Key Point
Platelet and MK apoptosis may be involved in the pathogenesis of ITP through a mechanism associated to CLU and its regulators, glucose-regulated protein 78, and glucose-regulated protein 94, potentially by activating Bax.
Visual Abstract
Abnormalities in the apoptosis pathway have been implicated into the pathogenesis of various autoimmune diseases, including immune thrombocytopenia (ITP). Our data suggest that mechanisms associated with impaired clusterin-mediated apoptosis might play a role in the pathophysiology of ITP platelets and their production by MKs. Platelet-rich plasma from 10 patients with ITP compared with healthy controls was used for the apoptosis proteomic profiling and clusterin (CLU) expression validation by reverse transcription polymerase chain reaction. We used the megakaryoblastic (MEG-01) cell line, treated for 2 hours with plasma from patients with newly diagnosed ND), chronic ITP or healthy controls and pan-caspase inhibitor (Z-VAD-FMK), apoptosis inducer ABT-737, rotenone (Rot), or rapamycin (Rap). Our apoptosis proteomic profiling revealed significantly increased expression levels of certain apoptotic genes such as CLU, phospho-p53 (S46), procaspase-3, and cleaved caspase 3 (CASP-3) in patients with ITP compared with healthy controls. Treatment with pan-caspase inhibitor or Rap had a significant downregulatory effect at messenger RNA (mRNA) level for CLU; CASP-3, -8, and -9; p53; and B-cell lymphoma-2–associated X protein (BAX) in ITP plasma–treated cells in comparison with control plasma–treated MEG-01 cells, whereas Rot or ABT-737 had opposite effects. We observed a significant downregulation of mRNA expression levels of these apoptotic markers in ITP plasma–treated and CLU or glucose-regulated protein 78 small interfering RNA–transfected MEG-01 cells. Our results indicate an upregulation of CLU and BAX in platelets and MKs which may contribute to deciphering the cause of platelet destruction in ITP disease.
Introduction
Immune thrombocytopenia (ITP) is an acquired autoimmune disease with a mostly benign course in children. ITP can occur in both children and adults and is more common in women than in men.1 It can present with severe mucocutaneous, in rare occasions even life-threatening bleeding symptoms.2 Classically, ITP is primarily caused by the production of autoantibodies against platelet cell surface molecules, such as the glycoprotein 2b/3a complex.2,3 In addition to autoantibody-mediated mechanisms, T-cell–mediated destruction of platelets, antiplatelet antibodies, and impaired megakaryopoiesis have been described as potential contributors to the low platelet counts that hallmark this disorder.3-5 Moreover, in vitro culture studies of both pediatric and adult ITP have indicated that ITP plasma could suppress both megakaryocytopoiesis and thrombopoiesis.6 This highlights that the pathophysiology of ITP is far more complex than previously thought.
Depending on its clinical course, ITP is classified into 3 categories: (1) newly diagnosed (ND) ITP, which is an acute transient form lasting up to 3 months; (2) persistent ITP (up to 12 months); and (3) chronic form (>12 months).3 In children, spontaneous remission is more common, resulting in a lower probability of disease recurrence and chronicity, whereas adolescents and adults tend to relapse and to develop chronic disease more often. The current goal of therapy is to increase platelet counts to prevent of subsequent hemorrhages. First-line therapies include IV immunoglobulins, oral corticosteroids, and anti-D immunoglobulins.2 However, these treatments have limitations, because they cannot impede or decrease the number of patients that develop persistent or chronic ITP.7
In nucleated cells, 2 major apoptosis pathways are known: the intrinsic apoptosis pathway, regulated by proapoptotic and antiapoptotic members of the B-cell lymphoma 2 (BCL-2) family in the mitochondria, and the extrinsic pathway that is initiated by the interaction between death ligands and death receptors (DR) (eg, Fas/CD95, tumor necrosis factor α [TNFα], TNF receptor 1, TNF receptor 2, the TNF–related apoptosis-inducing ligand receptors, and DR4 and DR5, and is regulated by death–inducing signaling complexes.8,9 Each of these pathways can be triggered by specific stimuli that first lead to activation of initiator caspases, that is, CASPASE-08 (CASP-8) and/or CASP-10 (extrinsic pathway) and CASP-9 (intrinsic pathway), and then of executor CASP-3, -6, and -7. The BCL-2 family of critical regulators and effectors are divided in 3 groups: (1) the multidomain killers BAK1 and B-cell lymphoma-2–associated X protein (BAX), as effectors of the intrinsic pathway; (2) prosurvival proteins (BCL2, BCL-XL, BCL2L2, MCL1, and BCL2A1) that prevent the activation of BAK1 and BAX; and (3) proapoptotic BH3-only proteins (Bcl-2-interacting mediator of cell death [BIM], BH3-interacting domain death agonist [BID], BCL2-associated agonist of cell death [BAD], p53 upregulated modulator of apoptosis [PUMA], BH3-only protein [NOXA], Bcl-2-modifying factor [BMF], Bcl-2-interacting killer [BIK], and activator of apoptosis harakiri [HRK]). Defects in apoptotic cell clearance can induce autoimmunity.10,11 The apoptotic signals change the permeability of the outer mitochondrial membrane, leading to the release of the apoptotic factors from the mitochondrial intermembrane space, such as cytochrome c, second mitochondria derived activator of caspases/direct IAP binding protein with low PI (SMAC/Diablo), high-temperature requirement HtrA2 serine protease (OMI), endonuclease G, and apoptosis-inducing factor.12,13 In platelets, as anucleated cells, apoptosis is mainly controlled by the intrinsic pathway through the interaction between proapoptotic Bcl-2 homologous antagonist/killer (BAK) and Bcl-2-like protein 4 (BAX) and the antiapoptotic prosurvival B-cell lymphoma-extra large (BCL-XL).14 The proapoptotic and proapoptotic multidomain of the BCL-2 family proteins regulates the entire platelet life span, whereas BID and BIM are dispensable for BAX activation and mitochondrial apoptosis.15 However, we, and others, have not been able to demonstrate a role of receptor activation or of a “death ligand” initiating platelet apoptosis via the extrinsic apoptosis pathway.9 It remains unclear which or how proapoptotic signals induce apoptosis in platelets or even in their precursors, megakaryocytes (MKs).16 Previous studies, also from our laboratory, have reported the role of platelet apoptosis in the pathogenesis of ITP, demonstrating CASP-3, -8, and -9 activation in acute ITP8 and increased phosphatidylserine (PS) exposure in chronic ITP.8,14,17 A murine model of ITP has shown that the profound thrombocytopenia resulting from the injection of anti-glycoprotein 2b antibodies is accompanied by apoptosis-like events in the platelets.18 Further studies are needed to fully understand the mechanisms underlying these observations.
Clusterin (CLU) is a stress-activated chaperone that is highly expressed in Alzheimer disease and cancer and has been implicated in the pathogenesis of several protein aggregopathies and cancer entities.19,20CLU can limit the severity of certain autoimmune diseases such as autoimmune myocarditis, systemic lupus erythematosus, and rheumatoid arthritis.21CLU binds to histones expressed by late apoptotic cells and is involved in their clearance.21 Intracellular CLU localized to endoplasmic reticulum (ER) or mitochondria can directly bind to the proapoptotic BAX protein.22,23 During stressful conditions, it can inhibit apoptosis by alleviating protein aggregation, and enhance Akt phosphorylation and transactivation of NF-κB.24 Whether CLU is a prosurvival or a proapoptotic molecule remains unclear, because it is likely to have dual functions.23CLU inhibitors are currently being investigated in clinical trials for lung and prostate cancer.24CLU has different isoforms with distinct cellular or subcellular localizations in the brain.25 The CLU gene comprises 11 exons (of which 2 are untranslated), spanning a region of 18 115 base pairs in humans. Transcription of CLU results in the production of 3 messenger RNA (mRNA) isoforms in humans and mice.25 The 2 primary isoforms, CLU1 and CLU2, first reported in the human brain, share exon 2 to 9 but differ in exon 1 and proximal promoters and present an increased expression in Alzheimer disease neuropathology.19,23CLU was detected in α-granules of platelets and in bone marrow–derived MKs, most probably produced and packaged into the α-granules during MK development.26 Previous reports showed that CLU can interact with glucose-regulated protein (GRP78), a central regulator of unfolded protein response, playing an important role in cellular adaptation and survival under stress conditions, in particular in ER stress.27,28
We first investigated which markers of the extrinsic and intrinsic apoptosis pathway may be involved in platelet apoptotic responses in ND and chronic ITP. We then validated the observations from the apoptotic proteomic array and further explored whether (1) CLU may be involved in apoptosis mechanisms seen in ITP platelets and MKs treated with ITP plasma, and (2) these effects could be reversed by RNA interference using small interfering RNA (siRNA) transfections targeting CLU and GRP78.
Materials and methods
Patient characteristics
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the ethics committee of Canton Zurich, Switzerland (protocol code reference STV 11/07 and 2023-01147). Written informed consent was obtained from all healthy blood donors as well as from patients with ITP, or their legal guardians. All patients were recruited at the University Children’s Hospital Zurich, Zurich, Switzerland. All methods are reported in accordance with ARRIVE (Animal Research: Reporting of In Vivo Experiments) guidelines.
All patients fulfilled the criteria for primary ITP (as defined elsewhere8,11). In total, we enrolled 12 patients with ND ITP presenting a median platelet count of 6.35 × 109/L ± 5.79 × 109/L, a median age of 12.36 ± 5.20 years, and a sex ratio of 8 females (F) to 4 males (M); and 15 patients with chronic ITP (median platelet count of 77.60 ± 86.46 [× 109/L]); median age of 11.0 ± 4.14 years; sex ratio of 8:7 [F:M]) presenting a persistent low platelet count (<100 × 109/L), which lasted longer than 1 year. Children with ND and chronic ITP received a dose of 0.8 g of IV immunoglobulin per kg of body weight (0.8 g/kg per dose). In addition, we included 11 healthy pediatric controls with a median platelet count of 263.5 × 109/L ± 125.5 × 109/L and a median age of 10.5 ± 7.01 years (5:6 [F:M]; as summarized in Table 1). Overall, the plasma from healthy control derived from younger individuals than the one from the patients with ND or chronic ITP.
Summary of patient characteristics
| . | No. of patients . | Median age (y) . | Median platelet count . | Sex ratio (F:M) . |
|---|---|---|---|---|
| Acute ITP | 12 | 12.36 ± 5.20 | (6.35 ± 5.79) × 109/L | 8:4 |
| Chronic ITP | 15 | 11.0 ± 4.14 | (77.60 ± 86.46) × 109/L | 8:7 |
| Healthy controls | 11 | 10.5 ± 7.01 | (263.5 ± 125.5) × 109/L | 5:6 |
| . | No. of patients . | Median age (y) . | Median platelet count . | Sex ratio (F:M) . |
|---|---|---|---|---|
| Acute ITP | 12 | 12.36 ± 5.20 | (6.35 ± 5.79) × 109/L | 8:4 |
| Chronic ITP | 15 | 11.0 ± 4.14 | (77.60 ± 86.46) × 109/L | 8:7 |
| Healthy controls | 11 | 10.5 ± 7.01 | (263.5 ± 125.5) × 109/L | 5:6 |
Preparation of PRP
The citrated blood was centrifuged at 150g for 20 minutes at room temperature (RT). Platelet-rich plasma (PRP) was collected in a fresh 15-mL Falcon tube and was centrifuged at 150g for 10 minutes at RT. The supernatant was carefully pipetted into a fresh tube, leaving the PRP pellet undisturbed. The PRP pellet was further processed or placed into a freezer at −80°C.
Peripheral blood mononuclear cell (PBMC) isolation
The blood was diluted 1:1 with RPMI 1640 medium (Sigma-Aldrich, Merck KGaA, Darmstadt, Germany) and then resuspended. The diluted blood was carefully added to 5 mL of Ficoll (Sigma-Aldrich) in a 15-mL tube. The tube was centrifuged for 20 minutes at 800g. After the centrifugation, the supernatant was carefully removed and the PBMC monolayer was collected and placed in a new 15-mL tube. The cells were once washed with phosphate-buffered saline and centrifuged for 5 minutes at 400g at RT. RPMI 1640 medium (1 mL) was added to the PBMC pellet, resuspended, and the cells were counted.
Proteomic apoptosis profiling
We used the proteome profiler human apoptosis array kit from R&D Systems (Minneapolis, MN; catalog no. ARY009) to assess apoptosis and apoptosis-related proteins, according to manufactures instructions. In brief, the PRP pellet was lysed in lysis buffer and the protein concentration was measured using a Pierce bicinchoninic acid protein assay kit (Thermo Fisher Scientific, Schlieren, Switzerland; catalog no. 23225) and a Tecan Infinite 200 plate reader (Tecan Group Ltd, Männedorf, Switzerland). The membranes were blocked with blocking solution and incubated overnight with the cell lysate (37.5 μg). After the washing steps, the membranes were incubated with the biotinylated detection antibody cocktail and conjugated with streptavidin–horseradish peroxidase. To detect the signal intensity after adding chemiluminescent reagents we used the ChemiDoc Touch Imaging System (Bio-Rad, Hercules, CA). For the analysis of the results we used Bio-Rad ImageLab 5.2.1 software.
RNA extraction and quantitative reverse transcription polymerase chain reaction (qRT-PCR) analysis
Total RNA was extracted from platelets and PBMCs using an RNeasy mini kit from Qiagen (catalog no. 74104; Qiagen Inc, Germantown, MD) as recommended by the manufacturer. The extracted RNA was reverse transcribed using the QuantiTect Reverse Transcription Kit from Qiagen (catalog no. 205311) as recommended by the manufacturer. qRT-PCR was performed in 96-well fast thermal cycling plates (Thermo Fisher Scientific, Schlieren, Switzerland) using PowerUp SYBR Green Master Mix (Thermo Fisher Scientific; catalog no. A25742) and primers with specific sequences as mentioned in supplemental Table 1 and Table 2.
List of primers used to perform qRT-PCR
| Gene name . | Primer sequence . |
|---|---|
| Clusterin _total_forward | 5′-CTA TCT GCG GGT CAC CA-3′ |
| Clusterin _total_reverse | 5′-CTC AGT GAC ACC GGA AGG AAC-3′ |
| Clusterin_1_ forward | 5′-GCG AGC AGA GCG CTA TAA AT-3′ |
| Clusterin_1_ reverse | 5′-GAT GCG GTC ACC ATT CAT C-3′ |
| Clusterin_2_ forward | 5′-AGA TGG ATT CGG TGT GAA GG-3′ |
| Clusterin_2_ reverse | 5′-GAT GCG GTC ACC ATT CAT C-3′ |
| 18S_forward | 5′-GTT CCG ACC ATA AAC GAT GCC-3′ |
| 18S_reverse | 5′-TGG TGG TGC CCT TCC GTC AAT-3′ |
| Clusterin | qHsaCED0043884 (Bio-Rad) |
| GRP78 | qHsaCED0001784 (Bio-Rad) |
| GRP94_forward | 5′-ACT GTT GAG GAG CCC ATG GAG-3′ |
| GRP94_reverse | 5′-GCT GAA GAG TCT CGC GGG AAA-3′ |
| Bax_forward | 5′-CCA GCA AAC TGG TGC TCA AG-3′ |
| Bax_reverse | 5′-GGA GGC TTG AGG AGT CTC AC-3′ |
| CASP-3_forward | 5′-TCT GGT TTT CGG TGG GTG TG-3′ |
| CASP-3_reverse | 5′-CGC TTC CAT GTA TGA TCT TTG-3′ |
| CASP-8_forward | 5′-CTG CTG GGG ATG GCC ACT GTG-3′ |
| CASP-8_reverse | 5′-TCG CCT CGA GGA CAT CGC TCT-3′ |
| CASP-9 | qHsaCED00444 (Bio-Rad) |
| p53_forward | 5′-GTG GAA GGA AAT TTG CGT-3′ |
| p53_reverse | 5′-CCA GTG TGA TGA TGG TGA-3′ |
| Gene name . | Primer sequence . |
|---|---|
| Clusterin _total_forward | 5′-CTA TCT GCG GGT CAC CA-3′ |
| Clusterin _total_reverse | 5′-CTC AGT GAC ACC GGA AGG AAC-3′ |
| Clusterin_1_ forward | 5′-GCG AGC AGA GCG CTA TAA AT-3′ |
| Clusterin_1_ reverse | 5′-GAT GCG GTC ACC ATT CAT C-3′ |
| Clusterin_2_ forward | 5′-AGA TGG ATT CGG TGT GAA GG-3′ |
| Clusterin_2_ reverse | 5′-GAT GCG GTC ACC ATT CAT C-3′ |
| 18S_forward | 5′-GTT CCG ACC ATA AAC GAT GCC-3′ |
| 18S_reverse | 5′-TGG TGG TGC CCT TCC GTC AAT-3′ |
| Clusterin | qHsaCED0043884 (Bio-Rad) |
| GRP78 | qHsaCED0001784 (Bio-Rad) |
| GRP94_forward | 5′-ACT GTT GAG GAG CCC ATG GAG-3′ |
| GRP94_reverse | 5′-GCT GAA GAG TCT CGC GGG AAA-3′ |
| Bax_forward | 5′-CCA GCA AAC TGG TGC TCA AG-3′ |
| Bax_reverse | 5′-GGA GGC TTG AGG AGT CTC AC-3′ |
| CASP-3_forward | 5′-TCT GGT TTT CGG TGG GTG TG-3′ |
| CASP-3_reverse | 5′-CGC TTC CAT GTA TGA TCT TTG-3′ |
| CASP-8_forward | 5′-CTG CTG GGG ATG GCC ACT GTG-3′ |
| CASP-8_reverse | 5′-TCG CCT CGA GGA CAT CGC TCT-3′ |
| CASP-9 | qHsaCED00444 (Bio-Rad) |
| p53_forward | 5′-GTG GAA GGA AAT TTG CGT-3′ |
| p53_reverse | 5′-CCA GTG TGA TGA TGG TGA-3′ |
Cell cultures
The human megakaryoblast leukemic cell line MEG-01 (Sigma-Aldrich, ECACC 94012401) was maintained in RPMI 1640 medium (Gibco, Thermo Fisher Scientific) supplemented with 10% of heat-inactivated fetal bovine serum (Sigma-Aldrich; catalog no. B9433). Cells were cultured in an incubator at 37°C in a 5% CO2 humidified atmosphere.
siRNA transfection and plasma treatment
For siRNA transfections and chemical treatments, 40 000 cells per well were plated in a 24-well plate. MEG-01 cells were incubated with siRNAs gene against CLU (sc-43688) and GRP78 (sc-29338), or negative control siRNA (sc-44230, using siRNA transfection reagent [sc-29528], siRNA transfection medium [sc-36868], and siRNA dilution buffer [sc-29527], purchased from Santa Cruz Biotechnology Inc (Dallas, TX) for 48 hours according to the manufacturer’s instructions. Two hours before harvesting, 40 μL of PRP plasma from a healthy control, a patient with ND ITP, or a patient with chronic ITP were added to the cells. Cells were then collected and stored at −80°C until further processing.
MEG-01 cells were incubated with pan-CASP inhibitor Z-VAD-FMK (R&D Systems, MN; catalog no. FMK001) up to a concentration of 10 μM, 10 μM rapamycin (Rap; BioGems International Inc, Westlake Village, CA), or 10 μM rotenone (Rot; Sigma-Aldrich, catalog no. R8875), or with 7 μM ABT-737 (MedChemExpress, Monmouth Junction, NJ; catalog no. HY-5097) for 2 hours. Afterward, 20 μL of plasma of a patient with ND ITP, a patient with chronic ITP, or a healthy control was added to the cells and incubated for 2 hours. Harvested cells were stored at −80°C until further processing.
Statistical analysis
Our data were statistically analyzed by using Prism 8.00 (GraphPad Software, San Diego, CA). Data are presented as mean ± standard deviation from 3 independent experiments, unless otherwise mentioned. Data were analyzed using t tests or 1-way analysis of variance, followed by multiple comparisons tests to compare the means between the groups, and P value < .05 was considered significant.
Results
Proteomic apoptosis profiling in ITP
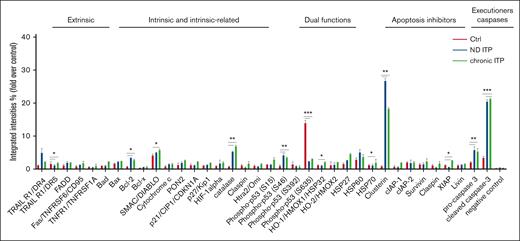
We investigated the apoptotic proteomic profiling of platelets in both patients with ND and those with chronic ITP compared with healthy controls (Figure 1). Our analysis revealed significant upregulation of several proteins involved in intrinsic or extrinsic apoptotic regulation, such as BCL-2, X-linked inhibitor of apoptosis protein, TNF-related apoptosis-inducing ligand R1/death receptor 4, CLU, second mitochondria-derived activator of CASP/direct inhibitor of apoptosis protein-binding protein (SMAC/DIABLO), phospho-p53 (S46), procaspase-3, and cleaved CASP-3 in ITP platelets when compared with healthy control platelets (Figure 1). This observation suggests that apoptotic mechanisms could be activated in the platelets from both patients with ND ITP and/or those with chronic ITP compared with platelets from healthy controls.
Apoptosis proteomic profiling in healthy controls, and patient with either ND or chronic ITP. Platelets isolated from fresh PRP were analyzed on a human apoptosis antibody array for the presence of apoptotic proteins (extrinsic, intrinsic, intrinsic-related, dual functions, and apoptosis inhibitors and executioners). The integrated densities of the dot blot assays were calculated according to the dot blot analysis (Bio-Rad ImageLab 5.2.1) and were normalized to the positive control (representing 100%).
Apoptosis proteomic profiling in healthy controls, and patient with either ND or chronic ITP. Platelets isolated from fresh PRP were analyzed on a human apoptosis antibody array for the presence of apoptotic proteins (extrinsic, intrinsic, intrinsic-related, dual functions, and apoptosis inhibitors and executioners). The integrated densities of the dot blot assays were calculated according to the dot blot analysis (Bio-Rad ImageLab 5.2.1) and were normalized to the positive control (representing 100%).
CLU gene expression in ITP platelets and PBMCs
We validated the CLU protein expression results in platelets isolated from both patients with ND ITP and those with chronic ITP at mRNA level by qRT-PCR (Figure 2). Our results showed a significant increase in CLU levels, including the 2 isoforms CLU1 and CLU2, in both groups of patients with ITP compared with healthy controls.
mRNA expression levels of CLU and its isoforms CLU1 and CLU2 in platelets from groups of patients with ITP compared with healthy controls. Total RNA from platelets was isolated and subjected to qRT-PCR with 18S ribosomal RNA as internal control. Relative mRNA levels of each sample normalized to healthy controls are shown as mean ± standard deviation (SD). Statistical analyses were performed using 1-way analysis of variance (ANOVA) followed by multiple comparisons tests to compare the mean ranks between the groups. Means are significantly different with P < .001 (highly significant).
mRNA expression levels of CLU and its isoforms CLU1 and CLU2 in platelets from groups of patients with ITP compared with healthy controls. Total RNA from platelets was isolated and subjected to qRT-PCR with 18S ribosomal RNA as internal control. Relative mRNA levels of each sample normalized to healthy controls are shown as mean ± standard deviation (SD). Statistical analyses were performed using 1-way analysis of variance (ANOVA) followed by multiple comparisons tests to compare the mean ranks between the groups. Means are significantly different with P < .001 (highly significant).
Moreover, we also observed that CLU levels were significantly lower in PBMCs from patients with ND or chronic ITP compared with healthy controls, suggesting that the increased expression is rather specific to platelets (supplemental Figure 1).
CLU activation mechanisms in ITP plasma–treated MKs (MEG-01 cell line)
Next, we performed a further in-depth molecular analysis to determine which mechanisms are involved in the CLU–mediated apoptotic death of platelets in ITP disease and whether MKs, as precursors of platelets, show similar changes as in platelets.
We used the megakaryoblastic cell line MEG-01 first treated with ITP or control plasma. In line with the proteomic data, we were able to confirm the upregulation of all investigated proteins, being significant for CLU, GRP-94, CASP-3, CASP-8, and BAX in the MEG-01 cell line treated with plasma derived from patients with ITP compared with plasma isolated from blood of healthy individuals (Figure 3).
Effect of ITP vs control plasma treatment on the expression of apoptosis genes in MEG-01 cells. Apoptosis genes were significantly upregulated in ITP plasma–treated compared with the control plasma–treated and untreated cell conditions. Relative mRNA levels of each sample normalized to control condition (pan-caspase inhibitor [CI]) are shown as mean ± SD. Statistical analyses were performed using 1-way ANOVA followed by multiple comparisons tests to compare the mean ranks between the groups.
Effect of ITP vs control plasma treatment on the expression of apoptosis genes in MEG-01 cells. Apoptosis genes were significantly upregulated in ITP plasma–treated compared with the control plasma–treated and untreated cell conditions. Relative mRNA levels of each sample normalized to control condition (pan-caspase inhibitor [CI]) are shown as mean ± SD. Statistical analyses were performed using 1-way ANOVA followed by multiple comparisons tests to compare the mean ranks between the groups.
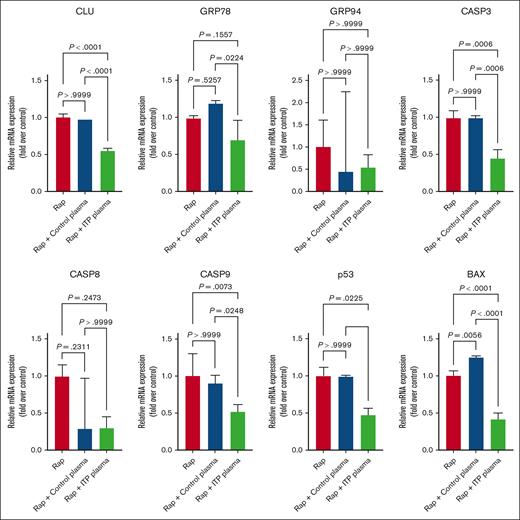
To determine the effects of different apoptosis inducers on MEG-01 treated cells, we first used the mechanistic target of rapamycin (mTOR) complex 1 inhibitor Rap, which is known to induce apoptosis and autophagy in platelets.29-31 We observed a significant downregulation at mRNA level for CLU; caspase-3 (CASP-3), -8, and -9; TP53; and BAX in ITP plasma–treated cells in comparison with the control plasma–treated or non-plasma-treated cells (Figure4). The mRNA levels of GRP78 were significantly downregulated in ITP plasma vs control plasma–treated cells only, whereas the downregulation of the mRNA levels of CASP-8 in ITP plasma was not significant (Figure 4).
Effects of Rap on apoptosis genes in MEG-01 cells.CLU, CASP-3, TP53, and BAX were significantly downregulated after ITP plasma and Rap treatment compared with in the other 2 control conditions. Relative mRNA levels of each sample normalized to control condition (Rap) are shown as mean ± SD. Statistical analyses were performed using 1-way ANOVA followed by multiple comparisons tests to compare the mean ranks between the groups.
Effects of Rap on apoptosis genes in MEG-01 cells.CLU, CASP-3, TP53, and BAX were significantly downregulated after ITP plasma and Rap treatment compared with in the other 2 control conditions. Relative mRNA levels of each sample normalized to control condition (Rap) are shown as mean ± SD. Statistical analyses were performed using 1-way ANOVA followed by multiple comparisons tests to compare the mean ranks between the groups.
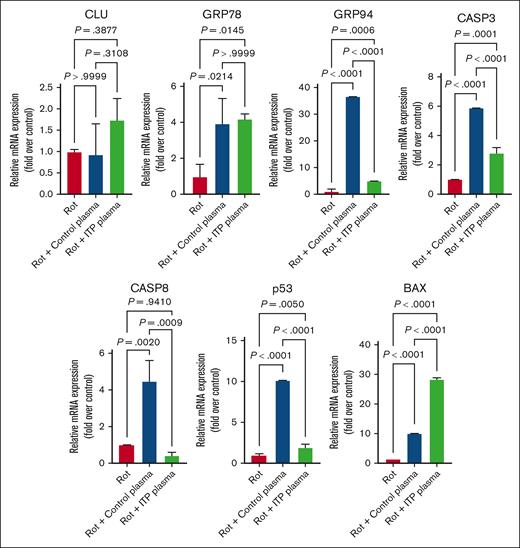
Secondly, we used Rot, a known apoptosis inducer,32,33 to assess changes in MEG-01–treated cells. In contrast to Rap, we detected a significant upregulation at mRNA level for GRP78, CASP-3, p53, and BAX in control and ITP plasma–treated cells in comparison with control cells. The mRNA expression of CLU in ITP plasma–treated cells was increased compared with control plasma but it did not show a significance (Figure 5). The mRNA levels of CASP-8 were decreased in ITP plasma–treated cells compared with control plasma– and Rot-only–treated cells (Figure 5).
Effects of Rot on apoptosis genes in MEG-01 cells. Rot treatment had increasing effects on all analyzed apoptosis genes except CASP-8 in both control and ITP plasma groups compared with in the control condition. Relative mRNA levels of each sample normalized to control condition (Rot) are shown as mean ± SD. Statistical analyses were performed using 1-way ANOVA followed by multiple comparisons tests to compare the mean ranks between the groups.
Effects of Rot on apoptosis genes in MEG-01 cells. Rot treatment had increasing effects on all analyzed apoptosis genes except CASP-8 in both control and ITP plasma groups compared with in the control condition. Relative mRNA levels of each sample normalized to control condition (Rot) are shown as mean ± SD. Statistical analyses were performed using 1-way ANOVA followed by multiple comparisons tests to compare the mean ranks between the groups.
Next, to elucidate whether the apoptotic death could be CASP mediated, we performed suppressing apoptosis experiments by treating with pan-CASP inhibitors (Z-VAD-FMK; Promega) and inducing apoptosis experiments by treating with ABT-737, an inhibitor of BCL-2 family proteins, that prevents the sequestration of proapoptotic molecules. We quantified the transcription levels of the aforementioned apoptotic regulatory markers in both untreated and treated cells (Figure 6). As expected, ITP plasma–treated cells exhibited an upregulation of all apoptosis markers expression upon ABT-737 treatment, which could be reversed when using the pan-CASP inhibitor (Figure 7). These results indicate that the apoptosis-induced mechanisms in ITP could be CASP and BCL-2 dependent.
Effects of ABT737 on apoptosis genes in MEG-01 cells. Apoptosis genes were significantly upregulated after ITP plasma and ABT737 treatment compared with in the other 2 control conditions. Relative mRNA levels of each sample normalized to control condition (ABT737) are shown as mean ± SD. Statistical analyses were performed using 1-way ANOVA followed by multiple comparisons tests to compare the mean ranks between the groups.
Effects of ABT737 on apoptosis genes in MEG-01 cells. Apoptosis genes were significantly upregulated after ITP plasma and ABT737 treatment compared with in the other 2 control conditions. Relative mRNA levels of each sample normalized to control condition (ABT737) are shown as mean ± SD. Statistical analyses were performed using 1-way ANOVA followed by multiple comparisons tests to compare the mean ranks between the groups.
Effects of CI on apoptosis genes. Apoptosis genes were significantly downregulated in ITP and control plasma and CI treatment compared with in the control condition. Relative mRNA levels of each sample normalized to control condition are shown as mean ± SD. Statistical analyses were performed using 1-way ANOVA followed by multiple comparisons tests to compare the mean ranks between the groups.
Effects of CI on apoptosis genes. Apoptosis genes were significantly downregulated in ITP and control plasma and CI treatment compared with in the control condition. Relative mRNA levels of each sample normalized to control condition are shown as mean ± SD. Statistical analyses were performed using 1-way ANOVA followed by multiple comparisons tests to compare the mean ranks between the groups.
CLU and GRP78 siRNA knockdown in ITP plasma–treated MEG-01 cells
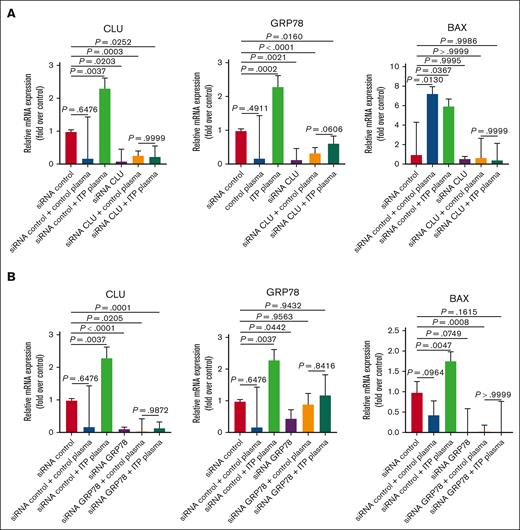
Next, we investigated the CLU function in apoptosis by blocking its expression through RNA interference, using CLU siRNA in both ITP plasma– and healthy control plasma–treated MEG-01 cells. The transfected cells were tested for the expression of CLU, GRP78, and BAX, showing a significant downregulation after ITP plasma treatment compared with untreated or control plasma treatment (Figure 8A). To further investigate whether targeting GRP78 has similar effects to that of CLU, we performed siRNA transfections against GRP78 in the ITP plasma– and healthy control plasma–treated MEG-01 cells. We observed similar significant downregulatory effects on the mRNA expression of CLU, GRP78, and BAX (Figure 8B), suggesting that these 2 proteins could have synergistic effects and may be involved in similar mechanisms underlying the pathogenesis of ITP.
Effects of siRNA transfections targeting CLU and GRP78 in MEG-01 cells. mRNA levels of CLU, GRP78, and BAX determined by qRT-PCR in MEG-01 cells treated with plasma from patients with ITP compared with plasma from healthy controls, and transfected with siRNA targeting (A) CLU or (B) GRP78 are shown. Statistical analyses were performed using 1-way ANOVA followed by multiple comparisons tests to compare the mean ranks between groups. Expression levels are shown as mean; error bars show SDs and P values.
Effects of siRNA transfections targeting CLU and GRP78 in MEG-01 cells. mRNA levels of CLU, GRP78, and BAX determined by qRT-PCR in MEG-01 cells treated with plasma from patients with ITP compared with plasma from healthy controls, and transfected with siRNA targeting (A) CLU or (B) GRP78 are shown. Statistical analyses were performed using 1-way ANOVA followed by multiple comparisons tests to compare the mean ranks between groups. Expression levels are shown as mean; error bars show SDs and P values.
Discussion
The pathogenic mechanisms underlying ITP, a highly complex autoimmune disease characterized by low platelet counts, remain poorly understood. We initially used a proteomic approach to investigate the protein expression of specific apoptotic markers in platelets from both patients with ND ITP and those with chronic ITP compared with healthy controls. Subsequently, we selected the highly upregulated CLU to further study which potential molecular mechanisms may lead to apoptosis CLU activation, by using different apoptosis inhibitors or inducers as well as siRNA knockdown targeting CLU.
The involvement of either MK or platelet apoptosis in the pathogenesis of ITP has been the subject of previous research (as reviewed elsewhere34), yielding contradictory or inconsistent results, for example, reporting that various regulators such as AKT signaling, BCL-XL and BAX-mediated apoptosis, or the PI3K/AKT/mTOR pathway may be involved.14,35-40 Our prior research reported the activation of CASP and PS exposure in ITP platelets,8,9,11,41 but the exact mechanism is not yet fully revealed. In the current study, we observed that ITP plasma treatment of the megakaryoblastic cell line MEG-01, resulted in the activation of certain apoptotic markers, including CLU, an effect that could be reversed using either chemical inhibitors or siRNA transfection. Another previous study using plasma treatment, showed decreased expression of BCL-XL and increased expression of BAX, and activity of CASP-3 in ITP.37 Deng et al42 indicated a significant increase in both surface-exposed PS in platelets and in the expression levels of BAK and BAX in patients with chronic ITP compared with healthy controls. These results suggest an increased activation of the apoptosis pathway in ITP platelets, aligning with our results. However, it has been shown previously that ITP plasma is not only involved in platelet destruction but may also inhibit platelet production, because autoantibodies from the majority of the patients with ITP can inhibit in vitro proplatelet formation by MKs and their subsequent ability to produce platelets.43,44
CLU has been implicated in several disease mechanisms, affecting pathways such as BAX-mediated apoptosis,22PI3K-AKT, and GSK-3β signaling,45 as well as oxidative stress.46,47 We provide evidence, to our knowledge, for the first time, that CLU–mediated proapoptotic mechanisms could be involved in the pathogenesis of ITP. We validated our proteomic results by confirming the upregulation of CLU at both mRNA and protein levels in ITP platelet samples compared with healthy controls. This upregulation was specific to ITP platelets and not observed in PBMCs, implying that CLU could trigger an apoptotic response in platelets specifically. CLU has previously been detected predominately in human platelets, also by immunohistochemistry and in MKs from the bone marrow by in situ hybridization.26,48 Although Tschopp et al26 concluded that CLU is likely produced during MK development, our evidence at mRNA level suggest a CLU activation and regulation in platelets as well as in MKs in ITP compared with in healthy control samples. Mechanistically, GRP78 and CLU are known to directly interact under ER stress conditions, facilitating its redistribution to the mitochondria and trafficking.27,28 Interestingly, we could show an increase in the expression of 2 ER luminal chaperones, GRP78 and GRP94, in MKs treated with ITP plasma compared with control plasma. Moreover, we could downregulate their mRNA expression to control levels using Rap or siRNA targeting either CLU or GRP78, suggesting that they could act synergistically. Thereby, our results suggest that the ER-stress–induced apoptosis might play a role in the mechanisms underlying the upregulation of CLU in ITP platelets (as highlighted in supplemental Figure 2).
Interestingly, Zhang et al22 showed that CLU can inhibit apoptosis by interfering with the activation of BAX in mitochondria. Specifically, CLU was found to interact with conformation-altered BAX upon treatment with chemotherapeutic drugs. In agreement with this, our experiments showed the same response pattern of CLU and BAX in MEG-01 cells treated with ITP plasma and apoptosis inducers Rot or ABT-737, suggesting that the elevated levels of CLU could interfere with BAX proapoptotic activity. Thus, it will be of interest to elucidate whether disrupting the CLU–BAX interaction may be a possible strategy not only in cancer therapy but also in ITP disease or other autoimmune disorders (as highlighted in supplemental Figure 2).
Rap, also named sirolimus, specifically targets mTOR, a conserved serine-threonine kinase member of the PI3K kinase family regulating cell survival, proliferation, and differentiation.49 It has been reported as a treatment for primary and secondary ITP, as well as for other autoimmune disorders such as autoimmune lymphoproliferative syndrome, systemic lupus erythematosus, or primary antiphospholipid syndrome.49 Because clinical evidence supporting the use of Rap in ITP is growing, we interrogated its effects on key apoptotic genes expression including CLU in the MEG-01 cell line treated with ITP plasma. The downregulation at mRNA levels of all investigated apoptosis genes including CLU, GRP78, CASP-3 and -8, BAX, and TP53 upon ITP plasma and Rap treatment vs their upregulation after ABT-737 or Rot treatment might suggest that Rap could have an antiapoptotic effect in ITP. These results could be supported by the positive effects of Rap in the clinical context of ITP, however it is in contrast with other reports on various cell types, such as retinoblastoma cells,50 rhabdomyosarcoma cell lines,51 or in childhood acute lymphoblastic leukemia cells,52 in which Rap was reported to induce apoptosis. This discrepancy might be because of different targeting mechanisms other than mTOR, such as PI3K/AKT signaling, or p53-dependent mechanisms. Therefore, future studies are required to elucidate the impact of these apoptogenic stimuli on MK or platelet survival.
In conclusion, our findings strongly suggest that the modulation of CLU and its regulators, GRP78 and GRP94, might have a role in the pathogenesis of ITP, potentially through a BAX-mediated mechanism. Moreover, we were able to downregulate the ITP plasma–induced apoptotic effects on MEG-01 cells via RNA interference targeting CLU and its interactors GRP78 and BAX. Our study provides new insights that could contribute to a better understanding of apoptosis-induced molecular mechanisms underlying ITP pathogenesis and that could be used to identify promising molecular targets with therapeutic potential.
Acknowledgments
The authors thank Nadine Goelz, Rosa Hinselmann, Claudia Gowin, David Graber, and Susanne Staubli for their excellent technical support.
This study was funded by the Vontobel Foundation (F.D.F.) and the Jacques & Gloria Gossweiler Foundation (F.D.F. and M. Schmugge).
Authorship
Contribution: F.D.F. and M. Schmugge conceived and coordinated the study and supervised the project; T.S., C.B., and F.D.F. designed and conducted the experiments and conducted data analysis; and T.S. and F.D.F. drafted and refined the manuscript, with contributions from all authors.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Francesca D. Franzoso, Division of Hematology, Children's Research Center, University Children's Hospital Zurich, Steinwiesstr 75, 8032 Zurich, Switzerland; email: francesca.franzoso@kispi.uzh.ch.
References
Author notes
Data are available on request from the corresponding author, Francesca Daniela Franzoso (francesca.franzoso@kispi.uzh.ch).
The full-text version version of this article contains a data supplement.



![Effect of ITP vs control plasma treatment on the expression of apoptosis genes in MEG-01 cells. Apoptosis genes were significantly upregulated in ITP plasma–treated compared with the control plasma–treated and untreated cell conditions. Relative mRNA levels of each sample normalized to control condition (pan-caspase inhibitor [CI]) are shown as mean ± SD. Statistical analyses were performed using 1-way ANOVA followed by multiple comparisons tests to compare the mean ranks between the groups.](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodvth/1/3/10.1016_j.bvth.2024.100012/3/m_bvth_vth-2024-000148-gr3.jpeg?Expires=1767747793&Signature=ylpbaKxpHHGy9z-7dZi4kqf1hWSQ5JB-ZPsfn68cfsEYQaykca98NttYgMnea4vaD3UfOR-TBSPpBQhbfRAfXwfPGLtOuJpSHP~s94zEtTME~N7P~umevAUMVrDLLTt79Lx8QJ5vzkTFvnWmC~F4n0f2KoEeEJMHaNK8T6IDuSrErs3lBncsP5QaV~i2bEdWLifPJztS3~Mw86Rt6A9y8f0QFL16frcbLpZZWKN23TltngMWMZIA3Ce2yJfzMhW7ookdzuAxEWEYPTMNW0cnanDdUJqIUsNJxbzqpt2G3eyRau8RBPXxbjZ7Ir3UXCctoTkelCpkWPJcWxUi-7HXLA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)