warm and moist environment of the nasal cavity. However, in a sheep model following endoscopic nasal surgery, Lee and Ananda31 observed no synechiae and enhanced histological regeneration of sinonasal mucosa at 6 weeks. These findings suggest that the hydrogel persists long enough to support mucosal healing without sloughing off, although this requires further investigation to be determined. Comparison to placebo has also yet to be considered. These are important elements to consider to best elucidate RADA16’s potential application and optimization.
Limitations of this study include its retrospective design and small sample size that limits the generalizability of our findings. Another important limitation exists regarding the primary outcome tool of the ESS as a recall instrument, as previous studies have reported a significant placebo effect related to its use.32 Additionally, retrospective observational studies of other agents (eg, doxycycline) have similarly shown significant improvements in ESS, but failed to be redemonstrated in randomized controlled trials (ie, NOSTRIL trial).33 Prospective randomized trials are crucial to adequately determine the benefit of RADA16 in HHT-related epistaxis management. Additionally, future research should investigate the ideal frequency of dosing and the potential for patient self-administration at home.
We have demonstrated the novel application of RADA16 hydrogel as a low-risk option for managing HHT-related epistaxis. Within the treatment algorithm for HHT-related epistaxis, RADA16 hydrogel may prove to fit as a conservative, outpatient intervention that can be offered before considering more invasive or higher-risk treatments.
Authorship
Contribution: A.A. performed data acquisition, data interpretation, and wrote and approved the final version of the manuscript; E.I. performed data acquisition, data interpretation, and wrote the manuscript; L.J. and A.P. performed data acquisition and wrote the manuscript; N.C., C.E., A.A., M.Z., J.K.M., C.A., and B.C.L. performed data acquisition, study design, and manuscript writing; and J.M.J. performed data acquisition, study design, manuscript writing, and approved the final version of the manuscript.
Conflict-of-interest disclosure: J.M.J., B.C.L., and J.K.M. are all consultants for 3-D Matrix, the manufacturer of the RADA16 hydrogel used in this study. The remaining authors declare no competing financial interests.
Correspondence: Jeb M. Justice, Department of Otolaryngology – Head &Neck Surgery, University of Florida, 1345 Center Dr, M228 MSB PO Box 100264 Gainesville, FL 32610; email: jeb.justice@ent.ufl.edu.
References
Author notes
Data are available on request from the corresponding author, Jeb M. Justice (jeb.justice@ent.ufl.edu).