Key Points
Patients with CM-TMA, despite remission, have significant white matter changes on MRI and lower scores on neurocognitive testing.
These changes suggest more research is required to determine optimal therapy for these patients to preserve neurocognitive status.
Visual Abstract
Complement-mediated thrombotic microangiopathy (CM-TMA) is a rare, life-threatening thrombotic microangiopathy caused by a defect in the alternative complement pathway. It is associated with renal failure and acute encephalopathy, but long-term neurocognitive effects are uncertain. Using magnetic resonance imaging (MRI) and neurocognitive tests, we can further evaluate the long-term neurocognitive complications in CM-TMA and compare them with controls. In this study, we analyzed microstructural changes in the cerebral white matter and neurocognitive testing results of patients with CM-TMA. Seven adult patients with CM-TMA in remission and 6 healthy controls were included. All patients were treated with C5 complement blockade. They were followed-up for 12 months after study entry. All patients had consecutive MRI scans (standard-of-care and quantitative sequences) to assess for white matter changes and concurrent neurocognitive testing. Patients with CM-TMA had increased white matter signal intensity in most regions of the brain compared with controls. This was accompanied by increased depression and neurocognitive dysfunction (impaired concentration, short-term memory, and verbal memory). These findings were also present up to 12 months after the initial study visit. In summary, patients with previous CM-TMA were found to have significant, albeit nonspecific, cerebral white matter abnormalities, with impaired memory and concentration. Larger studies with longitudinal follow-up to assess neurocognitive complications in CM-TMA are required. This trial was registered at Clinical Trials Ontario (ctontario.ca; project ID: 1318).
Introduction
Complement-mediated thrombotic microangiopathy (CM-TMA), frequently referred to as atypical hemolytic uremic syndrome (HUS), is a rare cause of primary TMA characterized by microangiopathic hemolytic anemia and organ failure, most commonly renal failure. A genetic defect in the alternative complement pathway can be identified in 40% to 60% of patients with CM-TMA1,2 These defects in the alternative complement pathway may involve gain-of-function mutations (complement component 3 [C3] and complement factor B), loss-of-function mutations (complement factors I and H, membrane-cofactor protein, thrombomodulin, and complement factor H–related protein), or autoantibodies (to complement factor H [CFH]).1 Most patients do not respond to plasma exchange or infusion. Sixty-five percent of these patients progress to dialysis or permanent kidney damage within 1 year.2 Eculizumab, a humanized monoclonal antibody that inhibits the formation of C5a and C5b from C5 in the terminal complement pathway (thus blocking formation of the membrane attack complex, sC5b-9), is an effective treatment, showing greatly improved hematologic parameters and renal function, with up to 80% of patients on dialysis recovering renal function in clinical studies.3-5
Neurological complications are not uncommon in these patients but may not be widely screened for radiographically.6 Although eculizumab has been known to rapidly stabilize acute neurological manifestations, there is a paucity of data regarding long-term neurologic outcomes of adults with CM-TMA, who are often only prescribed eculizumab for 6 to 12 months. Previous data on patients with thrombotic thrombocytopenic purpura (TTP), which has a similar clinical presentation to CM-TMA, shows evidence of long-term cognitive and functional decline.7,8 Therefore, the objective of this study is to evaluate long-term neurocognitive and brain imaging outcomes in patients with CM-TMA treated with C5 inhibition.
In this prospective observational study, we assessed neurocognitive function and structural injuries in patients with a clinical diagnosis of CM-TMA who achieved remission while relating the location of quantitative and qualitative magnetic resonance imaging (MRI) changes to neurocognitive testing. We hypothesized that patients with CM-TMA have an increased risk of depression and neurocognitive changes despite hematologic remission correlating with the pathological changes in the brain. Characterizing the pathophysiology and progression of cerebrovascular injury in CM-TMA may help to guide future targeted therapies.
Methods
Study design
This was a multicenter prospective observational study conducted at London Health Sciences Centre and St. Joseph’s Health Care London. Patients and controls were enrolled from London Health Sciences Centre (London, ON, Canada), University Health Network (Toronto, ON, Canada), St. Michael’s Hospital (Toronto, ON, Canada), and Kingston Health Sciences Centre (Kingston, ON, Canada) between 2016 and 2023. All patients and controls provided written informed consent. Ethics approval for this study was provided by Clinical Trials Ontario (project ID: 1318).
Eligibility criteria
Eligible patients were aged ≥18 years and enrolled after being in hematologic remission from CM-TMA for ≥1 month. Hematologic remission was defined as the normalization of platelet count (≥150 × 109/L) and lactate dehydrogenase (LDH) levels for >30 days.9 Patients were diagnosed with CM-TMA if the following criteria were present at time of the initial episode: thrombocytopenia with platelet count of <150 × 10⁹/L, microangiopathic hemolytic anemia (presence of schistocytes on peripheral blood smear), elevated LDH at >1.25× the upper limit of normal, and/or tissue biopsy which showed TMA features. Patients were excluded if they had any of the following: (1) an alternative cause of TMA (eg, TTP, disseminated intravascular coagulation, malignant hypertension, or Shiga toxin–producing Escherichia coli–associated HUS [STEC-HUS]), (2) secondary CM-TMA (owing to HIV/AIDS, hematopoietic stem cell and solid organ transplant, drugs, pregnancy, infection, and autoimmune disease), (3) solid organ malignancy within 5 years of presentation, (4) malignant hypertension, or (5) pregnancy at time of presentation. Patients were also excluded if they had an internal device not compatible with MRI or if they were unable to do the scan owing to claustrophobia. All patients received C5 inhibitors. Six healthy controls with no significant medical comorbidities were recruited using posters placed in a clinical area. They all consented to the study.
Data collection
Demographic and clinical data were obtained from medical charts, including: age, sex, treatment during the initial episode of CM-TMA, and a disintegrin and metalloproteinase with a thrombospondin type 1 motif 13 (ADAMTS13) activity. Further information was collected at baseline (at the time of the acute episode), and between month 0 (the initial visit; conducted at least 1 month after remission) and month 12, including complete blood count, peripheral blood film (as reported by the hematologist), serum creatinine, LDH, and genetic and functional complement testing results if available. At months 0 and 12, each patient had neurocognitive and depression assessments, and brain MRI. The 6 healthy controls underwent brain MRI and neurocognitive testing during 1 visit.
MRI
MRI scans were obtained at St. Joseph’s Health Care London using a 3T MRI (Siemens Biograph mMR) with a conformal 32-channel head receive coil. The imaging protocol included sequences based on a standard-of-care stroke protocol, and 3 quantitative MRI sequences examining white matter integrity and noncontrast cerebral blood flow.
The standard-of-care imaging included 5 sequences that assessed gross pathology in the brain. T₁-weighted (longitudinal relaxation time) imaging examined general anatomy and evidence of atrophy. T₂-weighted (transverse relaxation time) imaging with fluid attenuated inversion recovery (T₂-FLAIR) assessed acute pathology such as white matter hyperintensities. White matter hyperintensities are lesions demonstrating high signal on T₂-FLAIR assessed in at least 2 planes. They are nonspecific but could represent leukoaraiosis. Diffusion-weighted images detected ischemic lesions. Susceptibility-weighted images evaluated regions of abnormal susceptibility such as microbleeds and late-stage hemorrhage. Vasculature was examined using magnetic resonance angiography. Scans were read by the study radiologists (M.T.J. and L.T.), who were blinded as to whether the individual was a patient or healthy control.
We used myelin water imaging to assess in vivo myelin integrity using the multicomponent-driven equilibrium steady-state observations of T₁ and T₂ (mcDESPOT) technique.10 mcDESPOT uses a 3-component tissue model that generates whole-brain T₁ relaxation, T₂ relaxation, and myelin water fraction (MWF) maps by accounting for the effects of several types of water protons (myelin water, intracellular and extracellular water, and cerebrospinal fluid). Detailed explanations of MRI sequence parameters and models are included in supplemental Data.
Depression and cognitive testing
At months 0 and 12, patients were asked to complete the online Cambridge Brain Sciences (CBS) cognitive assessment, a web-based neurocognitive test series that evaluates global cognition and domain-specific function.11 It includes 12 nonverbal, culturally independent tests, which cover the cognitive domains of memory, reasoning, concentration, and planning or executive function.11 CBS has demonstrated efficacy in evaluating cognitive function in several large-scale population-based studies.11,12 Results for our patients and controls were compared with an age- and sex-matched normative data set provided by CBS with >10 000 healthy participants. The scores and percentages were then assessed as falling within 1 standard deviation (SD), within 2 SDs, or >2 SDs of the healthy age-matched sex-matched control group. Scores of >2 indicated significant changes whereas scores of 1 to 2 showed changes approaching significance.
Patients also completed the Montgomery-Åsberg Depression Rating Scale (MADRS) to evaluate for signs of depression.13 The scale includes 10 categories. Each category is scored from 0 to 6. The sum of all categories signifies the severity of depression, with a score of 0 to 6 being normal, 7 to 19 being mild, 20 to 34 being moderate, and >34 being severe. The controls completed the Patient Health Questionnaire (PHQ-9) for depression assessment.
Statistical analysis
Means, SDs, medians, and interquartile ranges (25th and 75th percentiles) were calculated for demographic data. SPSS software version 29 (IBM Corp, https://www.ibm.com/spss) was used to perform statistical analyses. For quantitative MRI parameters of patients with CM-TMA, z scores were calculated and compared with an average of the 6 controls, with a z score of >3.1 corresponding to a significant result of P < .01. The z scores were used to determine a significant increase in T₁ and T₂ signals, or a significant decrease in MWF values. z score maps were created for each metric using Statistical Parametric Mapping’s “ImCalc” function. z scores were also calculated for CBS scores of patients and controls compared with age- and sex-matched control data provided by the CBS. In this instance, a z score of >2 indicated a significant result of >2 SDs from the mean.
Results
Controls
Six controls were enrolled. Fifty percent were female. The median age was 44 years (range, 24-66). None of the controls scored ≥2 SDs below the mean on CBS testing. Fifty percent of controls scored 1 to 2 SDs below the mean on at least 1 test pertaining to reasoning and concentration. None of the controls had evidence of depression on the PHQ-9 questionnaire. The controls did not have genetic testing done for variants in complement proteins.
Patients
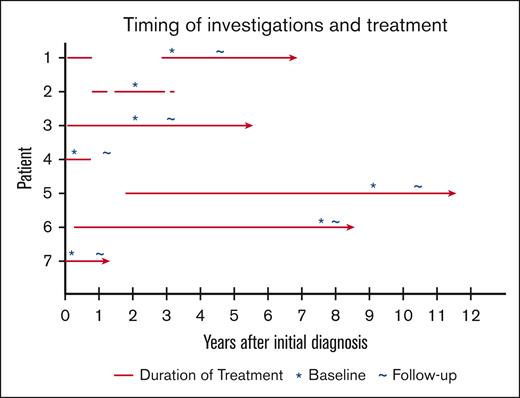
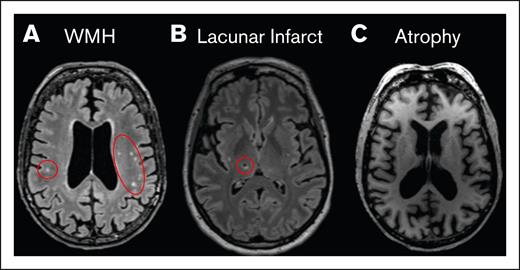
Seven patients with CM-TMA were enrolled, with clinical and demographic characteristics depicted in Table 1; 71% were female. Median age at diagnosis was 40 (range, 16-70). Two patients had major comorbidities (Patients 2 and 5), with 3 of 5 remaining patients only having hypertension as a significant comorbidity. Six were treated with eculizumab, and 1 received ravulizumab. See Figure 1 for timing of treatment initiation and study visits. One patient died before the follow-up visit was completed, owing to stroke in the context of possible CM-TMA relapse. Neuroimaging for all patients showed abnormalities as detailed hereafter and in Table 2. See Figure 2 for z score maps for myelin water imaging and Figure 3 for representative images from the standard-of-care MRI scans. Very few areas on MWF maps reached the z score significance threshold and thus are not included in this report. All patients had abnormalities in their neurocognitive testing, as detailed hereafter and in Table 3. Three patients had at least mild depression during the initial visit. Neurocognitive results for controls are listed in Table 4.
Clinical characteristics of patients at time of first episode of CM-TMA
| Baseline characteristics . | Patient 1 . | Patient 2 . | Patient 3 . | Patient 4 . | Patient 5 . | Patient 6 . | Patient 7 . |
|---|---|---|---|---|---|---|---|
| Age, y | 16 | 70 | 40 | 46 | 51 | 35 | 28 |
| Sex | Female | Male | Female | Male | Female | Female | Female |
| Hemoglobin, g/L | 97 | 56 | 67 | 74 | 70 | 68 | 93 |
| Platelet count, × 10⁹/L | 46 | 17 | 63 | 110 | 88 | 29 | 55 |
| LDH, U/L∗ | 1697 | 656 | 1167 | 1444 | 289 | 761 | 905 |
| Serum creatinine, μmol/L | 163 | 583 | 499 | 1358 | 135 | 318 | 201 |
| ADAMTS 13 activity, %† | N/A | 68 | 82 | 45 | 50 | N/A | 86 |
| C3, g/L‡ | 0.84 | 1.2 | 0.84 | 0.9 | 0.67 | 0.13 | 1.15 |
| C4, g/L§ | 0.2 | 0.25 | 0.2 | 0.19 | 0.13 | 0.04 | 0.23 |
| CH50, U/L|| | 50 | NA | >60 | 74 | 58 | <10 | 86 |
| SC5b-9, ng/mL¶ | Elevated | NA | 235 | NA | NA | NA | NA |
| Genetic mutations | Factor H# | C3 | None | None | None | Factor H | None |
| Plasma exchange, n | 9 | 8 | 4 | 4 | 7 | 5 | 0 |
| Time when first course of complement inhibitor started, months after diagnosis | 1 | 4 | <1 | <1 | 9 | 2 | <1 |
| Baseline characteristics . | Patient 1 . | Patient 2 . | Patient 3 . | Patient 4 . | Patient 5 . | Patient 6 . | Patient 7 . |
|---|---|---|---|---|---|---|---|
| Age, y | 16 | 70 | 40 | 46 | 51 | 35 | 28 |
| Sex | Female | Male | Female | Male | Female | Female | Female |
| Hemoglobin, g/L | 97 | 56 | 67 | 74 | 70 | 68 | 93 |
| Platelet count, × 10⁹/L | 46 | 17 | 63 | 110 | 88 | 29 | 55 |
| LDH, U/L∗ | 1697 | 656 | 1167 | 1444 | 289 | 761 | 905 |
| Serum creatinine, μmol/L | 163 | 583 | 499 | 1358 | 135 | 318 | 201 |
| ADAMTS 13 activity, %† | N/A | 68 | 82 | 45 | 50 | N/A | 86 |
| C3, g/L‡ | 0.84 | 1.2 | 0.84 | 0.9 | 0.67 | 0.13 | 1.15 |
| C4, g/L§ | 0.2 | 0.25 | 0.2 | 0.19 | 0.13 | 0.04 | 0.23 |
| CH50, U/L|| | 50 | NA | >60 | 74 | 58 | <10 | 86 |
| SC5b-9, ng/mL¶ | Elevated | NA | 235 | NA | NA | NA | NA |
| Genetic mutations | Factor H# | C3 | None | None | None | Factor H | None |
| Plasma exchange, n | 9 | 8 | 4 | 4 | 7 | 5 | 0 |
| Time when first course of complement inhibitor started, months after diagnosis | 1 | 4 | <1 | <1 | 9 | 2 | <1 |
NA, not available.
Upper limit of normal is 214 U/L.
ADAMTS13 activity of >10% was defined as ruling out TTP (enzyme-linked immunosorbent assay; reference range, 41%-130% [Technozym, Vienna, Austria]).
Lower limit of normal is 0.90 g/L.
Lower limit of normal is 0.10 g/L.
Normal range is 20 to 55 U/L.
Normal range is 58 to 239 ng/mL.
The mutation in question was Y402, which is a variant of uncertain significance.
Timing of investigations and complement inhibitor treatment for patients with CM-TMA. The red line denotes the duration and timing of eculizumab or ravulizumab treatment in relation to when the diagnosis of CM-TMA was made. Asterisks (∗) identify the timing of the first study visit and tildes (∼) refer to the timing of the follow-up visit. The arrowheads indicate ongoing treatment. For patient 5, the time of diagnosis refers to the initial diagnosis of TTP (before kidney transplant), as this was likely unrecognized CM-TMA.
Timing of investigations and complement inhibitor treatment for patients with CM-TMA. The red line denotes the duration and timing of eculizumab or ravulizumab treatment in relation to when the diagnosis of CM-TMA was made. Asterisks (∗) identify the timing of the first study visit and tildes (∼) refer to the timing of the follow-up visit. The arrowheads indicate ongoing treatment. For patient 5, the time of diagnosis refers to the initial diagnosis of TTP (before kidney transplant), as this was likely unrecognized CM-TMA.
Summary of findings of standard-of-care MRI in patients with CM-TMA
| . | Month 0 . | Month 12 . | ||
|---|---|---|---|---|
| White matter hyperintensities . | Other radiologic findings . | White matter hyperintensities . | Other radiologic findings . | |
| Patient 1 | Spots (5) | None | Spots (2) | None |
| Patient 2 | Spots (≥30) | Atrophy | NA | NA |
| Patient 3 | Spots (5) | Chronic lacunar infarcts (2) | Spots (6) | Unusual spot in midbrain, 1.1 cm pineal cyst, chronic lacunar infarct (1) |
| Patient 4 | Spots (30-40) | Atrophy, aneurysm (1 mm) | Spots (≥20) | Global atrophy, blood products, chronic lacunar infarct (1) |
| Patient 5 | Spots (10) | Mild supratentorial volume loss, bilateral lacunar infarcts (basal ganglia) | Spots (12) | Mild supratentorial volume loss, bilateral lacunar infarcts (basal ganglia) |
| Patient 6 | Spots (≥20) | Chronic lacunar infarcts (3-4) | Spots (≥20) | Chronic lacunar infarcts (3-4) |
| Patient 7 | Spots (3) | None | Spot (1) | None |
| . | Month 0 . | Month 12 . | ||
|---|---|---|---|---|
| White matter hyperintensities . | Other radiologic findings . | White matter hyperintensities . | Other radiologic findings . | |
| Patient 1 | Spots (5) | None | Spots (2) | None |
| Patient 2 | Spots (≥30) | Atrophy | NA | NA |
| Patient 3 | Spots (5) | Chronic lacunar infarcts (2) | Spots (6) | Unusual spot in midbrain, 1.1 cm pineal cyst, chronic lacunar infarct (1) |
| Patient 4 | Spots (30-40) | Atrophy, aneurysm (1 mm) | Spots (≥20) | Global atrophy, blood products, chronic lacunar infarct (1) |
| Patient 5 | Spots (10) | Mild supratentorial volume loss, bilateral lacunar infarcts (basal ganglia) | Spots (12) | Mild supratentorial volume loss, bilateral lacunar infarcts (basal ganglia) |
| Patient 6 | Spots (≥20) | Chronic lacunar infarcts (3-4) | Spots (≥20) | Chronic lacunar infarcts (3-4) |
| Patient 7 | Spots (3) | None | Spot (1) | None |
Lesions were qualitative and mostly located in the frontal lobe and in the periventricular area. Spots are small nonspecific areas of white matter hyperintensity measuring 2 to 15 mm. Chronic lacunar infarcts are regions of necrosis typically measuring 2 to 15 mm.
NA indicates that results are not available.
z score maps for myelin water imaging of patients with CM-TMA vs controls. In T1 and T2 z score maps, red regions indicate significant increases (z score of less than −3.1). Regions used in myelin water imaging are overlaid and color-coded.
z score maps for myelin water imaging of patients with CM-TMA vs controls. In T1 and T2 z score maps, red regions indicate significant increases (z score of less than −3.1). Regions used in myelin water imaging are overlaid and color-coded.
Representative image of radiologic findings. (A) T₂-FLAIR image of multiple WMHs (red circles). (B) T₂-FLAIR image of a chronic lacunar infarct (red circle). (C) MPRAGE image showing whole-brain volume loss (atrophy). MPRAGE, magnetization-prepared rapid acquisition with gradient echo; WMH, white matter hyperintensities.
Representative image of radiologic findings. (A) T₂-FLAIR image of multiple WMHs (red circles). (B) T₂-FLAIR image of a chronic lacunar infarct (red circle). (C) MPRAGE image showing whole-brain volume loss (atrophy). MPRAGE, magnetization-prepared rapid acquisition with gradient echo; WMH, white matter hyperintensities.
CBS SD and MADRS scores for patients with CM-TMA
| Visit . | Patient 1 . | Patient 2 . | Patient 3 . | Patient 4 . | Patient 5 . | Patient 6 . | Patient 7 . | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 . | 2 . | 1 . | 2 . | 1 . | 2 . | 1 . | 2 . | 1 . | 2 . | 1 . | 2 . | 1 . | 2 . | |
| CBS cognitive test | ||||||||||||||
| Reasoning | ||||||||||||||
| Odd 1 out | −0.96 | −0.21 | −0.52 | NA | −0.27 | 0.66 | 0.67 | 0.42 | −0.50 | NA | 1.36 | 0.20 | −1.20 | −2.20∗ |
| Polygons | −0.36 | −1.46 | −1.44 | NA | −1.16 | −0.36 | −0.23 | −0.94 | −0.41 | −1.39 | −1.43 | 0.43 | −1.19 | −0.51 |
| Spatial planning | −0.39 | −0.49 | −1.23 | NA | −0.43 | 1.19 | 0.45 | −0.18 | 0.52 | −0.10 | −0.08 | 0.43 | −1.57 | −1.08 |
| Rotations | −0.99 | 0.43 | −1.95 | NA | NA | 0.27 | −0.65 | −0.09 | −1.72 | −1.20 | −1.22 | −1.72 | −1.84 | −0.64 |
| Short-term memory | ||||||||||||||
| Spatial span | −1.04 | −1.05 | −1.10 | NA | 0.47 | 0.47 | −1.92 | −0.91 | −1.15 | −1.15 | 0.47 | 1.54 | −1.05 | −1.05 |
| Monkey ladder | −0.69 | −0.69 | −1.62 | NA | −0.33 | −0.33 | −1.46 | −1.46 | 0.99 | 0.99 | 0.58 | −0.33 | −1.60 | −0.69 |
| Token search | −0.51 | −0.99 | −2.21∗ | NA | 0.90 | 0.41 | 0.12 | −0.78 | −1.42 | 0.15 | −1.55 | −0.57 | 0.93 | 0.45 |
| Pair associates | −1.39 | −1.39 | −0.21 | NA | 0.72 | 0.72 | 2.49 | −1.04 | 1.17 | −0.55 | 0.72 | 2.49 | 0.33 | 2.05 |
| Verbal memory | ||||||||||||||
| Grammatical reasoning | −0.44 | −0.25 | −1.06 | NA | 0.07 | −0.71 | 0.02 | −0.19 | −0.38 | −0.61 | −2.67∗ | −1.89 | −1.38 | −0.06 |
| Digit span | −0.77 | −1.46 | −1.29 | NA | −0.66 | −1.33 | −0.19 | −0.19 | 0.77 | −1.22 | −1.33 | −1.33 | −1.46 | −0.77 |
| Concentration | ||||||||||||||
| Feature match | −1.15 | −2.28∗ | −0.95 | NA | −0.42 | −1.11 | −2.27∗ | −1.41 | −0.54 | −2.73∗ | 0.10 | −0.14 | −1.08 | −0.05 |
| Double trouble | 0.13 | −1.55 | −1.16 | NA | 0.12 | 0.69 | −0.45 | −0.73 | NA | −0.32 | −0.95 | −1.58 | 0.27 | −0.08 |
| MADRS score | ||||||||||||||
| Total | 7 | 16 | 8 | NA | 25 | 12 | 5 | 3 | 4 | 6 | 2 | 1 | 5 | 8 |
| Visit . | Patient 1 . | Patient 2 . | Patient 3 . | Patient 4 . | Patient 5 . | Patient 6 . | Patient 7 . | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 . | 2 . | 1 . | 2 . | 1 . | 2 . | 1 . | 2 . | 1 . | 2 . | 1 . | 2 . | 1 . | 2 . | |
| CBS cognitive test | ||||||||||||||
| Reasoning | ||||||||||||||
| Odd 1 out | −0.96 | −0.21 | −0.52 | NA | −0.27 | 0.66 | 0.67 | 0.42 | −0.50 | NA | 1.36 | 0.20 | −1.20 | −2.20∗ |
| Polygons | −0.36 | −1.46 | −1.44 | NA | −1.16 | −0.36 | −0.23 | −0.94 | −0.41 | −1.39 | −1.43 | 0.43 | −1.19 | −0.51 |
| Spatial planning | −0.39 | −0.49 | −1.23 | NA | −0.43 | 1.19 | 0.45 | −0.18 | 0.52 | −0.10 | −0.08 | 0.43 | −1.57 | −1.08 |
| Rotations | −0.99 | 0.43 | −1.95 | NA | NA | 0.27 | −0.65 | −0.09 | −1.72 | −1.20 | −1.22 | −1.72 | −1.84 | −0.64 |
| Short-term memory | ||||||||||||||
| Spatial span | −1.04 | −1.05 | −1.10 | NA | 0.47 | 0.47 | −1.92 | −0.91 | −1.15 | −1.15 | 0.47 | 1.54 | −1.05 | −1.05 |
| Monkey ladder | −0.69 | −0.69 | −1.62 | NA | −0.33 | −0.33 | −1.46 | −1.46 | 0.99 | 0.99 | 0.58 | −0.33 | −1.60 | −0.69 |
| Token search | −0.51 | −0.99 | −2.21∗ | NA | 0.90 | 0.41 | 0.12 | −0.78 | −1.42 | 0.15 | −1.55 | −0.57 | 0.93 | 0.45 |
| Pair associates | −1.39 | −1.39 | −0.21 | NA | 0.72 | 0.72 | 2.49 | −1.04 | 1.17 | −0.55 | 0.72 | 2.49 | 0.33 | 2.05 |
| Verbal memory | ||||||||||||||
| Grammatical reasoning | −0.44 | −0.25 | −1.06 | NA | 0.07 | −0.71 | 0.02 | −0.19 | −0.38 | −0.61 | −2.67∗ | −1.89 | −1.38 | −0.06 |
| Digit span | −0.77 | −1.46 | −1.29 | NA | −0.66 | −1.33 | −0.19 | −0.19 | 0.77 | −1.22 | −1.33 | −1.33 | −1.46 | −0.77 |
| Concentration | ||||||||||||||
| Feature match | −1.15 | −2.28∗ | −0.95 | NA | −0.42 | −1.11 | −2.27∗ | −1.41 | −0.54 | −2.73∗ | 0.10 | −0.14 | −1.08 | −0.05 |
| Double trouble | 0.13 | −1.55 | −1.16 | NA | 0.12 | 0.69 | −0.45 | −0.73 | NA | −0.32 | −0.95 | −1.58 | 0.27 | −0.08 |
| MADRS score | ||||||||||||||
| Total | 7 | 16 | 8 | NA | 25 | 12 | 5 | 3 | 4 | 6 | 2 | 1 | 5 | 8 |
Visit 1 occurred at the time of the initial study visit (month 0), and visit 2 occurred 12 months afterward unless data were unavailable for that time. This applies to patient 1’s MADRS score at visit 1, which was done at month 6, and patient 6’s MADRS and CBS scores at visit 2, which were done at month 6. The numbers under the CBS section of the chart indicate SD from age- and sex-matched controls. Under the MADRS section of the chart, total score of 0 to 6 denotes no depression; 7 to 19, mild depression; 20 to 34, moderate depression; and >34, severe depression.
NA indicates not applicable; in these cases, either patient data were missing or the score was interpreted to be due to patient error.
Scores >2 SDs below the mean.
CBS SD and PHQ-9 scores for healthy controls
| . | Number of SDs below the mean . | ||||
|---|---|---|---|---|---|
| Frequency (%) | <1 | 1-2 | ≥2 | ||
| CBS cognitive test | |||||
| Reasoning | |||||
| Odd 1 out | 5 (83.33) | 1 (16.67) | 0 | ||
| Polygons | 3 (50) | 3 (50) | 0 | ||
| Spatial planning | 5 (83.33) | 1 (16.67) | 0 | ||
| Rotations | 5 (100) | 0 | 0 | ||
| Short-term memory | |||||
| Spatial span | 4 (66.67) | 2 (33.33) | 0 | ||
| Monkey ladder | 6 (100) | 0 | 0 | ||
| Token search | 6 (100) | 0 | 0 | ||
| Pair associates | 6 (100) | 0 | 0 | ||
| Verbal memory | |||||
| Grammatical reasoning | 4 (66.67) | 2 (33.33) | 0 | ||
| Digit span | 5 (83.33) | 1 (16.67) | 0 | ||
| Concentration | |||||
| Feature match | 3 (50) | 3 (50) | 0 | ||
| Double trouble | 5 (83.33) | 1 (16.67) | 0 | ||
| PHQ-9 score | |||||
| Frequency (%) | None-minimal | Mild | Moderate | Moderately severe | Severe |
| 6 (100) | 0 | 0 | 0 | 0 | |
| . | Number of SDs below the mean . | ||||
|---|---|---|---|---|---|
| Frequency (%) | <1 | 1-2 | ≥2 | ||
| CBS cognitive test | |||||
| Reasoning | |||||
| Odd 1 out | 5 (83.33) | 1 (16.67) | 0 | ||
| Polygons | 3 (50) | 3 (50) | 0 | ||
| Spatial planning | 5 (83.33) | 1 (16.67) | 0 | ||
| Rotations | 5 (100) | 0 | 0 | ||
| Short-term memory | |||||
| Spatial span | 4 (66.67) | 2 (33.33) | 0 | ||
| Monkey ladder | 6 (100) | 0 | 0 | ||
| Token search | 6 (100) | 0 | 0 | ||
| Pair associates | 6 (100) | 0 | 0 | ||
| Verbal memory | |||||
| Grammatical reasoning | 4 (66.67) | 2 (33.33) | 0 | ||
| Digit span | 5 (83.33) | 1 (16.67) | 0 | ||
| Concentration | |||||
| Feature match | 3 (50) | 3 (50) | 0 | ||
| Double trouble | 5 (83.33) | 1 (16.67) | 0 | ||
| PHQ-9 score | |||||
| Frequency (%) | None-minimal | Mild | Moderate | Moderately severe | Severe |
| 6 (100) | 0 | 0 | 0 | 0 | |
A total of 6 healthy controls were enrolled. The frequency of controls scoring <1, 1 to 2, and ≥2 SDs below the mean are reported. The SDs were calculated with reference to age- and sex-matched controls. The scores for only 5 of 6 patients were enrolled for the rotations section of the CBS. Under the PHQ-9 section of the chart, a total score of 0 to 4 denotes no depression; 5 to 9, mild depression; 10 to 14, moderate depression; 15 to 19, moderately severe depression; and 20 to 27, severe depression.
Patient 1
This patient is a 23-year-old woman with a history of hypertension, diagnosed with CM-TMA at the age of 16 years. She presented with seizure, blindness, STEC-negative bloody diarrhea, posterior reversible encephalopathy syndrome, cerebral microhemorrhage, and acute kidney injury (AKI) requiring dialysis. She achieved full hematologic and renal remission with eculizumab and was able to come off hemodialysis after her first course of eculizumab. She had a Y402 genetic mutation associated with factor H but not known to be a pathogenic variant. She completed 9 months of eculizumab, which was paused for 2 years because of renal recovery. It was restarted because of worsening neuroimaging in the context of memory impairment and depression after other causes were ruled out. She had computed tomography (CT) imaging showing improvement in blood–brain barrier permeability after restarting eculizumab. On her T₂-FLAIR MRI sequence done on the same day as the CT scan, there were 5 hyperintense spots on the initial scan and 2 hyperintense spots 12 months after. The z score maps showed small areas of significantly increased T₂ values at month 0. This increased on both T₁ and T₂ maps at month 12 but was still overall present in small amounts. Her initial MADRS score was mild (7) and was also mild, albeit increased (16), at month 12. Her CBS score showed worsening concentration over 12 months, scoring >2 SDs below the mean at month 12 in 1 of 2 tests assessing concentration. Overall, despite self-reported improvement in memory and ongoing eculizumab treatment, patient 1 demonstrated worsening neurocognitive results over 12 months, with overall equivocal change in neuroimaging.
Patient 2
This 72-year-old man, a retired university professor with a history of systemic lupus erythematosus, monoclonal gammopathy of uncertain significance, and bicytopenia, was diagnosed with CM-TMA at the age of 70 years. He presented with worsening anemia, thrombocytopenia, fever, and AKI. He developed chronic renal failure requiring dialysis despite eculizumab treatment. He had a C3 genetic mutation. He received 2 years of eculizumab with a brief 2-month interruption owing to pancytopenia with a new diagnosis of myelodysplastic syndrome on bone marrow biopsy. Eculizumab was restarted owing to worsening thrombocytopenia but paused after a total treatment course of 2 years because he was unable to receive further government funding for the medication. A month after eculizumab was stopped, he was admitted to hospital with stroke and thrombocytopenia. Eculizumab was restarted owing to stroke, but he died shortly afterward from aspiration pneumonia. He only completed 1 study visit. His standard-of-care scan showed >30 areas of hyperintensity, mostly in the frontal lobe and periventricular areas, and atrophy. This was more than expected for his age. He developed multiple strokes and transient ischemic attacks after his diagnosis of CM-TMA, which could explain his significant structural findings. His z score map showed large amounts of increased T₁ in the periventricular area, cingulate, and frontal lobe. Large amounts of T₂ signal were diffusely present. His MADRS score indicated mild depression (8). His CBS score showed significant impairment in short-term memory, scoring >2 SDs below the mean in 1 of 4 applicable tests, and 1 to 2 SDs below the mean in 2 of 4 tests. His impairment in reasoning and verbal memory was approaching significance, with scores 1 to 2 SDs below the mean in 3 of 4 and 2 of 2 applicable tests, respectively. Although he did not have a follow-up study visit at 12 months, patient 2 demonstrated significant neurocognitive and MRI changes despite eculizumab, which could be affected by his interruption in treatment, history of systemic lupus erythematosus, previous strokes, and myelodysplastic syndrome.
Patient 3
Patient 3, a 46-year-old woman with hypertension, was diagnosed with CM-TMA at the age of 40 years. She presented with hypertensive emergency, respiratory failure, STEC-negative diarrhea, altered mentation, and AKI. She achieved hematologic and renal remission with ravulizumab, a humanized monoclonal antibody inhibitor of C5 in the complement pathway. She was enrolled in a ravulizumab clinical trial at the time of her diagnosis.14 She continued on ravulizumab throughout and after the study period. She had no rare pathogenic variants in complement proteins. She developed membranous nephropathy afterward but has remained clinically stable. She has also developed memory loss, depression, and mild heart failure. On her MRI, there were 5 hyperintense spots and 2 chronic lacunar infarcts on her initial scan, and 6 spots and 1 chronic infarct 12 months after. They were located primarily in the periventricular regions, with 1 in the brain stem and 1 in the temporal lobe. z score maps showed small areas of scattered increases in T₁ and T₂ at month 0. This was slightly decreased at month 12. Her MADRS score was moderate (25) at month 0 and improved to mild (12) at month 12. Her CBS score showed no significant deficits over 12 months. Therefore, patient 3 overall showed improvement or stability in neuroimaging and neurocognitive tests.
Patient 4
Patient 4 is a previously healthy 49-year-old man with CM-TMA diagnosed at the age of 46 years. He presented with seizures, strokes, and AKI requiring dialysis. He remained dialysis dependent despite receiving eculizumab for 9 months. He had no underlying genetic mutations detected. On his MRI, he had >30 hyperintense spots, a 1-mm aneurysm, and atrophy on his initial scan. At 12 months, he had ≥20 hyperintensities and 1 chronic lacunar infarct; these were located throughout the brain. z score maps showed a strong increase in T₁ in the subcortical and periventricular region with improvement by month 12. There was a diffuse increase in T₂ that improved by month 12. His MADRS score was normal (5) at month 0 and also normal (3) at month 12. His CBS score showed significant impairment in concentration in 1 of 2 applicable tests at month 0, with his score improving by month 12. Therefore, despite stopping eculizumab and ongoing need for dialysis, patient 4 was stable or improved in all investigations except for a new lacunar infarct on structural neuroimaging.
Patient 5
Patient 5 is a 63-year-old woman diagnosed with CM-TMA after kidney transplant with previous renal failure attributed to TTP (diagnosed at the age of 51 years). She presented 1 month after her transplant with AKI, hypotension, STEC-negative bloody diarrhea, and abdominal pain. Her AKI resolved with eculizumab without requiring dialysis. She remained on eculizumab throughout the study period and remained in renal and hematologic remission. She had no detectable genetic mutations. On her initial MRI, there were 10 scattered hyperintense spots with bilateral remote basal ganglia lacunar infarcts and mild supratentorial volume loss. There were 2 additional hyperintense spots in the frontal lobe at month 12. The z score map shows scattered areas of increased T₁ at month 0, which was similar at month 12. There were scattered areas of increased T₂ at month 0 that slightly grew at month 12. Her MADRS score was normal (4) at month 0 and normal (6) at month 12. Her CBS score showed significant impairment in concentration in 1 of 2 applicable tests at month 12 compared with baseline testing. Two of 3 tests for reasoning were approaching significant impairment at month 12. Overall, she demonstrated worsening neurocognitive and quantitative MRI results over time with ongoing eculizumab therapy, stable structural MRI findings, and maintenance of clinical remission.
Patient 6
This patient is a previously healthy 44-year-old woman diagnosed with CM-TMA at the age of 35 years. She presented with seizures, posterior reversible encephalopathy syndrome, hypertension, AKI, and cerebral microhemorrhages. She achieved hematologic and renal remission with eculizumab. She never required dialysis. She had a likely pathogenic factor H mutation. On her MRI, there were ≥20 hyperintense spots and 3 to 4 chronic lacunar infarcts. This was unchanged during her follow-up visit. z score maps showed rare areas of increase in T₁ with minimal growth at follow-up. There were scattered areas of increase in T₂ with improvement on follow-up scan. Her MADRS score was normal (2) at month 0 and normal (1) on follow-up visit. CBS score showed significant impairment in verbal memory at month 0, scoring >2 SDs below the mean in 1 of 2 applicable tests, and 1 to 2 SDs below the mean in the remaining test. By the time of the follow-up visit, both tests for verbal memory had scores of 1 to 2 SDs below the mean.
Patient 7
This patient is a 28-year-old woman diagnosed with CM-TMA 1 month postpartum. She had a history of hypertension. She presented with abdominal pain, headache, vision changes, hypertension, and AKI. She demonstrated hematologic resolution with eculizumab but ongoing renal impairment, but she has not required any dialysis. There were no genetic mutations detected. Her initial MRI showed 3 hyperintense spots in the frontal lobe, with only 1 detected on follow-up imaging. Initial z score maps showed increased T₁ primarily in the frontal and insular areas. There were areas of increased T₂ primarily in the frontal lobe. Follow-up maps were similar with the exception of slightly increased areas of T₂ signal. Her MADRS score was normal (5) at first but increased to mild (8) by the follow-up visit. Her CBS score approached significant impairment (1-2 SDs below the mean) in all tests pertaining to reasoning and verbal memory at month 0. By month 12, there was 1 reasoning category showing significant impairment, but her impairment in verbal memory had normalized.
Summary
Of 7 study participants, 5 had neurologic symptoms documented at the time of diagnosis of CM-TMA, yet all patients had detectable abnormalities on MRI and/or cognitive testing. MRI white matter changes were more frequent in patients with CM-TMA than in healthy controls. These changes often occurred in the periventricular and subcortical areas, primarily in the frontal lobe, cingulate, and insula. There were 3 patients with no depression, 3 with mild depression, and 1 with moderate depression. None of the patients had severe depression. The mood scores improved in 3 patients and worsened in 3 patients. The cognitive results using CBS scores showed that concentration and short-term memory were affected in 4 of 7 patients with CM-TMA with a z score of >2 SDs below the reference population, both indicative of frontal lobe involvement.15 The 6 healthy controls did not have any z scores of >2 SDs below the reference population. Three patients also had deficiencies in verbal memory (with 1 patient having a z score of >2 SDs below the mean for 1 test, and the other 2 patients having z scores of 1-2 SDs below the mean for all tests), indicative of issues with the hippocampus, temporal lobe, and prefrontal cortex.16 One patient had significant deficiencies in reasoning whereas 3 other patients had results approaching significance, suggesting frontal lobe involvement. All had scores of 1 to 2 SDs below the mean in at least 1 category. Of 6 patients with serial study visits, 4 patients showed interval stability or improvement (1 was off eculizumab, 2 were still undergoing treatment with eculizumab, and the other was being treated with ravulizumab). Of 7 patients, 3 had a genetic mutation identified. Two of the patients were on chronic anticomplement blockade. Despite treatment, their imaging studies did not show improvement.
Discussion
This study investigated structural changes on brain MRI and neurocognitive changes in patients with a clinical diagnosis of primary CM-TMA. It has already been recognized that survivors with inherited TTP, another TMA, demonstrate chronic neurocognitive impairment. Previous work showed that quantitative and qualitative changes in MRI parameters can be correlated with cognitive impairment.17 A report by Alwan et al showed that patients with TTP with intellectual impairment primarily had frontal lobe involvement on MRI.18 Hannan et al used the same brain MRI protocols used in our study to demonstrate chronic white matter changes in patients with acquired TTP, particularly in the frontal lobe and cingulate cortex.19
T₁ and T₂ changes have been associated with pathologies altering the structure and chemical make-up of neurological tissue by reflecting properties such as pH, protein and fat composition, local tissue density, water content and diffusion, and paramagnetic properties.20-22 These correlate with white matter edema, axonal loss, neuroinflammation, and early demyelination.23-25
There are a few studies investigating neurological complications in children with both HUS and CM-TMA. One study of pediatric patients with diarrheal CM-TMA who received eculizumab detected abnormalities on diffusion-weighted imaging in the basal ganglia as well as the white matter of the centrum semiovale, deep white matter, and corpus callosum, all of which resolved 6 weeks after symptom onset. Cognitive testing ≥6 months after onset of symptoms was unremarkable.26 Tavasoli et al found residual neurological involvement in pediatric patients with CM-TMA (more pronounced than in STEC-HUS), along with a positive correlation with hypertension. Most patients with acute neurologic involvement in the study developed persistent neurologic complaints, including neurodevelopmental delay, movement disorders, speech delay, structural damage, and neuropsychological problems.27 A study of neurologic involvement in adults with epidemic STEC-HUS found T2 hyperintensities on MRI in the dorsal pontine base, thalamus, mesiotemporal lobe, and juxtacortical and periventricular white matter; these lesions largely resolved on follow-up imaging.28
Several of our patients had white matter changes in the frontal lobe and cognitive impairment in frontal lobe functions such as concentration and short-term memory. Our patients also had abnormalities in depression testing, which can be related to cingulate and insula abnormalities detected on their scans. These 2 regions play a key role in emotion and behavior regulation.29,30 Some had significant burden to periventricular and subcortical white matter, which has been found in multiple studies to affect executive function and processing speed and that affects the cognitive domains assessed by the CBS in this study.31 White matter hyperintensities were common in our patients on structural MRI regardless of age. These lesions are significant because they have been associated with mental decline, strokes, and cognitive diseases if left untreated.32 Several patients in our study have already shown signs of similar cognitive decline.
The 2 study participants (patients 2 and 5) whose complement inhibitor therapy was initiated a few months after diagnosis demonstrated increased T1 and T2 signal on z score maps compared with patients with timely initiation of complement inhibitors. These 2 patients had more significant comorbidities than the other patients, which could have affected the MRI findings. These 2 patients also were on dialysis (although patient 4 had received a transplant before entering the study), which could have led to confounding of neurocognitive testing owing to increased cognitive impairment and cerebral ischemia among dialysis patients assessed in previous studies.33,34 Although patient 5 developed worsening neurocognitive findings over time, patient 4, who was on dialysis at time of the 2 study visits, although he had increased MRI abnormalities compared with other study patients, improved in his testing results over time. This suggests that there are other factors at play. After all, even the patients with few or no comorbidities developed abnormalities on MRI and/or cognitive testing. Further studies in the future should assess the difference in neurological outcomes with various new upcoming anticomplement blockers (such as anti-C3 and factor B blockers with anti-C5 blockers) while controlling for confounding factors such as history of hemodialysis.
The 3 patients in our study with genetic mutations did not show any improvement in neuroimaging despite treatment with complement blockade. Patient 1 had rapidly worsening clinical symptoms and possible blood–brain barrier leakage. This was improved with restarting anti-C5 complement blockade, but progression continued. The 5 patients who had no genetic mutations had stable or improving neurocognitive and imaging outcomes, except patient 4, who had recurrence of disease after renal transplant. Therefore, CFH and C3 mutations and patients with recurrent disease were at high risk of worsening neurological outcomes. Caution should be used in stopping treatment in this scenario, and close monitoring with neuroimaging studies should be considered. One caveat is that 2 of the patients without genetic mutations (patients 5 and 6) were diagnosed with CM-TMA >7 years before entering the study and thus the stability in their MRI findings could be attributed to chronicity of previous neurostructural changes. Patient 7 had few MRI changes, likely because very little time passed between her diagnosis of atypical HUS and entry into the study.
Complement is responsible for clearing excess neurotransmitters and old proteins and helps to regulate neurogenesis in adults. Complement components and immunoglobulin G are able to cross a blood–brain barrier that has increased permeability, which may occur with increased age, mitochondrial dysfunction, and oxidative stress. Complement contributes to anti–aquaporin-4–positive neuromyelitis optica, multiple sclerosis, acute disseminating encephalomyelitis, some autoimmune encephalopathies, and possibly some neurodegenerative conditions.35,36 It is unclear in our study why there are persistent neurologic abnormalities despite previous successful treatment with complement inhibitors, in particular, whether this is indicative of persistent low-level abnormal complement activation, chronic damage from previous neurologic stress, or owing to other chronic diseases.
Previous analogous work on persistent neurological abnormalities in patients with TTP despite hematologic remission found that there was abnormal increase in blood–brain barrier permeability as detected by CT perfusion imaging.37 Patient 1 had CT perfusion imaging showing abnormal blood–brain barrier permeability, suggesting that this is a possible component of the mechanism of persistent neurologic injury in patients with CM-TMA.
The strengths in this study lie in investigating long-term cognitive abnormalities in successfully treated patients with CM-TMA using both standard cognitive and depression testing, as well as structural and quantitative MRI. Although 1 of the hallmarks of TTP is neurologic involvement, cerebral assessment is not routine in CM-TMA unless there is an obvious focal deficit. This study’s findings demonstrate that neurologic involvement is not uncommon in CM-TMA and may need to be considered in patients’ treatment plans, including timing of drug discontinuation.
The chief limitation of the study is its small sample size. Thus, we were unable to robustly correlate neuroimaging with features such as duration of disease, age at diagnosis, genetic mutations in the complement pathway, and duration of complement inhibitor therapy. We were unable to match study patients with controls in terms of age, sex, comorbidities, and socioeconomic status owing to the rarity of some of our patients’ comorbidities as well as our initial recruitment strategy for controls. Cognitive testing scores were also not adjusted for socioeconomic status and educational background because these data were not collected. As well, some patients had other comorbidities expected to affect their neurologic and cognitive testing. Furthermore, there was much variation among study patients in terms of time from diagnosis to study entry, time from start and stop of treatment, and renal outcome, making it challenging to draw firm conclusions regarding the exact impact of CM-TMA and complement inhibitor treatment on neurocognition. We did not collect data regarding blood–brain barrier permeability, which could have been used to relate white matter abnormalities with the possibility of complement and other inflammatory mediators crossing the blood–brain barrier. Moreover, data were missing for patient 3 because he died before he could complete his follow-up visit. The MADRS score was used to assess depression in patients with CM-TMA whereas the controls were assessed using the PHQ-9 questionnaire because we were in the process of transition to using the latter test at the time of enrollment of controls.
In conclusion, our study provides preliminary evidence that there are persistent neurologic and neurocognitive complications in survivors of CM-TMA months to years after achieving remission, even with successful treatment with eculizumab. Our data suggest that neuroimaging and cognitive testing may be used to assess length of complement inhibition treatment. The relationship between brain anatomy and neurocognitive outcomes requires further assessment and analysis with greater patient sample sizes. Targeted treatment options, in particular complement inhibitors, have greatly improved hematologic and renal outcomes, but more work will be required to determine optimal therapy to prevent future neurologic deficits.
Acknowledgments
The authors thank Kerri Gallo for help as a research coordinator.
This work was supported by grants from the Department of Medicine of Western University, London, ON, Canada; the Physicians' Services Incorporated Foundation; and Answering TTP.
The authors dedicate this article to the late Michael T. Jurkiewicz, who made a significant contribution to this study.
Authorship
Contribution: S.-H.H.S., K.P., and C.J.P. designed and conducted the study; J.M. assisted in designing the study protocol; J.D.T. and J.T. designed the imaging protocols and obtained MRI images; M.T.J., L.T., F.H., and P.K.K. analyzed the clinical data; and all authors had access to the primary trial data and contributed to the writing of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for P.K.K. is Division of Nephrology, Department of Medicine, Health Sciences North, Sudbury, ON, Canada.
Michael T. Jurkiewicz died on 9 October 2023.
Correspondence: Shih-Han Susan Huang, Division of Nephrology, Department of Medicine, Western University, 800 Commissioners Rd E, Rm A2-344, London, ON N6A 5W9, Canada; email: shuang45@uwo.ca.
References
Author notes
Presented in abstract form at the Department of Medicine Resident Research Day at Schulich School of Medicine & Dentistry, Western University, London, ON, Canada, 19 May 2023.
Original data are available on request from the corresponding author, Shih-Han Susan Huang (shuang45@uwo.ca).
The full-text version of this article contains a data supplement.