Key Points
Fibrinogen activity decreases rapidly after injury.
Fibrinogen repletion increases quantity but does not improve activity in injured patients.
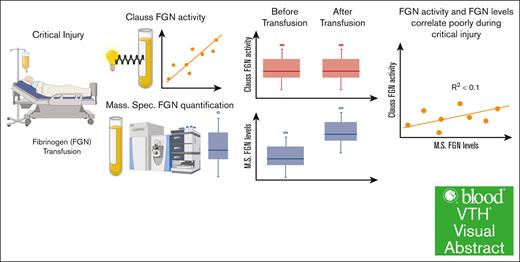
Visual Abstract
Trauma patients who sustain severe tissue injury and hemorrhage often receive fibrinogen repletion to avert coagulopathy and achieve hemostasis. However, fibrinogen supplementation has not shown a benefit in trauma patients with coagulopathy. The von Clauss functional fibrinogen assay is the primary indication for fibrinogen transfusion. This assay, however, infers fibrinogen levels optically via in vitro clot formation time and does not directly measure the quantity or quality of plasma fibrinogen. We hypothesized that the Clauss fibrinogen activity assay does not accurately reflect true fibrinogen levels in severely injured patients. Here, we demonstrate normal baseline plasma fibrinogen levels as measured by mass spectrometry despite coagulopathic Clauss values in severely injured patients. This discrepancy is most significant in patients with coagulopathy (international normalized ratio of >1.3) or with high shock, and persists even after fibrinogen repletion. These data highlight the need to reevaluate clinical testing of fibrinogen activity and transfusion criteria for the critically injured, and indicate that correcting shock and the oxidative, inflammatory milieu of trauma may be more effective at improving fibrinogen activity. This trial was registered at www.ClinicalTrials.gov as #NCT01838863.
Introduction
Trauma-induced coagulopathy is induced by the combination of tissue injury and hypoperfusion and is marked by impaired blood clotting, which increases mortality.1,2 One driver of the early hypocoagulable state in trauma-induced coagulopathy is dysfibrinogenemia.2 Fibrinogen measurements drop early after severe injury, and early dysfibrinogenemia strongly correlates with mortality.3-5 Traditionally, this dysfibrinogenemia is understood to be fibrinogen depletion and is treated with fibrinogen replacement.2 However, multiple studies of fibrinogen replacement have failed to show a benefit in outcomes in severely injured patients.6-11
Recent studies have shown that sterile inflammation, which is rapid and robust in onset after injury, drives oxidation or degradation that deactivates fibrinogen.12 Several of these studies have focused on oxidation of a methionine on the fibrinogen α-chain.12,13 This oxidation impairs fibrin polymerization at low concentrations of oxidized peptide, and this peptide is increased in the most severely injured patients.13 Additionally, oxidation is known to impair fibrin polymerization and modify clot dynamics.14
The von Clauss assay is the most common clinical test used to infer fibrinogen levels.2,15 This is a functional test that relies on fibrinogen activation by thrombin to estimate a concentration optically, and is based on the clotting dynamics of the fibrin meshwork that is formed.16 This assay was developed for diagnosis and monitoring of bleeding disorders but was validated in otherwise healthy patients,17 and to our knowledge its accuracy has not been assessed in severely injured patients. Therefore, we hypothesized that the von Clauss assay does not accurately reflect fibrinogen concentration in severely injured patients with coagulopathy.
Methods
Patients, sample collection, and von Clauss assay
This study used data collected as part of the COMBAT, single-center, randomized clinical trial (ClinicalTrials.gov identifier: NCT01838863). The institutional review board of the University of Colorado and Denver Health Medical Center approved the research protocol under emergency research waiver of consent. The inclusion criteria for COMBAT were previously published.18 In brief, adult trauma patients with hypotension in the field due to hemorrhage were randomized to receive either 2 units of fresh frozen plasma (FFP) or normal saline as per standard of care before hospital arrival. Blood samples were collected in the field and immediately upon hospital arrival, followed by samples at 2, 4, 6, 12, 24, 48, 72, and 168 hours. Clinical laboratory analysis of samples was conducted at each time point including Clauss fibrinogen measured via an STA Compax Max Hemostasis Analyzer (Diagnostica Stago, Parsippany, NJ). The trial was terminated early because of lack of significant differences in survival between treatment arms and a risk of increased need for transfusion products in the FFP group. A cohort of 100 prospectively collected healthy volunteers was used as a control group.
Sample measurement (proteomics)
Whole-blood samples were collected on ice, centrifuged to yield platelet-free plasma, and flash frozen in liquid nitrogen for proteomic analysis, as described in our previous work.19 Briefly, proteins were isolated and digested with trypsin after reduction and alkylation of cysteines. After reversed phase cleanup of the digests, the samples were subjected to liquid chromatography separation followed by electrospray ionization–tandem mass spectrometry (MS) characterization (TIMS TOF Pro; Bruker Daltonics, Bremen, Germany). Raw data files were converted to peak lists and queried for peptide identification against the human proteome (Uniprot database). Peptide identifications were summed (area under the curve) to generate protein level measurements as label-free quantifications, and presented here as units of relative intensity. This method and workflow has been used previously by our group and others to quantify plasma fibrinogen.20,21
Outcome measures
Total fibrinogen concentration was analyzed as the aggregate of the 3 fibrinogen chains (FGA, FGB, FGG) identified in our proteomics analysis. Covariates included the study group (prehospital plasma and control), to which patients were randomized, the new injury severity score (NISS) of ≥25, base deficit of >10 mEq/L, and prothrombin international normalized ratio (INR) of >1.3. These cutoffs were based on previous studies including the original description of the COMBAT randomized control trial.
Statistical analyses
Analyses were performed using Mann-Whitney U, Kruskal-Wallis, Wilcoxon, or Friedman tests, as appropriate. Correlations were performed using Spearman test, and simple linear regression was used to evaluate relationships between fibrinogen concentration and activity. Proteomic analyses were conducted in MetaboAnalyst 5.0 (Genome Canada, Ottawa, ON, Canada). All other analyses were conducted in GraphPad Prism 9.3.0 (GraphPad, San Diego, CA). All tests were 2-tailed with significance defined as P < .05.
Results
In total, 118 trauma patients were included in this analysis across 11 time points in addition to 97 healthy controls totaling 865 blood samples collected, which were processed for standard clinical assays as well as MS proteomics, as previously described (Figure 1A).22 Blood samples were collected until patient discharge to home, other care facility, or death (Figure 1B). Patients were young (median age, 33 years), >80% male, with 50% penetrating injury, and median NISS of 27%, and 11% mortality (Figure 1A). Patients were classified according to their tissue NISS (NISS > 25 = high trauma [HT]) and shock (base excess less than −10 = high shock [HS]) into low shock–low trauma (LSLT), LSHT, HSLT, and HSHT groups to further determine the effects of shock and tissue injury on fibrinogen measurements (Figure 1C).
Trauma patient plasma collected out to 7 days after injury was analyzed by standard clinical assays and MS proteomics. (A) Patient baseline characteristics and demographics showing median (range) or proportion of specified variable. (B) Number of samples collected per time point. (C) Scatterplot of patients specified by tissue injury (NISS, x-axis) or shock (base excess [BE], y-axis) upon hospital arrival into shock-trauma (ST) groups of LSLT, LSHT, HSLT, or HSHT. Diamonds indicate death. BMI, body mass index; BPM, beats per minute; ICU, intensive care unit; SBP, systolic blood pressure; TBI, traumatic brain injury.
Trauma patient plasma collected out to 7 days after injury was analyzed by standard clinical assays and MS proteomics. (A) Patient baseline characteristics and demographics showing median (range) or proportion of specified variable. (B) Number of samples collected per time point. (C) Scatterplot of patients specified by tissue injury (NISS, x-axis) or shock (base excess [BE], y-axis) upon hospital arrival into shock-trauma (ST) groups of LSLT, LSHT, HSLT, or HSHT. Diamonds indicate death. BMI, body mass index; BPM, beats per minute; ICU, intensive care unit; SBP, systolic blood pressure; TBI, traumatic brain injury.
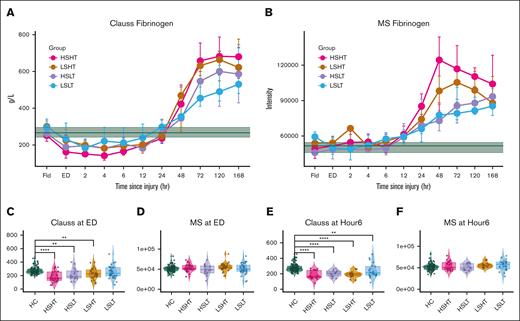
Patient plasma field Clauss activity and MS levels were the same as healthy control (HC) levels (Figure 2; supplemental Figure 1). Then Clauss activity decreased in all injured patients compared with HC and baseline levels from the emergency department (ED) to ∼24 hours after injury, with the most severely injured HSHT and least severely injured LSLT patients having the most and least perturbed Clauss levels, respectively. However, MS levels remained stable and within HC and baseline levels during this acute and subacute injury time. After ∼24 hours after injury, Clauss activity and MS fibrinogen levels showed an acute phase response increase. These trends persisted even when adjusting for D-dimers, which were elevated in HT patients at most time points (supplemental Figure 2).
Discrepancy between fibrinogen Clauss activity and MS level persists throughout acute and subacute severe injury. (A) Time series plot of Clauss fibrinogen activity displaying median activity ± IQR by ST group across time. Green is HC median level ± IQR. (B) Time series plot of MS fibrinogen levels displaying median levels ± IQR by ST group across time. Green is HC median level ± IQR. (C-F) Clauss fibrinogen activity and MS fibrinogen levels at ED and hour 6 after injury by ST group. Significance determined as ∗∗P < .005, ∗∗∗∗P < .0005 by Kruskal-Wallis test.
Discrepancy between fibrinogen Clauss activity and MS level persists throughout acute and subacute severe injury. (A) Time series plot of Clauss fibrinogen activity displaying median activity ± IQR by ST group across time. Green is HC median level ± IQR. (B) Time series plot of MS fibrinogen levels displaying median levels ± IQR by ST group across time. Green is HC median level ± IQR. (C-F) Clauss fibrinogen activity and MS fibrinogen levels at ED and hour 6 after injury by ST group. Significance determined as ∗∗P < .005, ∗∗∗∗P < .0005 by Kruskal-Wallis test.
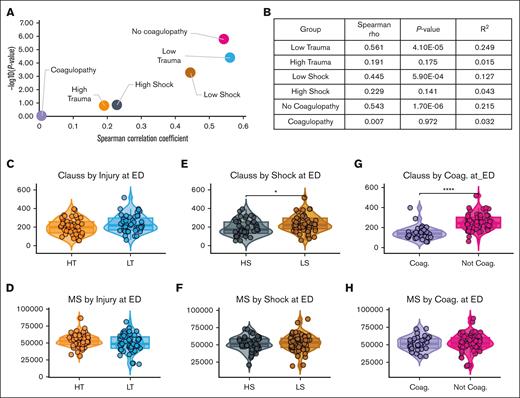
To further evaluate factors driving this discrepancy, we correlated Clauss activity with MS fibrinogen levels by shock, tissue injury, and coagulopathy (INR > 1.3). Minimally injured patients with low shock, low trauma, and no coagulopathy had strong correlations between Clauss fibrinogen activity and MS fibrinogen levels at ED (Figure 3A-B). However, patients with severe injury including HT, HS, or coagulopathy had weak to no correlation between Clauss activity and MS fibrinogen levels, indicating that fibrinogen activity and concentration were decoupled in severely injured trauma patients, with quality (Clauss) decreasing more than quantity (MS).
Clauss activity and MS fibrinogen levels correlate poorly with coagulopathy (Coag.), shock, and tissue injury. (A) Scatterplot of Spearman rho correlation values by P value for patients with/without coagulopathy, shock, or tissue injury, and (B) Spearman rho correlation and P values for associated groups. (C-H) Box plot comparisons of Clauss fibrinogen activity and MS fibrinogen levels by (C-D) tissue injury, (E-F) shock, and (G-H) coagulopathy upon ED arrival. Significance determined as ∗P < .05, ∗∗∗∗P < .005 by Wilcoxon signed-rank test.
Clauss activity and MS fibrinogen levels correlate poorly with coagulopathy (Coag.), shock, and tissue injury. (A) Scatterplot of Spearman rho correlation values by P value for patients with/without coagulopathy, shock, or tissue injury, and (B) Spearman rho correlation and P values for associated groups. (C-H) Box plot comparisons of Clauss fibrinogen activity and MS fibrinogen levels by (C-D) tissue injury, (E-F) shock, and (G-H) coagulopathy upon ED arrival. Significance determined as ∗P < .05, ∗∗∗∗P < .005 by Wilcoxon signed-rank test.
Clauss activity and MS fibrinogen levels were not significantly different between patients with HT and those with LT (Figure 3C-D). Patients with HS had significantly lower levels of Clauss activity (P = .015; median, 195 mg/dL; interquartile range [IQR], 141-259 mg/dL; vs 236 mg/dL [IQR, 200-302]) compared with LS, but there were no differences in MS fibrinogen levels between these shock groups (P = .73; Figure 3E-F). Similarly, patients with coagulopathy had significantly lower Clauss activity than patients without coagulopathy (P = 2.1e−9; 127 mg/dL [IQR, 106-156] vs 233 mg/dL [IQR, 200-302]), whereas there was no difference in MS fibrinogen levels between patients with and patients without coagulopathy (P = .62; Figure 3G-H). These trends persisted with significantly lower Clauss activity in coagulopathic patients from the field to 12 hours after injury, beyond which there were no significant differences (supplemental Figure 3). However, there were no significant differences between these patient groups in MS fibrinogen levels, except higher MS levels in coagulopathic patients at 48 and 72 hours after injury (supplemental Figure 3).
Next, we sought to identify the clinical relevance of these findings by correlating Clauss and MS fibrinogen levels with clinical laboratory values, transfusion events, and patient outcomes. At ED, low Clauss fibrinogen correlated with HT, HS (low base excess), and mortality, as well as more days in the intensive care unit and on a ventilator (Table 1). Low ED Clauss fibrinogen was also associated with worse coagulopathy (increased INR and partial thromboplastin time [PTT], increased clot initiation, and depressed angle and MA). Additionally, patients with the lowest ED Clauss fibrinogen required transfusions of the most blood products (whole red blood cells [RBCs], FFP, platelets, and cryoprecipitate) by 6 and 24 hours after injury. Conversely, MS fibrinogen did not correlate with most variables except weak positive correlations with PTT and thromboelastography angle (clot assembly) and moderate correlation with maximum amplitude of clot strength (clot size).
Fibrinogen correlations with clinical and laboratory outcomes
| . | ED Clauss . | ED MS . | 6-h Clauss . | 6-h MS . | ||||
|---|---|---|---|---|---|---|---|---|
| Spearman R . | P value . | Spearman R . | P value . | Spearman R . | P value . | Spearman R . | P value . | |
| NISS | −0.26 | .01 | 0.03 | .75 | −0.19 | .11 | 0.09 | .39 |
| Base excess | 0.31 | <.001 | 0.06 | .57 | −0.05 | .75 | 0.01 | .94 |
| INR | −0.67 | <.001 | −0.06 | .57 | −0.76 | <.0001 | −0.09 | .45 |
| PTT | −0.38 | <.001 | 0.16 | .11 | −0.45 | <.001 | −0.09 | .45 |
| Death | −0.33 | <.001 | 0.08 | .41 | −0.21 | .08 | −0.05 | .67 |
| ICU days | 0.31 | <.001 | −0.01 | .92 | 0.39 | <.001 | −0.14 | .19 |
| Vent. days | 0.35 | <.001 | 0.03 | .79 | 0.46 | <.001 | −0.06 | .56 |
| 6-h RBC | −0.37 | <.001 | 0.04 | .67 | −0.52 | <.001 | 0.13 | .23 |
| 6-h FFP | −0.35 | <.001 | 0.01 | .94 | −0.33 | .01 | 0.13 | .22 |
| 6-h Plt | −0.31 | .00 | 0.00 | .97 | −0.35 | .00 | 0.29 | .01 |
| 6-h Cryo | −0.24 | .02 | 0.04 | .68 | −0.07 | .57 | 0.29 | .01 |
| 24-h RBC | −0.35 | <.001 | 0.07 | .50 | −0.51 | <.001 | 0.14 | .20 |
| 24-h FFP | −0.35 | <.001 | −0.01 | .91 | −0.39 | <.001 | 0.11 | .32 |
| 24-h Plt | −0.26 | .01 | 0.01 | .91 | −0.07 | .57 | 0.23 | .03 |
| 24-h Cryo | −0.26 | .01 | 0.05 | .59 | −0.07 | .55 | 0.30 | .00 |
| . | ED Clauss . | ED MS . | 6-h Clauss . | 6-h MS . | ||||
|---|---|---|---|---|---|---|---|---|
| Spearman R . | P value . | Spearman R . | P value . | Spearman R . | P value . | Spearman R . | P value . | |
| NISS | −0.26 | .01 | 0.03 | .75 | −0.19 | .11 | 0.09 | .39 |
| Base excess | 0.31 | <.001 | 0.06 | .57 | −0.05 | .75 | 0.01 | .94 |
| INR | −0.67 | <.001 | −0.06 | .57 | −0.76 | <.0001 | −0.09 | .45 |
| PTT | −0.38 | <.001 | 0.16 | .11 | −0.45 | <.001 | −0.09 | .45 |
| Death | −0.33 | <.001 | 0.08 | .41 | −0.21 | .08 | −0.05 | .67 |
| ICU days | 0.31 | <.001 | −0.01 | .92 | 0.39 | <.001 | −0.14 | .19 |
| Vent. days | 0.35 | <.001 | 0.03 | .79 | 0.46 | <.001 | −0.06 | .56 |
| 6-h RBC | −0.37 | <.001 | 0.04 | .67 | −0.52 | <.001 | 0.13 | .23 |
| 6-h FFP | −0.35 | <.001 | 0.01 | .94 | −0.33 | .01 | 0.13 | .22 |
| 6-h Plt | −0.31 | .00 | 0.00 | .97 | −0.35 | .00 | 0.29 | .01 |
| 6-h Cryo | −0.24 | .02 | 0.04 | .68 | −0.07 | .57 | 0.29 | .01 |
| 24-h RBC | −0.35 | <.001 | 0.07 | .50 | −0.51 | <.001 | 0.14 | .20 |
| 24-h FFP | −0.35 | <.001 | −0.01 | .91 | −0.39 | <.001 | 0.11 | .32 |
| 24-h Plt | −0.26 | .01 | 0.01 | .91 | −0.07 | .57 | 0.23 | .03 |
| 24-h Cryo | −0.26 | .01 | 0.05 | .59 | −0.07 | .55 | 0.30 | .00 |
Cryo, cryoprecipitate; ICU, intensive care unit; Plt, platelet; Vent., ventilator.
At 6 hours, correlations between Clauss fibrinogen and both coagulopathy (INR, PTT) and poor outcomes (death, intensive care unit, and ventilator days) persisted. At 6 hours after injury, Clauss fibrinogen levels continued to negatively correlate with RBC, FFP (6 and 24 hours after injury), and platelet transfusions (6 hours after injury). Interestingly, there was no significant association with cryoprecipitate transfusions, indicating that fibrinogen repletion over the first 6 hours failed to improve fibrinogen activity in these patients. At 6 hours after injury, MS fibrinogen levels positively correlated with 6- and 24-hour postinjury platelet and cryoprecipitate transfusions, both rich sources of fibrinogen. MS correlated weakly or not at all with other variables at this time.
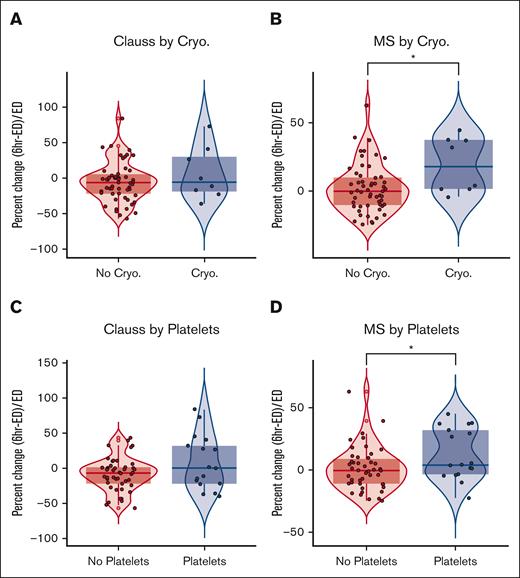
Because low Clauss activity in trauma patients with hemorrhage is an indication for fibrinogen transfusion to control bleeding, we evaluated how platelet and cryoprecipitate transfusions affected Clauss activity and MS fibrinogen levels after the first 6 hours of injury. Of 63 patients with both Clauss and MS fibrinogen measurements in the field/ED and at 6 hours, 8 received at least 1 pooled FFP cryoprecipitate transfusion (median, 2 units; range, 1-8). In patients who received cryoprecipitate, MS fibrinogen levels increased by 18.0% (IQR, 36%), whereas there was no change in patients who did not receive cryoprecipitate (median, −0.31%; IQR, 20%; P < .05; Figure 4). In contrast, Clauss activity decreased in both groups over the first 6 hours of injury. Patients who received cryoprecipitate had a median change of −5.6% in their Clauss activity (IQR, 48.4) whereas Clauss activity decreased by −6.3% in patients who did not receive cryoprecipitate (IQR, 26.7), and there was no significant difference between groups. For platelets, in the 20 patients who received at least 1 platelet transfusion (median, 1; range, 1-8) MS fibrinogen increased by a median of 4.0% (IQR, 35.5) and there was a decrease in 0.6% (IQR, 19.9) in the no-platelet group. In contrast, Clauss fibrinogen was unchanged (0%; IQR, 54.0) in patients who received platelet transfusion, whereas Clauss fibrinogen was decreased by −6.7% (IQR, 23.1) in patients who did not receive platelet transfusion. Lastly, there were no significant differences in Clauss activity or MS fibrinogen when patients who received FFP or RBCs were compared with those who did not.
Fibrinogen supplementation increases MS fibrinogen levels but does not increase Clauss activity. (A) Percent change from ED to 6 hours after injury of (A) Clauss activity after cryoprecipitate transfusion, (B) MS fibrinogen levels after cryoprecipitate transfusion, (C) Clauss activity after platelet transfusion, and (D) MS fibrinogen levels after platelet transfusion.
Fibrinogen supplementation increases MS fibrinogen levels but does not increase Clauss activity. (A) Percent change from ED to 6 hours after injury of (A) Clauss activity after cryoprecipitate transfusion, (B) MS fibrinogen levels after cryoprecipitate transfusion, (C) Clauss activity after platelet transfusion, and (D) MS fibrinogen levels after platelet transfusion.
Discussion
Here, we demonstrate that trauma-induced dysfibrinogenemia is driven by fibrinogen inactivation and not depletion. Cryoprecipitate, and, to a lesser extent, platelet transfusions increase fibrinogen concentration but not activity. This demonstrates that simply increasing the concentration of fibrinogen is insufficient to correct the dysfibrinogenemia of trauma. This supports the findings of White et al that patients with even low levels of oxidized fibrinogen demonstrate coagulopathy, indicating that even a small amount of dysfunctional fibrinogen can impair fibrin polymerization and clotting.13
The failure to increase activity with fibrinogen replacement may be because of inadequate dosing. One transfusion of 10 pooled units of cryoprecipitate contains at least 1.5 g of cryoprecipitate. Rourke et al demonstrated that in spiking experiments 6 to 12 g of cryoprecipitate is required to significantly increase fibrinogen activity above admission levels.3 This may be, in part, because of the need to dilute dysfunctional fibrinogen, which causes coagulopathy at low concentrations.13 In contrast, the massive doses of cryoprecipitate required to achieve this benefit may predispose these patients to thrombotic complications.11
The potential mechanisms of trauma-induced dysfibrinogenemia remain poorly understood. Multiple posttranslational modifications have been shown to affect fibrinogen function.23 Fibrinogen oxidation after trauma decreases Clauss fibrinogen activity.13,24 Acetylation and glycosylation have been shown to inhibit polymerization,23 whereas acetylation also increases susceptibility to fibrinolysis.25 Additionally, massive tissue plasminogen activator release occurs in HSHT patients,26 which degrades fibrinogen as well as fibrin.27 Fibrin degradation products correlate with coagulopathy and may be driving some component of trauma-induced dysfibrinogenemia.28 However, the discrepancy between fibrinogen activity (Clauss) and concentration (MS) persists after correcting for elevated fibrin degradation products in patients with HSHT. This indicates that trauma-induced dysfibrinogenemia is likely driven by a combination of degradation and inactivation.
There are several limitations of this study. Patients were not randomized, and patients received cryoprecipitate as part of a massive transfusion protocol thromboelastography-based resuscitation. Therefore, only patients in shock with severe injury received fibrinogen repletion. Additionally, there were limited numbers of patients at later time points with both Clauss and MS fibrinogen measurements, precluding analysis of the impact of fibrinogen repletion on complications or clinical outcomes. Finally, there are several known confounders of the von Clauss assay, including thrombin inhibitors and fibrin degradation products, and we were unable to control for these factors here. Future studies will consider these limitations as well as mechanistic studies underlying the dysfibrinogenemia after trauma. Results from such research should advance a standardized clinical approach to accurately quantify dysfibrinogenemia in severely injured patients as has been proposed.29
In conclusion, we found that the von Clauss assay underestimates fibrinogen levels in the most severely injured patients and cryoprecipitate transfusion increases fibrinogen concentration but not activity. Because dysfibrinogenemia may not respond to repletion, correcting shock and the oxidative milieu of trauma may be more effective at improving fibrinogen activity.
Acknowledgments
This study was supported by funds from the National Institutes of Health, National Institute of General Medical Sciences, award nos. RM1GM131968 and T32 GM008315.
Authorship
Contribution: All authors conceptualized the study; M.D., C.E., C.S., E.E.M., M.J.C., K.C.H., and A.D. were responsible for data curation; M.D., C.E., K.C.H., and A.D. were responsible for formal analysis; all authors contributed to study investigation; M.D. and C.E. developed the methodology, were responsible for data validation and visualization, and wrote the manuscript; and C.S., E.E.M., M.J.C., K.C.H., and A.D. supervised the study.
Conflict-of-interest disclosure: A.D. and K.C.H. are founders of Omix Technologies Inc. A.D. is a scientific advisory board member for Hemanext Inc and Macopharma Inc. The remaining authors declare no competing financial interests.
Correspondence: Kirk C. Hansen, Biochemistry and Molecular Genetics, University of Colorado Anschutz Medical Campus, 12901 E 17th Ave, RC1-S Room 9120, Aurora, CO 80045; email: kirk.hansen@cuanschutz.edu.
References
Author notes
Data are available via ProteomeXchange with identifier PXD053799.
The full-text version of this article contains a data supplement.

![Trauma patient plasma collected out to 7 days after injury was analyzed by standard clinical assays and MS proteomics. (A) Patient baseline characteristics and demographics showing median (range) or proportion of specified variable. (B) Number of samples collected per time point. (C) Scatterplot of patients specified by tissue injury (NISS, x-axis) or shock (base excess [BE], y-axis) upon hospital arrival into shock-trauma (ST) groups of LSLT, LSHT, HSLT, or HSHT. Diamonds indicate death. BMI, body mass index; BPM, beats per minute; ICU, intensive care unit; SBP, systolic blood pressure; TBI, traumatic brain injury.](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodvth/1/3/10.1016_j.bvth.2024.100017/3/m_bvth_vth-2024-000166-gr1.jpeg?Expires=1769134948&Signature=piSrj6d7QAOIrlKt3igyknSKXuq5mXesr1oN1CiHYOcXJqnIl3MNTYu7j2SO~Bfc3G7E-tcPokbv~PGMcb0EKXbOQhmGTW1NqXTJtY8MPyxs-bxuOKvbpuks3XQ2X0vkeMwWuHkFYzQXReu8rXJdZT8Nl3yXFPHyYUWzk0WF6Z8GSSxMIQpt8m6ncwo3qVMku2olwoWBFKlcavR079R41xMuzZ8aIvOMu5J6guCeJdgyI7KE2Fw86uvnkYSrHTRy5SX5MPHBhe9X-7XV3stV2rzdKSBO9rvmw87JmT0VtjzjfjL-22isqiieBBCQzpzSrZauIFGNULEkUOQShjqs-Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)