Key Points
PTCy uniquely alters vascular biomarkers, with an increase in Ang-2 and a decrease in EGF at day +28 after transplant.
Tac/Sir regimen also alters vascular biomarkers, increasing follistatin and endoglin and decreasing VEGFR2.
Posttransplant cyclophosphamide (PTCy)–based graft-versus-host disease (GVHD) prophylaxis regimens are associated with very low rates of severe acute and chronic GVHD after hematopoietic cell transplant (HCT). However, concerns about cardiac and other organ toxicities persist. This study aimed to compare the vascular biomarker profile of PTCy with other GVHD regimens, including tacrolimus/sirolimus (Tac/Sir) and tacrolimus/methotrexate (Tac/MTX), to generate hypotheses for toxicity mitigation strategies. Plasma samples from day +28 after transplant were analyzed against pretransplant baseline measurements in patients receiving PTCy-based GVHD prophylaxis as part of Blood and Marrow Transplant Clinical Trials Network (BMT CTN) 1202 (n = 112) vs Tac/MTX (n = 98) and Tac/Sir (n = 95) regimens from BMT CTN 0402. Compared with Tac/MTX, PTCy was associated with increasing angiopoietin-2 levels and decreasing epidermal growth factor levels at day +28. In contrast, Tac/Sir displayed increasing follistatin and endoglin levels and decreasing vascular endothelial growth factor receptor 2 (VEGFR2) plasma levels after HCT. Across all cohorts, increasing epidermal growth factor was protective from nonrelapse mortality, and decreasing VEGFR2 was associated with subsequent development of extensive chronic GVHD. These distinct biomarker profiles offer insights that could guide strategies to mitigate unique GVHD prophylaxis–associated toxicities.
Introduction
Posttransplant cyclophosphamide (PTCy) has gained traction as an effective regimen for graft-versus-host disease (GVHD) prophylaxis in both the haploidentical and well-matched hematopoietic cell transplant (HCT) settings. Clinical trial findings, including those from the randomized, phase 3 Blood and Marrow Transplant (BMT) Clinical Trials Network (CTN) 1703 study, have established PTCy as a pivotal player in the GVHD prophylaxis landscape, given its marked reduction of both acute and chronic GVHD risk. However, cardiac and other organ toxicities have been reported with high-dose cyclophosphamide.1 Additionally, PTCy regimens may be associated with delayed neutrophil engraftment, prolonged transfusion dependence, and delayed immune reconstitution, including prolonged deficiency of total CD4+ cells and CD8+ central memory T-cell subsets.2-6 Thus, although PTCy offers significant benefits in GVHD prophylaxis, its toxicity profile requires further understanding. Vascular biomarker studies have identified circulating markers of angiogenesis that can be associated with pathologic inflammatory outcomes and organ dysfunction, including factors such as angiopoietin-2 (Ang-2) and the risk of cardiovascular disease, acute lung injury, and autoimmune diseases,7,8 as well as deficiency of epidermal growth factor (EGF) and renal disease.9 We thus sought to determine the association between circulating angiogenic markers and post-HCT outcomes to identify distinct toxicity profiles of PTCy compared with those of tacrolimus/sirolimus (Tac/Sir) and tacrolimus/methotrexate (Tac/MTX). These insights may prove crucial because they provide direction for mitigating unique toxicities associated with PTCy.
Study design
To investigate the potential effects of PTCy vs other GVHD prophylaxis regimens on the vascular/endothelial system and generate hypotheses regarding potential toxicity mitigation strategies, we compared the fold change (FC) of plasma vascular biomarkers at day 28 after transplant, consistent with prior studies,10 to pretransplant baseline in patients receiving PTCy-based GVHD prophylaxis collected under the biorepository BMT CTN 1202 (n = 112; NCT01879072) with those receiving Tac/MTX (n = 98) or Tac/Sir (n = 95) on BMT CTN 0402 (NCT00406393). Specifically, we measured plasma concentrations of Ang-2, EGF, follistatin, vascular endothelial growth factor-A (VEGF-A), endoglin, placental growth factor, and soluble VEGF receptor 1 (VEGFR-1) and VEGFR-2 using Milliplex magnetic bead panels (Millipore, Billerica, MA). Laboratory analyses were performed in the Cytokine Reference Laboratory at the University of Minnesota. Statistical significance was declared at P values of .006, using the Bonferroni correction to account for multiple comparisons. Mann-Whitney or Kruskal-Wallis tests were used to estimate the association of vascular biomarkers with relapse and GVHD outcomes. Cox proportional hazards models were used to estimate the independent association of vascular biomarkers on nonrelapse mortality (NRM). Statistical analyses were completed using JMP Pro 17 (SAS Corporation, Cary, NC). Figures were created using GraphPad Prism version 9.4.1 (GraphPad Software, Boston, MA).
Results and discussion
Table 1 presents baseline characteristics. BMT CTN 0402 required myeloablative conditioning and matched related donor peripheral blood stem cell transplantation. In contrast, BMT CTN 1202, an observational biorepository study, did not prescribe any specific transplant practice and thus included various allogeneic HCT settings, introducing more sample heterogeneity. Most PTCy samples were from patients who underwent reduced-intensity or nonmyeloablative conditioning, predominantly using haploidentical donors.
Patient baseline characteristics
| Characteristic . | PTCy (n = 112) . | Tac/Sir (n = 95) . | Tac/MTX (n = 98) . |
|---|---|---|---|
| Age, median (range), y | 56 (7-75) | 44 (18-60) | 41 (12-58) |
| Sex, n (%) | |||
| Male | 71 (63%) | 46 (48%) | 54 (55%) |
| Female | 41 (37%) | 49 (52%) | 44 (45%) |
| Year of HCT, range | 2013-2016 | 2007-2011 | 2006-2011 |
| Primary disease, n (%) | |||
| AML | 48 (43%) | 43 (45%) | 40 (41%) |
| MDS | 15 (13%) | 11 (12%) | 5 (5%) |
| ALL | 11 (10%) | 33 (35%) | 45 (46%) |
| Other | 38 (34%) | 8 (8%) | 8 (8%) |
| Conditioning intensity, n (%) | |||
| MA | 43 (38%) | 95 (100%) | 98 (100%) |
| RIC | 20 (18%) | 0 | 0 |
| NMA | 49 (44%) | 0 | 0 |
| Donor type, n (%) | |||
| Matched related donor | 15 (13%) | 95 (100%) | 95 (100%) |
| Unrelated donor | 22 (20%) | 0 | 0 |
| Haploidentical donor | 75 (67%) | 0 | 0 |
| Graft type, n (%) | |||
| Bone marrow | 39 (35%) | 0 | 0 |
| Peripheral blood | 73 (65%) | 95 (100%) | 98 (100%) |
| Characteristic . | PTCy (n = 112) . | Tac/Sir (n = 95) . | Tac/MTX (n = 98) . |
|---|---|---|---|
| Age, median (range), y | 56 (7-75) | 44 (18-60) | 41 (12-58) |
| Sex, n (%) | |||
| Male | 71 (63%) | 46 (48%) | 54 (55%) |
| Female | 41 (37%) | 49 (52%) | 44 (45%) |
| Year of HCT, range | 2013-2016 | 2007-2011 | 2006-2011 |
| Primary disease, n (%) | |||
| AML | 48 (43%) | 43 (45%) | 40 (41%) |
| MDS | 15 (13%) | 11 (12%) | 5 (5%) |
| ALL | 11 (10%) | 33 (35%) | 45 (46%) |
| Other | 38 (34%) | 8 (8%) | 8 (8%) |
| Conditioning intensity, n (%) | |||
| MA | 43 (38%) | 95 (100%) | 98 (100%) |
| RIC | 20 (18%) | 0 | 0 |
| NMA | 49 (44%) | 0 | 0 |
| Donor type, n (%) | |||
| Matched related donor | 15 (13%) | 95 (100%) | 95 (100%) |
| Unrelated donor | 22 (20%) | 0 | 0 |
| Haploidentical donor | 75 (67%) | 0 | 0 |
| Graft type, n (%) | |||
| Bone marrow | 39 (35%) | 0 | 0 |
| Peripheral blood | 73 (65%) | 95 (100%) | 98 (100%) |
AML, acute myeloid leukemia; ALL, acute lymphoblastic leukemia; MA, myeloablative; MDS, myelodysplastic syndrome; NMA, non-myeloablative; RIC, reduced intensity conditioning.
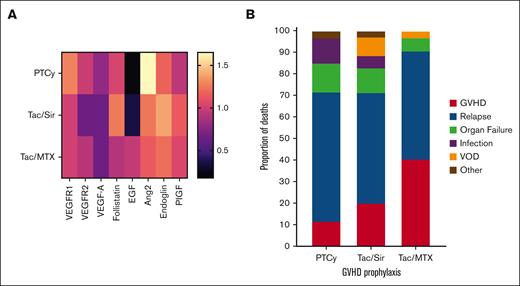
Figure 1A shows a heat map of the overall vascular biomarker profile of the 3 different GVHD prophylaxis regimens, with all statistical comparisons shown in Table 2. PTCy was associated with a unique profile of Ang-2, increasing a median of 65% from pretransplant baseline, and EGF decreasing a median of 82% from baseline, at day +28, compared with Tac/MTX (FC, 1.65 vs 1.23 [P < .001] and FC, 0.18 vs 0.95 [P = .002], respectively). Our previous research showed that Ang-2 levels are elevated after myeloablative conditioning.10 Therefore, had a larger proportion of PTCy recipients received myeloablative conditioning in this cohort, the effects on Ang2 after PTCy might have been even more pronounced than the already significant elevation we observed here. Ang-2 is secreted by endothelial cells stored in Weibel-Palade bodies and acts as an antagonist for the Tie2 receptor, leading to vascular permeability, especially in hypoxic or inflammatory conditions.11 Higher circulating levels of Ang-2 are associated with all-cause and cardiovascular mortality in the general population.12 Ang-2 is a vascular destabilizer, acutely worsening hypoxia and inflammation after cardiac ischemia, making it an potentially important therapeutic target after cardiac injury.13
GVHD prophylaxis vascular biomarker profile and differences in outcomes. (A) Heat map of the change in vascular biomarkers comparing the FC of day +28 after transplant to pretransplant baseline. Biomarkers that decrease over time are shown in darker colors; biomarkers that increase are shown in lighter colors. (B) Cause of death by GVHD prophylaxis regimen. PlGF, placental growth factor; VOD, veno-occlusive disease.
GVHD prophylaxis vascular biomarker profile and differences in outcomes. (A) Heat map of the change in vascular biomarkers comparing the FC of day +28 after transplant to pretransplant baseline. Biomarkers that decrease over time are shown in darker colors; biomarkers that increase are shown in lighter colors. (B) Cause of death by GVHD prophylaxis regimen. PlGF, placental growth factor; VOD, veno-occlusive disease.
FC of vascular biomarkers at day +28 after transplant compared with pretransplant baseline, ranked by highest to lowest changes observed after PTCy
| Vascular biomarker . | PTCy FC . | Tac/Sir FC . | Tac/MTX FC . | P value (PTCy vs Tac/Sir) . | P value (PTCy vs Tac/MTX) . | P value (Tac/Sir vs Tac/MTX) . |
|---|---|---|---|---|---|---|
| Ang-2 | 1.65 | 1.24 | 1.23 | .13 | <.001 | .04 |
| VEGF-R1 | 1.27 | 1 | 1 | .66 | .007 | .01 |
| Endoglin | 1.17 | 1.38 | 1.21 | .002 | .062 | .014 |
| Follistatin | 1.02 | 1.25 | 0.91 | .006 | .3 | .002 |
| VEGF-R2 | 0.98 | 0.66 | 0.92 | <.001 | .12 | <.001 |
| PlGF | 0.93 | 1.13 | 1.05 | .03 | .28 | .33 |
| VEGF-A | 0.78 | 0.65 | 0.65 | .59 | .47 | .94 |
| EGF | 0.18 | 0.35 | 0.95 | .02 | .002 | .42 |
| Vascular biomarker . | PTCy FC . | Tac/Sir FC . | Tac/MTX FC . | P value (PTCy vs Tac/Sir) . | P value (PTCy vs Tac/MTX) . | P value (Tac/Sir vs Tac/MTX) . |
|---|---|---|---|---|---|---|
| Ang-2 | 1.65 | 1.24 | 1.23 | .13 | <.001 | .04 |
| VEGF-R1 | 1.27 | 1 | 1 | .66 | .007 | .01 |
| Endoglin | 1.17 | 1.38 | 1.21 | .002 | .062 | .014 |
| Follistatin | 1.02 | 1.25 | 0.91 | .006 | .3 | .002 |
| VEGF-R2 | 0.98 | 0.66 | 0.92 | <.001 | .12 | <.001 |
| PlGF | 0.93 | 1.13 | 1.05 | .03 | .28 | .33 |
| VEGF-A | 0.78 | 0.65 | 0.65 | .59 | .47 | .94 |
| EGF | 0.18 | 0.35 | 0.95 | .02 | .002 | .42 |
PlGF, placental growth factor.
Duléry previously reported an incidence of 19% early cardiac events with PTCy, compared with 6% in patients not receiving PTCy.14 In this series, a decline in systolic function was most commonly observed (13%), with acute pulmonary edema, pericarditis, arrhythmia, and acute coronary syndrome being less common.14 A series from the MD Anderson Cancer Center reported a somewhat lower but still notable incidence of cardiotoxicity after PTCy at 7.4%, with non-PTCy regimens also associated with cardiac toxicity at 5.8%, suggesting host susceptibility and not just GVHD prophylaxis regimen may play a role.15 However, arrhythmias occurred in 5.6% in a series of young individuals undergoing well-HLA-matched myeloablative transplantation.16 In BMT CTN 1703, which was a randomized phase 3 trial conducted in the reduced-intensity setting, 4 patients (1.9%) died of early cardiac or multiorgan toxicity on the PTCy arm.3 Previous reports suggest that patients with preexisting cardiac morbidity have an increased risk of early cardiac events after PTCy.17 The potential role of Ang-2 in cardiovascular toxicity after PTCy thus requires further study.
Although levels of Ang-2 increase after PTCy, plasma levels of EGF, a protein that can improve endothelial function after injury, decrease.18 EGF functions to modulate cell proliferation and antiapoptosis pathways and has a known role in tissue regeneration and repair.10,19,20 We have previously shown that patients with low EGF in the early posttransplant period have a higher risk of death due to GVHD or organ damage.21 Thus, the elevated Ang-2 levels coupled with decreased EGF after PTCy could potentially create a clinical scenario that predisposes susceptible patients to severe impairment of endothelial and/or cardiopulmonary function, even in patients receiving reduced-intensity or nonmyeloablative conditioning.
At day +28, Tac/Sir was associated with a unique vascular biomarker profile, distinct from PTCy. As previously seen with sirolimus, markers of endothelial damage increased22 (follistatin by a median of 25% and endoglin by a median of 38%), whereas VEGFR2 decreased by a median of 44% (Table 1). We have previously shown that early increases in follistatin and endoglin are associated with NRM after allogeneic HCT.23 Low VEGFR2 has previously been observed in other settings of endothelial dysfunction, including dialysis and in pulmonary arterial hypertension.24,25
The only vascular biomarker associated with GVHD or relapse events irrespective of GVHD prophylaxis was VEGFR2, with which an early decrease was associated with the subsequent development of extensive chronic GVHD (FC, 0.75 vs 1.16 for no chronic GVHD; P = .003). A higher proportion of deaths was due to organ failure or infection with PTCy, and a higher proportion of deaths were due to veno-occlusive disease with Tac/Sir (Figure 1B). Among the biomarkers tested, only an increase in EGF was protective against NRM irrespective of GVHD prophylaxis regimen (hazard ratio, 0.88 per FC increase; 95% confidence interval, 0.70-0.99; P = .0077).
In conclusion, our findings illuminate the vascular biomarker profiles associated with GVHD prophylaxis regimens, emphasizing the unique impact of PTCy on Ang-2 and EGF. Important limitations of our study are the differences in donor source and conditioning intensity across studies; further studies are indicated to clarify the potential interaction of graft source, conditioning, and GVHD prophylaxis regimen. Despite this heterogeneity, the interplay between elevated Ang-2 and reduced EGF levels in the circulation after PTCy suggests potential risk factors for endothelial and cardiopulmonary dysfunction in susceptible patients.
Acknowledgments
The authors acknowledge the BMT CTN 0402 and 1202 study investigators and participating centers. They appreciate the assistance of Michael Ehrhardt in performing the circulating angiogenic factor analyses at the University of Minnesota Cytokine Reference Laboratory.
Support for the sample and data collection study was provided to the Blood and Marrow Transplant Clinical Trials Network from the National Heart, Lung, and Blood Institute (NHLBI) and the National Cancer Institute (NCI; U10HL069294). L.F.N. is supported by the National Institute of Child Health and Human Development (1K23 HD091369-01). S.G.H. is supported by the Women's Early Research Career award from the University of Minnesota, Department of Medicine. Statistical support was made possible by National Institutes of Health P30 CA77598 using the Biostatistics and Bioinformatics Shared Resource of the Masonic Cancer Center. Support for this study was provided to the Blood and Marrow Transplant Clinical Trials Network (grants U10HL069294 and U24HL138660) from the National Heart, Lung, and Blood Institute and the National Cancer Institute, along with contributions by Wyeth Pharmaceuticals Inc BMT CTN 0402 biospecimens were obtained from the NHLBI Biologic Specimen and Data Repository Information Coordinating Center (BioLINCC).
The manuscript was prepared using BMT CTN 0402 and 1202 Research Materials obtained from the BMT CTN Repository operated by the National Marrow Donor Program, and the content is solely the responsibility of the authors and does not necessarily represent the official views of the BMT CTN 0402 and 1202 protocol teams, the BMT CTN, the NHLBI, or the NCI.
Authorship
Contribution: L.F.N. and S.G.H. designed the project, analyzed the primary data, and wrote the manuscript; A.P.-M. performed the circulating angiogenic factor analyses and reviewed the manuscript; and all other authors offered critical review of the paper and approved the final manuscript.
Conflict-of-interest disclosure: S.G.H. receives research funding from Incyte and Vitrac Therapeutics; is a clinical trial adjudicator for CSL Behring; has given educational programs sponsored by Kadmon/Sanofi; and provided consultation services for Ossium. J.E.L. receives research funding from Genentech and VectivBio; provided consultation services for Bluebird Bio, Editas, Equillium, Incyte, Inhibrx, Kamada, Mesoblast, Sanofi, and X4 Pharmaceuticals; and also receives royalties from Viracor for a GVHD biomarker patient. The remaining authors declare no competing financial interests.
Correspondence: Shernan G. Holtan, Blood and Marrow Transplantation, Transplantation and Cellular Therapy, Roswell Park Comprehensive Cancer Center, Carlton and Elm Sts, Buffalo, NY 14263; email: shernan.holtan@roswellpark.org.
References
Author notes
Data are available from the corresponding author, Shernan Holtan (shernan.holtan@roswellpark.org), on request.