Oral iron (Fe) supplementation is one of the mainstays of treatment for iron deficiency anemia (IDA). However, its therapeutic effects are limited when there is poor absorption from the gastrointestinal tract. Vitamin C is hypothesized to improve uptake when combined as an adjunct agent. We aimed to determine the difference in hematologic outcomes in patients with IDA receiving oral iron, with or without vitamin C. MEDLINE, Embase, Web of Science, and Cochrane Central Register of Controlled Trials were searched from database inception to July 2023 for studies investigating the use of oral iron supplements with vitamin C in patients with IDA. The primary outcome was the change in serum hemoglobin (Hb). Secondary outcomes include change in serum ferritin, reticulocyte percentage, and incidence of adverse events. A total of 2231 studies were retrieved; 10 randomized control trials (n = 1782), and 1 prospective cohort study (n = 148) comprising 1930 patients were included. Vitamin C supplementation was associated with a significant increase in serum Hb level (mean differences [MDs], 0.14 g/dL; 95% confidence interval [CI], 0.08-0.20; P < .01; 10 studies, 1490 patients) and serum ferritin levels (MD, 3.23 μg/L; 95% CI, 1.63-4.84; P < .01; 9 studies, 1682 patients) in the iron plus vitamin C group compared with the iron-only group. The addition of vitamin C to iron supplementation was associated with a small and likely clinically insignificant increase in serum Hb. The results of this study do not support routine supplementation of oral iron therapy with vitamin C in the treatment of IDA.
Introduction
Anemia is a serious public health issue affecting one-third of the global population, with higher morbidity and mortality in patients affected.1-3 There are many etiologies of anemia, but the most prevalent is iron deficiency anemia (IDA), in which insufficient iron is available for erythrocyte production.4 This results in hemoglobin (Hb) concentrations below the required amount to meet physiological demand.5
Oral iron supplementation is the primary treatment for IDA. Commonly used iron supplements include ferrous fumarate, sulfate, and gluconate; these treatments differ based on the amount of elemental iron the body can absorb: 33%, 20%, and 12%, respectively.6 Absorption of oral iron supplements in the proximal gastrointestinal (GI) tract may be reduced in the context of concomitant use of inhibitors of absorption (eg, tea and calcium), coexisting inflammation with sequestration of iron, diseases of the intestinal mucosa (eg, inflammatory bowel disease and celiac disease), infection by Helicobacter pylori, and impaired secretion of gastric acid.6,7 This leads to reduced therapy efficacy. Intolerance is also highly prevalent and reduces the effectiveness of oral iron supplements because adverse effects such as constipation, nausea/vomiting, GI irritation, and/or poor taste can cause considerable discomfort for patients.8,9
Vitamin C is hypothesized to increase iron absorption by increasing the GI tract's acidity, which enhances the conversion of nonabsorbable ferric iron (Fe3+) to its absorbable ferrous (Fe2+) state.10,11 This has been investigated in a randomized controlled trial (RCT) by Patil et al, and the results suggest greater improvement in hematologic parameters such as Hb when iron is taken in a formulation with vitamin C.12 However, a more recent RCT by Li et al from 2020 found no difference in hematologic outcomes between patients with IDA taking iron with or without vitamin C.10 This RCT was responsible for influencing changes in IDA management guidelines by The British Society of Gastroenterology from a possible benefit of vitamin C coadministration to no longer recommending it.10,13,14
There exists uncertainty on the clinical benefit of vitamin C supplementation in IDA management. This systematic review and meta-analysis aimed to determine whether oral iron supplementation with vitamin C improves hematologic outcomes compared with supplementation without vitamin C for patients with IDA.
Methods
This systematic review adheres to the preferred reporting instructions for systematic reviews and meta-analysis.15 A study protocol was registered in the International Prospective Register of Systematic Reviews database (PROSPERO; CRD42023435260) before the initiation of data collection.
Search strategy
Electronic database searches were conducted on MEDLINE, Embase, Web of Science, and the Cochrane Central Register of Controlled Trials from database inception to July 2023 with the help of a librarian. A manual citation search and review of reference lists from studies with similar topics were performed. No language restrictions were applied. The full search strategy for each database is listed in supplemental Table 1.
Article screening and data abstraction
Studies from the initial search were first screened based on title and abstract. Studies with potential relevance were subsequently reviewed in full text. If they met the criteria of our systematic review, their data were extracted. Each stage was completed independently and in duplicate by 2 reviewers (J.D. and L.R.). Conflicts were resolved by an independent third reviewer (P.Y.L.).
Inclusion and exclusion criteria are listed in supplemental Table 2. Inclusion criteria included studies with patients who met the diagnostic criteria for IDA, comparative studies with a clear comparison of oral iron supplementation with vitamin C to oral iron supplementation alone, and studies reporting on at least one of the outcomes of interest.
Exclusion criteria included case reports, case series, review articles, animal studies or basic science studies, studies including patients receiving concomitant treatment with erythropoietin, studies including patients with active malignancies or myeloproliferative disorders (eg, colorectal cancer, myelodysplastic syndromes, acute myeloid leukemia, myeloproliferative neoplasms, gastric cancer, or endometrial cancer), or studies with no comparator group.
Study end points
The primary outcome was the change in serum Hb levels, presented as grams per deciliter. Secondary outcomes include the change in serum ferritin in micrograms per liter, change in reticulocyte percentage, and incidence of adverse events, including constipation, nausea/vomiting, GI irritation (as defined by vomiting, diarrhea, constipation, and abdominal pain), and poor taste. We used adverse event end points as they were defined by the study authors. If studies used different forms of measure for serum Hb and ferritin, they were manually converted to the desired measurement unit during data extraction.
Statistical analysis
Data analysis was performed using ReviewManager (RevMan) 5.4. Effect sizes were calculated using the Mantel-Haenszel fixed effects model and were represented by odds ratios (ORs) and mean differences (MDs) for binary end points and continuous end points, respectively, with 95% confidence intervals (CIs).15 I2 was calculated to assess heterogeneity between studies with values <30%, 30% to 60%, and >60%, representing low, moderate, and high degrees of heterogeneity, respectively.
Results
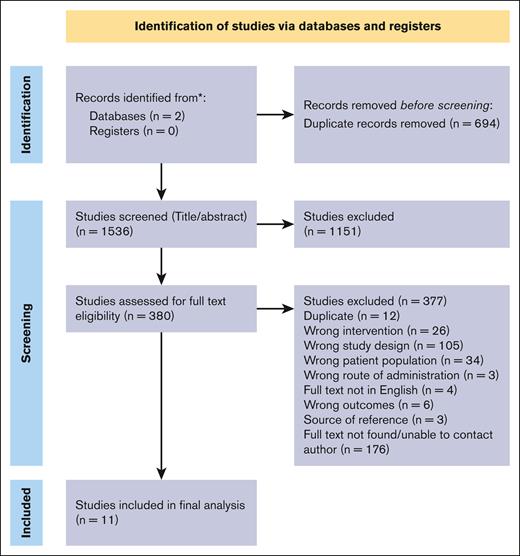
A total of 2231 studies were retrieved from electronic databases as well as manual citation searches.16 After article screening, 10 RCTs (n = 1782) and 1 prospective cohort study (n = 178) comprising 1930 patients were included in the final analysis.10,12,17-25 A summary of the searching process is included in Figure 1. The mean age of patients ranged from 1 to 39 years. The range of treatment duration across the studies was 45 to 180 days. The control formulations varied by study and included ferrous sulfate,17-20,22-25 iron complex,12 ferrous succinate,10 and colloidal iron.21 Most studies used ferrous ascorbate as the intervention therapy (6 of 11).12,19-22,24 The remaining studies used ferrous sulfate17 or ferrous fumarate18,25 with vitamin C. One study did not specify the formulation.23 A summary of the study characteristics can be found in Table 1.
PRISMA diagram displaying the results of the literature search conducted.
Study characteristics
| Study . | Country . | Study design . | Mean age (y) . | Male (%) . | Pregnant women (%) . | Severe anemia (Hb, ≤6 g/dL) (%) . | Intervention sample size . | Control sample size . | Intervention agent . | Control agent . | Duration of treatment (d) . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Astuti et al17 | Indonesia | RCT | 13.68 | 0 | 0 | ND | 11 | 11 | Ferrous sulfate + vitamin C (up to 100 mg daily) | Ferrous sulfate | 180 |
| Aycicek18 | Turkey | RCT | 7.1 | 52.81 | 0 | ND | 44 | 45 | Ferrous fumarate (5 mg/kg per day) + vitamin C (6.3 mg/kg per day) | Ferrous sulfate (5 mg/kg per day) | 45 |
| Chavan et al19 | India | RCT | 23.67 | 0 | 100 | 0 | 50 | 50 | Ferrous ascorbate (100 mg daily) + folic acid | Ferrous sulfate (60 mg bid) + folic acid | 90 |
| Gamad et al20 | India | RCT | 27.78 | 0 | 100 | 0 | 30 | 90 | Ferrous ascorbate (100 mg bid) + folic acid | Ferrous sulfate + folic acid, n = 30, Ferrous fumarate n = 30, and carbonyl iron + folic acid n = 30 (each 100 mg bid) | 56 |
| Ganguly21 | India | RCT | 5.14 | 65 | 0 | 26 | 41 | 39 | Ferrous ascorbate (3 mg/kg per day) + folic acid | Colloidal iron (3 mg/kg per day) + folic acid + vitamin B12 | 84 |
| Li et al10 | China | RCT | 38.3 | 3.18 | ND | ND | 220 | 220 | Ferrous succinate (100 mg tid) + vitamin C (200 mg tid) | Ferrous succinate (100 mg tid) | 90 |
| Panchal et al22 | India | Prospective cohort | 38.5 | 27.70 | 58.11 | ND | 62 | 86 | Ferrous ascorbate (100 mg daily) | Ferrous sulfate (200 mg daily) | 84 |
| Patil et al12 | India | RCT | 1.72 | 49 | 0 | ND | 50 | 50 | Ferrous ascorbate (6 mg/kg per day) | Iron polymaltose complex (6 mg/kg per day) | 90 |
| Rosado et al23 | Mexico | RCT | 1.78 | 47.73 | 0 | 0 | 48 | 40 | Unspecified oral iron (6.7 mg daily) + zinc + vitamin C (44.4 mg daily) | Ferrous sulfate (20 mg daily) | 112 |
| Singhal et al24 | India | RCT | 22.45 | 0 | 100 | ND | 50 | 200 | Ferrous ascorbate (100 mg daily) | Ferrous sulfate (100 mg daily) n = 50, ferrous fumarate (100 mg daily) n = 50, ferrous bisglycinate (30 mg daily) n = 50, and sodium feredetate (33 mg daily), n = 50 | 60 |
| Zlotkin et al25 | Ghana | RCT | 1.07 | 47.46 | 0 | ND | 246 | 247 | Ferrous fumarate (80 mg daily) + vitamin C (50 mg daily) | Ferrous sulfate (40 mg daily, divided into 3 doses) | 60 |
| Study . | Country . | Study design . | Mean age (y) . | Male (%) . | Pregnant women (%) . | Severe anemia (Hb, ≤6 g/dL) (%) . | Intervention sample size . | Control sample size . | Intervention agent . | Control agent . | Duration of treatment (d) . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Astuti et al17 | Indonesia | RCT | 13.68 | 0 | 0 | ND | 11 | 11 | Ferrous sulfate + vitamin C (up to 100 mg daily) | Ferrous sulfate | 180 |
| Aycicek18 | Turkey | RCT | 7.1 | 52.81 | 0 | ND | 44 | 45 | Ferrous fumarate (5 mg/kg per day) + vitamin C (6.3 mg/kg per day) | Ferrous sulfate (5 mg/kg per day) | 45 |
| Chavan et al19 | India | RCT | 23.67 | 0 | 100 | 0 | 50 | 50 | Ferrous ascorbate (100 mg daily) + folic acid | Ferrous sulfate (60 mg bid) + folic acid | 90 |
| Gamad et al20 | India | RCT | 27.78 | 0 | 100 | 0 | 30 | 90 | Ferrous ascorbate (100 mg bid) + folic acid | Ferrous sulfate + folic acid, n = 30, Ferrous fumarate n = 30, and carbonyl iron + folic acid n = 30 (each 100 mg bid) | 56 |
| Ganguly21 | India | RCT | 5.14 | 65 | 0 | 26 | 41 | 39 | Ferrous ascorbate (3 mg/kg per day) + folic acid | Colloidal iron (3 mg/kg per day) + folic acid + vitamin B12 | 84 |
| Li et al10 | China | RCT | 38.3 | 3.18 | ND | ND | 220 | 220 | Ferrous succinate (100 mg tid) + vitamin C (200 mg tid) | Ferrous succinate (100 mg tid) | 90 |
| Panchal et al22 | India | Prospective cohort | 38.5 | 27.70 | 58.11 | ND | 62 | 86 | Ferrous ascorbate (100 mg daily) | Ferrous sulfate (200 mg daily) | 84 |
| Patil et al12 | India | RCT | 1.72 | 49 | 0 | ND | 50 | 50 | Ferrous ascorbate (6 mg/kg per day) | Iron polymaltose complex (6 mg/kg per day) | 90 |
| Rosado et al23 | Mexico | RCT | 1.78 | 47.73 | 0 | 0 | 48 | 40 | Unspecified oral iron (6.7 mg daily) + zinc + vitamin C (44.4 mg daily) | Ferrous sulfate (20 mg daily) | 112 |
| Singhal et al24 | India | RCT | 22.45 | 0 | 100 | ND | 50 | 200 | Ferrous ascorbate (100 mg daily) | Ferrous sulfate (100 mg daily) n = 50, ferrous fumarate (100 mg daily) n = 50, ferrous bisglycinate (30 mg daily) n = 50, and sodium feredetate (33 mg daily), n = 50 | 60 |
| Zlotkin et al25 | Ghana | RCT | 1.07 | 47.46 | 0 | ND | 246 | 247 | Ferrous fumarate (80 mg daily) + vitamin C (50 mg daily) | Ferrous sulfate (40 mg daily, divided into 3 doses) | 60 |
bid, twice daily; ND, no data; tid, thrice daily.
Outcomes
A summary of the meta-analysis findings is shown in Table 2.
Summary of meta-analysis findings by outcome
| Outcome . | Iron + vitamin C (incidence/sample size) . | Iron with no vitamin C (incidence/sample size) . | Effect size . | No. of studies in systematic review (total sample size N = 1930) . |
|---|---|---|---|---|
| Primary outcome | ||||
| Change in serum Hb levels (g/dL) | NA/sample size | NA/sample size | MD, 0.14 (95% CI, 0.08-0.20; P < .01; I2 = 86%) | 10 (n = 1490) |
| Secondary outcomes | ||||
| Change in serum ferritin levels (μg/L) | NA/sample size | NA/sample size | MD, 3.23 (95% CI, 1.63-4.84; P < .01; I2 = 95%) | 9 (n = 1682) |
| Change in reticulocyte percentage (%) | NA/sample size | NA/sample size | MD, 0.22 (95% CI, 0.08-0.36; P <.01; I2 = 94%) | 3 (n = 620) |
| Adverse events outcomes | ||||
| Incidence of all adverse events (events/total sample) | 76/680 | 113/820 | OR, 0.71 (95% CI, 0.50-1.00; P = .05; I2 = 0%) | 3 (n = 1500) |
| Incidence of constipation (events/total sample) | 19/300 | 30/320 | OR, 0.83 (95% CI, 0.35-1.96; P = .66; I2 = 0%) | 3 (n = 620) |
| Incidence of nausea or vomiting (events/total sample) | 45/300 | 58/360 | OR, 0.80 (95% CI, 0.52-1.24; P = .32; I2 = 40%) | 3 (n = 660) |
| Incidence of GI irritation (events/total sample) | 12/80 | 25/140 | OR, 0.40 (95% CI, 0.18-0.92; P = .03; I2 = 0%) | 2 (n = 220) |
| Incidence of poor taste (events/total sample) | N/A | N/A | Not estimable | 0 |
| Outcome . | Iron + vitamin C (incidence/sample size) . | Iron with no vitamin C (incidence/sample size) . | Effect size . | No. of studies in systematic review (total sample size N = 1930) . |
|---|---|---|---|---|
| Primary outcome | ||||
| Change in serum Hb levels (g/dL) | NA/sample size | NA/sample size | MD, 0.14 (95% CI, 0.08-0.20; P < .01; I2 = 86%) | 10 (n = 1490) |
| Secondary outcomes | ||||
| Change in serum ferritin levels (μg/L) | NA/sample size | NA/sample size | MD, 3.23 (95% CI, 1.63-4.84; P < .01; I2 = 95%) | 9 (n = 1682) |
| Change in reticulocyte percentage (%) | NA/sample size | NA/sample size | MD, 0.22 (95% CI, 0.08-0.36; P <.01; I2 = 94%) | 3 (n = 620) |
| Adverse events outcomes | ||||
| Incidence of all adverse events (events/total sample) | 76/680 | 113/820 | OR, 0.71 (95% CI, 0.50-1.00; P = .05; I2 = 0%) | 3 (n = 1500) |
| Incidence of constipation (events/total sample) | 19/300 | 30/320 | OR, 0.83 (95% CI, 0.35-1.96; P = .66; I2 = 0%) | 3 (n = 620) |
| Incidence of nausea or vomiting (events/total sample) | 45/300 | 58/360 | OR, 0.80 (95% CI, 0.52-1.24; P = .32; I2 = 40%) | 3 (n = 660) |
| Incidence of GI irritation (events/total sample) | 12/80 | 25/140 | OR, 0.40 (95% CI, 0.18-0.92; P = .03; I2 = 0%) | 2 (n = 220) |
| Incidence of poor taste (events/total sample) | N/A | N/A | Not estimable | 0 |
N/A, not applicable.
Primary end point
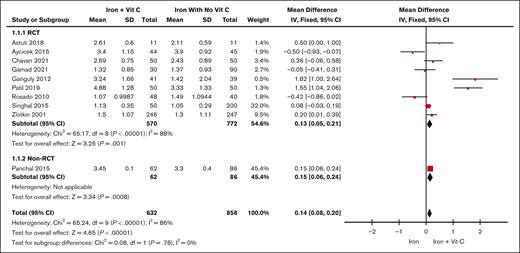
Ten studies (n = 1790)12,17-25 included in the final analysis measured serum Hb as an outcome. There was a statistically significant increase in serum Hb level in patients receiving iron plus vitamin C therapy compared with iron therapy alone (MD, 0.14 g/dL; z = 4.65; 95% CI, 0.08-0.20). The results are displayed in Figure 2. There was significant heterogeneity across the studies (I2 = 86%). The forest plot illustrates each study’s mean effect sizes and 95% CIs.
Forest plot of the change in serum Hb levels in iron vs iron plus vitamin C. The results are presented in the subgroups of RCT studies compared with non-RCT studies. Squares and horizontal lines represent the point estimate and the associated 95% CI. The diamond represents the pooled mean difference, with the center representing the point estimate and the width representing the associated 95% CI.
Forest plot of the change in serum Hb levels in iron vs iron plus vitamin C. The results are presented in the subgroups of RCT studies compared with non-RCT studies. Squares and horizontal lines represent the point estimate and the associated 95% CI. The diamond represents the pooled mean difference, with the center representing the point estimate and the width representing the associated 95% CI.
Secondary end points
Serum ferritin
Nine studies (n = 1682)10,17-21,23-25 included in the final analysis measured serum ferritin level as an outcome. There was a statistically significant increase in ferritin in the iron plus vitamin C group compared with the iron-only group (MD, 3.23 μg/L; z = 3.96; 95% CI, 1.63-4.84). The results are displayed in Figure 3. There was significant heterogeneity across these studies (I2 = 95%).
Forest plot of the change in serum ferritin levels (μg/L) iron vs iron plus vitamin C. The results are presented in the subgroups of RCT studies compared with non-RCT studies. Squares and horizontal lines represent the point estimate and the associated 95% CI. The diamond represents the pooled mean difference, with the center representing the point estimate and the width representing the associated 95% CI.
Forest plot of the change in serum ferritin levels (μg/L) iron vs iron plus vitamin C. The results are presented in the subgroups of RCT studies compared with non-RCT studies. Squares and horizontal lines represent the point estimate and the associated 95% CI. The diamond represents the pooled mean difference, with the center representing the point estimate and the width representing the associated 95% CI.
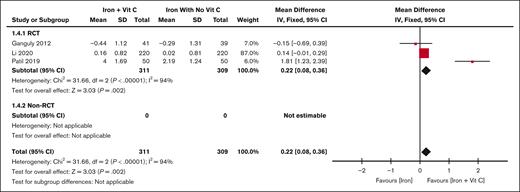
Reticulocyte percentage
Three studies (n = 620)10,12,20 measured reticulocyte percentage and found a significantly higher mean difference in the iron plus vitamin C patients than in patients receiving only iron , (MD 0.22%; z = 3.03; 95% CI, 0.08-0.36). The results are displayed in Figure 4. Significant heterogeneity was found between the 3 studies (I2 score of 95%).
Forest plot of the change in reticulocyte percentage iron vs iron plus vitamin C. The results are presented in the subgroups of RCT studies compared with non-RCT studies. Squares and horizontal lines represent the point estimate and the associated 95% CI. The diamond represents the pooled mean difference, with the center representing the point estimate and the width representing the associated 95% CI.
Forest plot of the change in reticulocyte percentage iron vs iron plus vitamin C. The results are presented in the subgroups of RCT studies compared with non-RCT studies. Squares and horizontal lines represent the point estimate and the associated 95% CI. The diamond represents the pooled mean difference, with the center representing the point estimate and the width representing the associated 95% CI.
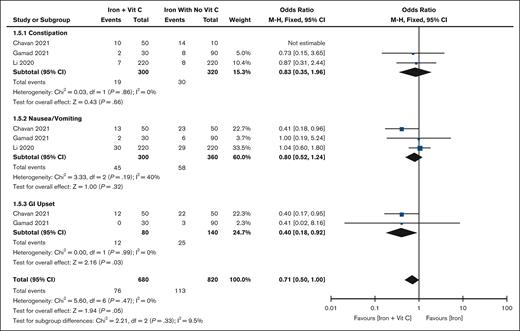
Adverse effects
Three studies (n = 660)10,19,20 reported adverse events. There were no significant differences in the overall incidence of adverse effects (OR, 0.71; 95% CI, 0.50-1.00), constipation (OR, 0.83; 95% CI, 0.35-1.96), or nausea/vomiting (OR, 0.80; 95% CI, 0.52-1.24). The results are displayed in Figure 5. However, the risk of GI irritation is significantly lower in the iron plus vitamin C group (OR, 0.40; 95% CI, 0.18-0.92) based on 2 included studies.19,20
Forest plot of adverse events iron vs iron plus vitamin C. The results are presented in the subgroups of RCT studies compared with non-RCT studies. Squares and horizontal lines represent the point estimate and the associated 95% CI. The diamond represents the pooled OR, with the center representing the point estimate and the width representing the associated 95% CI.
Forest plot of adverse events iron vs iron plus vitamin C. The results are presented in the subgroups of RCT studies compared with non-RCT studies. Squares and horizontal lines represent the point estimate and the associated 95% CI. The diamond represents the pooled OR, with the center representing the point estimate and the width representing the associated 95% CI.
Risk of bias
The results of the risk of bias assessment26,27 are displayed in supplemental Table 3. Six studies were deemed to have low concern of bias,10,19-21,23,24 whereas 3 studies were deemed to have some concern of bias,17,22,25 and 2 studies were deemed to have a high risk of bias.12,18 The first had a high risk of bias because it was an open-label RCT.18 The second was deemed to have a high risk of bias because the reported results came from a larger set of multiple eligible outcome measurements within the same outcome domain.12
Discussion
This systematic review and meta-analysis assessed the benefit of adding vitamin C to oral iron in the treatment of IDA. We found a statistically significant increase in Hb, ferritin, and reticulocyte percentage in favor of iron plus vitamin C therapy. There was no significant difference in the overall incidence of adverse events when comparing iron plus vitamin C with iron therapy, however the significant difference in GI irritation between the 2 groups was not an expected finding.
Although statistically significant, the absolute magnitude of difference between the 2 interventions was small. The mean difference in Hb was 0.14 g/dL. This difference is likely not clinically significant. Similarly, the increase in ferritin levels (3.23 μg/L) is likely not clinically relevant because the normal range for serum ferritin levels is very broad and a change such as that reported is unlikely to make a difference in the clinical presentation and management of patients.
Guidelines and routine clinical teaching and practice recommend vitamin C as an intervention along with iron.28, However, its use is solely based on the biological principle that vitamin C may enhance iron absorption by forming a chelate with ferric iron at acidic pH that then remains soluble at the alkaline pH of the duodenum.29,30 This allows for the enhancement of nonheme iron absorption, and iron absorption from vegetable meals is directly proportional to the quantity of ascorbic acid present.29,30 Our review suggests this theoretical benefit of adding vitamin C to oral iron therapy does not translate to clinically significant improvement in Hb. This may be because of the fact that oral iron alone is sufficient for Hb and iron storage recovery. Furthermore, the amount of vitamin C that exists in combination pills like ferrous ascorbate is not reported, however we were able to find a ferrous ascorbate supplement being sold online that included 80 mg of vitamin C. This finding, coupled with the results of this review, may mean that the absolute level of vitamin C used in supplementation is not enough to notice any appreciable difference compared with dietary vitamin C alone.10 Lastly, although our review found 2 studies that reported significantly lower rates of GI irritation with vitamin C supplementation, it is important to consider the additional cost of these supplements and the small sample size of patients that this finding is based off of.
The results of this meta-analysis are consistent with the current literature. A recent systematic review and meta-analysis by Loganathan et al found no statistical or clinical difference in the MD of Hb or serum ferritin between their intervention groups (ferrous ascorbate or oral iron and vitamin C) and control groups.31 The authors noted that the 4 studies they used to pool their data were of very low methodological quality. As a result, they concluded that there is insufficient evidence to support that oral vitamin C is an effective intervention to reduce anemia.31 Our review expands on this previous study through the inclusion of several more level-1 studies and the inclusion of studies with different therapy formulations, which provides greater statistical power and external validity of the results of this review. It is important to note that patients with symptomatic iron deficiency may not necessarily be anemic. Despite this, there does not seem to be any prospective studies that examine the effects of iron supplementation on symptomatic iron deficiency without anemia. Hence, our systematic review focused solely on patients who have anemia as a result of iron deficiency, and the results of the review apply to such patients.
Strengths and limitations
The key strengths of this review include the comprehensive and rigorous search and article screening process, as well as a clearly stated objective to answer a focused question of clinical relevance. Another strength of this review is the synthesis and analysis of several level-1 studies, with the majority of which being deemed to have a low risk of bias.
With respect to limitations, there was significant heterogeneity across the 11 studies included in our analysis. The I2 scores were >80% for the 4 outcomes of interest. There was also clinical heterogeneity, given that included patients ranged from infants to adults, patients had varying severity of anemia, and the formulations used varied from study to study, especially with respect to the amount of elemental iron and vitamin C used. Therefore, cautious interpretation of the results of this meta-analysis is warranted. Although 6 of 11 studies included were deemed to have a low risk of bias, 2 were deemed to have a high risk of bias, which decreases the internal validity of this review.
Conclusion
This systematic review and meta-analysis sought to determine whether oral iron supplementation with vitamin C improves hematologic outcomes compared with supplementation without vitamin C for patients with IDA. After statistical analysis was performed, we found that adding vitamin C to iron supplementation in patients with IDA significantly increases Hb and ferritin without increasing the risk of adverse effects. However, given the small actual difference, and the significant heterogeneity of the studies available, our findings do not support the routine use of vitamin C with oral iron for treatment of IDA.
Acknowledgments
A.E. is supported by the American Society of Hematology 2023 Hematology Opportunities for the Next Generation of Research Scientists Award. A.L. reports grants from the American Society of Hematology and the Canadian Society for Vascular Surgery.
Authorship
Contribution: J.D., G.S., and M.A.C. designed the research protocol; J.D., L.R., P.Y.L., and G.S. performed the research (data screening, collection, and extraction); J.D., L.R., P.Y.L., A.L., A.E., and M.A.C. analyzed and interpreted data; J.D., L.R., P.Y.L., A.L., A.E., G.S., and M.A.C. wrote the manuscript; and all authors made substantial contributions to the analysis and interpretation of data for the work, drafting the work, reviewing it critically for important intellectual content and giving final approval of the version to be published.
Conflict-of-interest disclosure: M.A.C., in the last 36 months, has received personal funding, including but not limited to preparation of educational material, participation in advisory boards, or providing expert testimony for Bayer, AstraZeneca, Pfizer, CSL Behring, Hemostasis Reference Laboratories, Syneos Health, and Eversana; has participated in various medicolegal activities relating to thrombosis, anticoagulant drugs, or other aspects of internal medicine and hematologic practice; has worked with multiple for-profit and not-for-profit entities such as UpToDate and medical communication companies; and holds the Leo Pharma Chair in Thromboembolism, endowed at McMaster University. The remaining authors declare no competing financial interests.
Correspondence: Joanna Deng, Temerty Faculty of Medicine, University of Toronto, 1 King’s College Cir, Toronto, ON, Canada, M5S 1A8; email: joanna.deng@mail.utoronto.ca.
References
Author notes
J.D. and L.R. contributed equally to this study.
Presented in abstract form at the 65th annual meeting of the American Society of Hematology, San Diego, CA, 9 December 2023.
Data are available on request from the corresponding author, Joanna Deng (joanna.deng@mail.utoronto.ca).
The full-text version of this article contains a data supplement.