Key Points
Using 423 days of patient data from 2 institutions, leukocytes and platelet counts measured during TTP are significantly concordant.
A rising total leukocyte count in a patient with TTP is not necessarily a worrisome trend but, rather, might be a welcome trend.
Visual Abstract
Thrombotic thrombocytopenic purpura (TTP) is a rare disorder involving pathological platelet–von Willebrand factor interaction, resulting in microvascular thrombosis. The consumption of platelets in TTP microthrombi results in severe thrombocytopenia which resolves with resolution of the microvascular thrombosis. Over the course of treatment, patient platelet counts often rise and fall multiple times before stable remission is achieved. On casual inspection, we noted that total leukocyte counts follow a pattern that appears to correspond to platelet counts. To explore whether changing leukocyte counts track significantly with changing platelet counts, we examined paired daily platelet counts and total leukocyte counts in 27 episodes of TTP in 13 patients across 2 institutions comprising 423 days of data. We modeled the nonlinear behavior in the data using a threshold mixed-effects regression model, in which we found a significant temporal relationship between total leukocyte counts and platelet counts. The model proved that, on a day when platelet counts were rising in previous days, the total leukocyte count is predictive of the rise in platelet count. The higher the total leukocyte count, the greater the rise in platelet count. Our results support the hypothesis that leukocytes play a role in the resolution of TTP microvascular thrombosis.
Introduction
Thrombotic thrombocytopenic purpura (TTP) is a rare microvascular thrombosis syndrome characterized by obstruction of microvasculature by platelet and von Willebrand factor (VWF)–rich microthrombi.1 The occurrence of platelet microthrombi is associated with severe thrombocytopenia and microangiopathic hemolytic anemia. The standard of care for treating TTP is daily plasma exchange, typically augmented by immunomodulatory drug therapy.2 The clinical course of TTP is monitored by laboratory tests usually including hemoglobin or hematocrit, lactate dehydrogenase, and platelet count (PLT). PLTs are often the most relied-upon tests to determine response to treatment and achievement of remission. A common definition of adequate response to treatment is the occurrence of 2 consecutive days of PLTs in the normal range. However, exacerbations of TTP, as evidenced by falling PLTs after a previous rise, are common. Before modern immunomodulatory therapies, an undulating course of rising and falling PLTs before eventual stable remission was frequent.
In the late 1990s, the metalloprotease ADAMTS13 was discovered and deficient activity of ADAMTS13 was established as a major cause of microvascular thrombosis in TTP.3,4 In the most common form of TTP, ADAMTS13 metalloprotease is targeted by autoantibodies that inhibit or clear the enzyme from circulation. The titers of autoantibodies and degree of suppression of ADAMTS13 activity have been linked to the length and durability of courses of treatment.5,6
Since the initial reports of the link between deficient activity of ADAMTS13 and TTP, a puzzling disconnect between ADAMTS13 activity and the clinical course of TTP has been noted in many patients.3,5 Many patients have been observed to achieve remission from TTP without apparent normalization of ADAMTS13 activity in plasma. This discordant observation of PLT recovery occurring despite persistent deficiency of ADAMTS13 activity suggests that other enzymes may regulate VWF activity sufficiently to abate the process of microvascular thrombosis. Exploring that hypothesis, previous studies showed that several neutrophil-derived proteases are able to cleave VWF substrate in vitro at or near the tyrosine-methionine peptide bond cleaved by ADAMTS13.7 This observation suggested a potential mechanism by which remission from clinical TTP might be achieved without concurrent normalization of ADAMTS13 activity. Since the initial observation of neutrophil protease cleavage of ADAMTS13, additional studies have confirmed the contribution of accessory enzymes in the regulation of VWF in certain disease states.8-10
Considering the potential of neutrophil proteases as accessory enzymes in the regulation of VWF activity in TTP, our casual inspection of medical records of patients with TTP revealed a remarkably undulating course of total leukocyte counts (TLCs) accompanying the rise and fall of PLTs in patients with TTP experiencing remissions and exacerbations. This observation prompted a systematic exploration of the hypothesis that TLCs correspond with PLTs during the course of TTP. Here, we provide statistical evidence that TLCs and PLTs measured during TTP are significantly concordant, supporting the possibility that leukocyte proteases downregulate VWF activity and abate the process of microvascular thrombosis and thrombocytopenia.
Methods
Data
Acquisition and use of data were approved by the human research subject committees of the University of Iowa and the University of Wisconsin. Clinical records of patients who received apheresis treatment were reviewed for the diagnosis of TTP. Patients who received plasma exchange treatment for TTP and whose medical records were retrievable in electronic format were entered into the study. In cases with multiple treatment episodes, all episodes were included. All patients achieved clinical remission in all episodes included in the study. The data included 6 patients at the University of Iowa with 19 total episodes, and 7 patients at the University of Wisconsin with 8 total episodes. PLTs and TLCs were obtained from daily complete blood counts and were recorded with corresponding dates. In cases of multiple complete blood counts per day, the earliest of the day was recorded. Leukocyte differential counts were not consistently available, so TLCs were used for this study. Each patient’s data therefore comprised 1 or more series of consecutive dates with corresponding TLCs and PLTs. In the statistical modeling, the entire time series of each patient (with multiple episodes for some patients) was used. A total of 423 days of data were available to fit the model.
Time lags in simple terms
Figure 1 is a schematic diagram to describe the lags for PLT in simple terms, in which the arrows between the lagged PLTs are placed in chronological order. In summary, PLT lag 0 is the PLT today, PLT lags 1 and 2 are the PLTs 1 day ago and 2 days ago, respectively.
A schematic diagram to describe the lag for PLT in simple terms. The arrows are placed in chronological order.
A schematic diagram to describe the lag for PLT in simple terms. The arrows are placed in chronological order.
Statistical approach
The TLCs of Iowa patients have a mean of 7.75 × 103/μL, a median of 7.3 × 103/μL, and a standard deviation (SD) of 2.49 × 103/μL. The TLCs of Wisconsin patients have a mean of 11.68 × 103/μL, a median of 10.3 k/μL, and an SD of 6.07 /× 103μL.
The PLTs of Iowa patients have a mean of 156.7 × 103/μL, a median of 137 × 103/μL, and an SD of 105.43 × 103/μL. The PLTs of Wisconsin patients have a mean of 114.4 × 103/μL, a median of 107 × 103/μL, and an SD of 76.61 × 103/μL. Although the TLCs of Iowa patients tend to be lower and less variable than the TLCs of Wisconsin patients, the PLTs of Iowa patients have a higher mean and median and are more variable than the PLTs of Wisconsin patients; see box plots in Figure 2.
The response variable is the log-transformed PLT and is assumed to be normally distributed. The response variable varies nonlinearly with respect to the (lagged) percentage change (increase or decrease) in PLTs between the 2 previous days, as demonstrated in Figure 3. The red curve in Figure 3 is a loess smoother that is added to the plot to highlight the nonlinear patterns in the data.
Plot of log PLTs vs lagged percentage change in PLTs between the last 2 consecutive days. The red curve is a loess smoother that illustrates the nonlinear dynamic structure in the data. The vertical line indicates the location of the estimated threshold parameter at −0.425%.
Plot of log PLTs vs lagged percentage change in PLTs between the last 2 consecutive days. The red curve is a loess smoother that illustrates the nonlinear dynamic structure in the data. The vertical line indicates the location of the estimated threshold parameter at −0.425%.
We model this nonlinear behavior using a generalized threshold regression model11-15 in which the mean of the response variable is assumed to be piecewise linear such that the behavior of the log PLTs is broken into 2 linear regression models based on where the value of the percentage change in PLTs in the last 2 days falls, whether this percent change is below a cutoff point (estimated by the model) or above the estimated cutoff point (we refer to the cutoff point as a threshold). If the lagged percent change in PLTs is below a threshold, the set of observations would belong to a domain called the “lower domain”; observations above the threshold belong to a domain referred to as the “upper domain.” In the lower domain, the log PLTs are modeled using a linear mixed-effects regression model defined by a set of explanatory variables that may differ from the linear mixed-effects regression model simultaneously produced in the upper domain.16 R software is used to fit the model.17
In terms of notations, let denote the PLTs for patient s, in hospital h, at time t. Let denote the TLCs for patient s, in hospital h, at time t. Let denote the (lagged) percent change (increase or decrease) in PLTs for patient s, in hospital h, at time t-1, that is, between the last 2 consecutive days (t-1 and t-2). The variable is called the threshold variable.
The parameter r is called the threshold parameter, is assumed to be unknown and is estimated. The intercept parameter and the regression parameter are the fixed effects in the linear submodel of the lower domain (ie, corresponding to a lagged percent change in PLTs less than or equal to r). The intercept parameter and the regression parameters are the fixed effects in the linear submodel of the upper domain (ie, corresponding to a lagged percent change in PLTs strictly greater than r).
The random effects are at the hospital level and account for variability between hospitals. They are assumed to be independent for different hospitals h, normally distributed with mean 0 and variance .
The within-group (ie, within-patient) error terms are assumed to be independent across hospitals h and for different patients s, and independent of the random effects . In addition, the error terms are assumed to be normally distributed with mean 0 and heteroscedastic hospital-varying variances that also depend on the value of the PLT of the previous day. By fitting this model, we see that the fitted values have more variability and are less predictable when the PLTs on the previous day (referred to as “lag-1 PLT”) are in a lower range than the fitted values when the lag-1 PLTs are in a higher range. We adjust for this by accounting for the error terms to have heteroscedastic variances varying per hospital and per the range of lag-1 PLTs (the cutoff point of which is estimated using our threshold model using a grid search of the lag-1 PLTs and is found to be 81.45 × 103/μL). Thus, we assume that the error variances are equal to if the patient is an Iowa patient and has a PLT on the previous day of ≤81.45 × 103/μL, if the patient is an Iowa patient and has a PLT on the previous day of >81.45 × 103/μL; and if the patient is a Wisconsin patient and has a PLT on the previous day of ≤81.45 × 103/μL, and if the patient is a Wisconsin patient and has a PLT on the previous day of >81.45 ×103/μL. The within-group serial correlation of the error terms is assumed to have an autoregressive correlation structure of order 2.16
Using a likelihood-based estimation approach, the threshold parameter r is estimated to be −0.00425 using a grid search between the 20th percentile and the 80th percentile of the lagged threshold variable, that is, the lagged percent change in PLTs. The remaining maximum likelihood estimates of the parameters in the fitted model are in Table 1, along with their standard errors and their 95% confidence intervals.
Maximum likelihood estimates of the parameters in the fitted model
| Variable . | Estimated value . | Asymptotic standard error . | Asymptotic 95% confidence interval . |
|---|---|---|---|
| Lower domain | |||
| Intercept | 0.964 | 0.149 | 0.672-1.26 |
| PLT of previous day (on the log scale) | 0.819 | 0.0310 | 0.758-0.880 |
| Upper domain | |||
| Intercept | 0.919 | 0.147 | 0.631-1.21 |
| PLT of previous day (on the log scale) | 0.749 | 0.0257 | 0.699-0.799 |
| TLC (on the log scale) | 0.213 | 0.0391 | 0.136-0.290 |
| Random effects | |||
| SD of the random effects | 0.00638 | 0.000496-0.0820 | |
| Error terms | |||
| Within-group standard error for Iowa patients with lower range of previous day PLT | 0.364 | 0.315-0.420 | |
| Within-group standard error for Wisconsin patients with lower range of previous day PLT | 0.504 | 0.409-0.621 | |
| Within-group standard error for Iowa patients with higher range of previous day PLT | 0.216 | 0.183-0.255 | |
| Within-group standard error for Wisconsin patients with higher range of previous day PLT | 0.179 | 0.144-0.223 | |
| AR(1) correlation parameter | 0.389 | 0.306-0.428 | |
| AR(2) correlation parameter | 0.151 | 0.0352-0.263 |
| Variable . | Estimated value . | Asymptotic standard error . | Asymptotic 95% confidence interval . |
|---|---|---|---|
| Lower domain | |||
| Intercept | 0.964 | 0.149 | 0.672-1.26 |
| PLT of previous day (on the log scale) | 0.819 | 0.0310 | 0.758-0.880 |
| Upper domain | |||
| Intercept | 0.919 | 0.147 | 0.631-1.21 |
| PLT of previous day (on the log scale) | 0.749 | 0.0257 | 0.699-0.799 |
| TLC (on the log scale) | 0.213 | 0.0391 | 0.136-0.290 |
| Random effects | |||
| SD of the random effects | 0.00638 | 0.000496-0.0820 | |
| Error terms | |||
| Within-group standard error for Iowa patients with lower range of previous day PLT | 0.364 | 0.315-0.420 | |
| Within-group standard error for Wisconsin patients with lower range of previous day PLT | 0.504 | 0.409-0.621 | |
| Within-group standard error for Iowa patients with higher range of previous day PLT | 0.216 | 0.183-0.255 | |
| Within-group standard error for Wisconsin patients with higher range of previous day PLT | 0.179 | 0.144-0.223 | |
| AR(1) correlation parameter | 0.389 | 0.306-0.428 | |
| AR(2) correlation parameter | 0.151 | 0.0352-0.263 |
AR, autoregressive.
The need for a heteroscedastic fit for the error terms is highlighted by the nonoverlapping 95% confidence intervals of the within-group standard errors for Iowa patients and Wisconsin patients in Table 1. The model shows high variability when the range of lag-1 PLTs are in the lower range of 81.45 × 103/μL or below; see results of heteroscedastic fit of the error terms in Table 1.
Model diagnostics evaluating the model and its fitting are in the supplemental Material.
Results
Dependent variable
To understand the relationship between TLCs and PLTs, the log-transformed PLT was the dependent variable assumed to be normally distributed. We wanted to know whether anything about log-transformed TLC (independent variable) told us anything about the (log) PLT, based on additional information on the PLTs in the last 2 days such as: the percent change in PLT from 2 days ago (PLT lag 2) to PLT from 1 day ago (PLT lag 1), and the actual value of PLT lag 1.
Threshold model
We used a threshold effects parametric model determined to optimize the fitting and interpretation of PLT data; see “Methods.” The threshold model identified 2 domains of PLT data based on the percentage change in (lagged) PLTs from 2 days ago to 1 day ago. The threshold derived from the model is at or near 0 (estimated at −0.00425), with upper domain data representing a positive percent change in lagged PLTs, and lower domain representing a negative percent change. In other words, the PLT data were grouped according to whether antecedent PLTs from 2 days (PLT lag 2) and 1 day ago (PLT lag 1) were rising (upper domain) or falling (lower domain).
Institutional consideration
The behavior of the data in the model gave similar results in the University of Wisconsin data and the University of Iowa data. Therefore, the overall interpretation of the data using the model is illustrated for both the University of Wisconsin and the University of Iowa data. The threshold mixed-effects model that allows for variation between hospitals and between patients and incorporates within-patient time dependence, is applied to both institutions’ data in both the upper and lower domains, and the conclusions derived from the model are consistent between the institutions and in both domains.
Modeling TLC with PLT
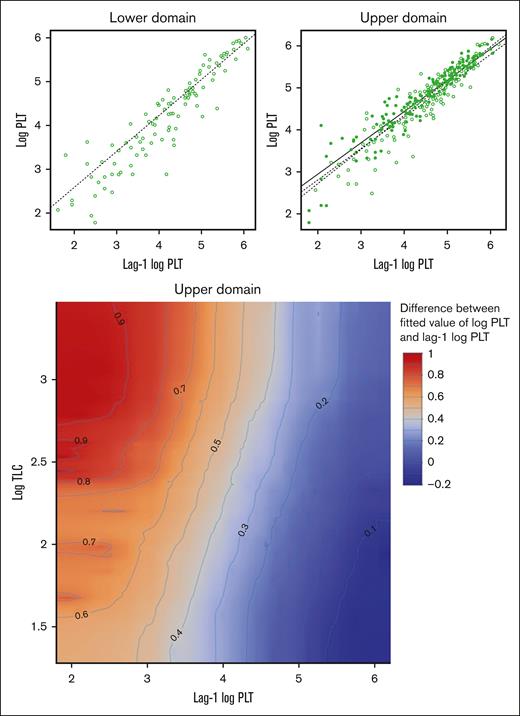
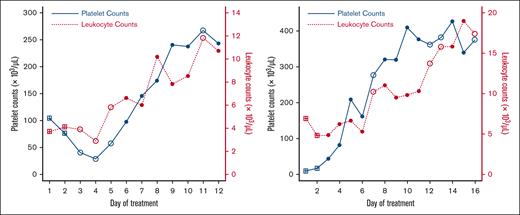
Using metrics of model quality or strength as a guide, we showed that the relationship between PLT and TLC is significant when we used today’s log-transformed TLC as the independent continuous variable. The plots in Figure 4 show the final model applied to both University of Wisconsin and University of Iowa in both the upper and lower domains of PLT data. These plots in Figure 4 represent an analytical scheme in which daily PLT data and TLC data are analyzed to determine a statistical association between rising and falling levels of each. The plot of the lower domain has 1 regression line which represents the association of the (log) PLT lag 1 on the (log) PLT lag 0, without any effect of TLC. The plot of the upper domain has 3 regression lines. These represent plots of (log) PLT lag 0 vs PLT lag 1 when the TLC was either above the mean (solid circles and solid line) or below the mean (open circles and dashed line). The dotted line represents the fitted line ignoring the effect of (log) TLC; that is, this line is the result of fitting a linear regression to the observations in the scatterplot of the upper domain (top right panel of Figure 4). The important features of these plots are that the solid and dashed lines have the same fixed-effects overall mean slopes between institutions (whereas the random effect accounts for variability across hospitals), and they have significantly different y-axis intercepts. The y-axis indicates the magnitude of PLT on the log scale. In simple terms, the model shows that (1) there is a significant statistical relationship between today’s TLC and PLTs (lag 0), and (2) today’s TLC is associated with a significantly larger increase in PLT lag 0 − PLT lag 1; and this is true when the PLT lag 2 to PLT lag 1 changes were rising (upper domain). The contour plot in the bottom panel of Figure 4 shows that the expected increment in PLT from yesterday to today, for a patient whose yesterday’s PLT is 7400 per μL and with rising PLT in the previous 2 days, will differ between at least 1822 and 2460 per μL, depending on the value of today’s TLC (as the value of TLC increases, the expected increment increases). However, for a patient whose yesterday’s PLT is 148 413 per μL and with rising PLT in the previous 2 days, this expected increment is between about 1105 and 1221 per μL. The regression lines and the contour plot in Figure 4 show that the impact of higher or lower TLCs is significant only in the upper domain, when PLTs are rising. The contour plot shows that the impact of TLCs on the expected increment in PLT from yesterday to today tends to be more pronounced in magnitude as yesterday’s PLT decreases. This concordance behavior between PLT and TLC can also be seen in the illustrative time-series plots of 1 episode in each of 2 patients (Figure 5).
Analytical scheme of the final fitted model in both the upper and lower domains of PLT data. The top panel shows a plot of log PLTs vs log PLTs of the previous day for the lower domain (left panel) and the upper domain (right panel). The dotted line is the fitted value of log PLTs without correcting for the effect of the TLCs. The dotted line in the lower domain is plotted using the parameter estimates in Table 1; however, the dotted line in the upper regime is plotted based on the results of fitting a linear regression to the observations in the plot. The open circles represent observations whose log TLCs are less than or equal to their overall mean value of 2.1. The solid circles represent the observations whose log TLCs are above their overall mean value of 2.1. The dotted line is the fitted line of log PLTs when log TLCs are fixed at 1.814, the mean value of the observations indicated in open circles. The solid line is the fitted line of log PLTs when log TLCs are fixed at 2.453, the mean value of the observations indicated in solid circles. The bottom panel shows a contour plot illustrating the effect of lag-1 log PLT and log TLC on the difference between the fitted value of log PLT and lag-1 log PLT, in the upper domain. In the upper domain, controlling for lag-1 log PLT (ie, for a fixed value of lag-1 log PLT), as log TLC increases, the expected difference between the fitted value of log PLT and lag-1 PLT increases and is positive.
Analytical scheme of the final fitted model in both the upper and lower domains of PLT data. The top panel shows a plot of log PLTs vs log PLTs of the previous day for the lower domain (left panel) and the upper domain (right panel). The dotted line is the fitted value of log PLTs without correcting for the effect of the TLCs. The dotted line in the lower domain is plotted using the parameter estimates in Table 1; however, the dotted line in the upper regime is plotted based on the results of fitting a linear regression to the observations in the plot. The open circles represent observations whose log TLCs are less than or equal to their overall mean value of 2.1. The solid circles represent the observations whose log TLCs are above their overall mean value of 2.1. The dotted line is the fitted line of log PLTs when log TLCs are fixed at 1.814, the mean value of the observations indicated in open circles. The solid line is the fitted line of log PLTs when log TLCs are fixed at 2.453, the mean value of the observations indicated in solid circles. The bottom panel shows a contour plot illustrating the effect of lag-1 log PLT and log TLC on the difference between the fitted value of log PLT and lag-1 log PLT, in the upper domain. In the upper domain, controlling for lag-1 log PLT (ie, for a fixed value of lag-1 log PLT), as log TLC increases, the expected difference between the fitted value of log PLT and lag-1 PLT increases and is positive.
Time-series plots of PLTs (solid blue) and TLCs (dotted red) for 1 episode in 2 patients illustrating concordance in changes in counts. The observations indicated with a square do not belong to any domain because the data at these time points have their lagged PLTs (lag 1 PLT and lag 2 PLT) missing. The observations indicated with an open circle belong to the lower domain (with lagged percent change in PLT less than or equal to −0.00425), and with a solid circle belong to the upper domain.
Time-series plots of PLTs (solid blue) and TLCs (dotted red) for 1 episode in 2 patients illustrating concordance in changes in counts. The observations indicated with a square do not belong to any domain because the data at these time points have their lagged PLTs (lag 1 PLT and lag 2 PLT) missing. The observations indicated with an open circle belong to the lower domain (with lagged percent change in PLT less than or equal to −0.00425), and with a solid circle belong to the upper domain.
When the PLT lag 1 is ≤81.45 × 103/μL, it is seen that the variability of the data is larger, and hence, the fitted values of the (log) PLTs have less accuracy than when the PLT lag 1 is >81.45 × 103/μL. The model adjusts for this aspect of the data.
In summary, the analysis shows that, when the PLT lag 2 to PLT lag 1 was rising (upper domain), today’s TLC has a significant probability of being accompanied by a larger positive change in PLT lag 1 to PLT lag 0, controlling for lag-1 PLT (ie, for a fixed lag-1 PLT), higher TLC, bigger increase in PLT, lower TLC, and smaller increase in PLT.
Discussion
This study was based on the clinical observation that during episodes of TTP both daily PLTs and TLCs varied markedly and seemingly correspondingly. In the often waxing and waning course of TTP, large fluctuations in PLTs are well known. However, large fluctuations in total TLCs (up to 5-fold in our data) have not been reported or explained. We pursued a possible correspondence between PLT and TLC with a statistical interrogation of the data, seeking the most informative model. The statistical model that emerged incorporates a threshold effect in which daily PLTs are either trending upward (upper domain) or downward (lower domain). We showed that when PLTs were trending upward, the percent increase in each daily PLT corresponded significantly with a corresponding increase in TLC. The absence of a significant correspondence between TLC changes and falling PLTs (lower domain) might be related to the far fewer number of falling PLT events. The observation that rising PLTs are accompanied by rising TLC is concordant with the observation that in the 27 TTP episodes analyzed in this study the mean TLC at presentation was 7.7 × 103/μL and at remission was 10.2 × 103/μL (P = .014; Student t test).
The importance of our work is that we found a statistical model that proves a temporal mathematical relationship between TLC and PLTs, and the model shows that higher current TLCs are associated with larger positive changes in PLTs when the PLTs are rising in the previous 2 days. So, when the anxious clinician is hoping for a higher PLT today in their patients with TTP, they should also be hoping for a higher TLC.
The reasons for these relationships could be many. A higher TLC being accompanied by a greater rise in PLT could, in some cases, be simply incidental to the effects of corticosteroids causing a rise in neutrophil counts while abating the autoimmune deficiency of ADAMTS13 activity. Although we did not have data to explore this possibility, the multiple episodes of rising and falling TLCs often observed during single episodes (Figure 5) suggest a more dynamic influence than corticosteroid-induced leukocytosis.
During an episode of TTP, a falling PLT may be assumed to reflect an increase in platelet-VWF microthrombosis. It is possible that evolving microthrombi consume leukocytes as well as platelets. And the opposite may be true as well. Therefore, our finding that rising PLTs are significantly associated with rising TLC might simply represent decreased consumption of leukocytes and platelets as the microvascular thrombosis resolves. If this phenomenon reflects some activity of leukocytes in TTP microthrombus formation, the question arises what function might they serve? It is known that leukocytes have both prothrombotic and antithrombotic effects in clot formation.18
The microthrombi in TTP are unusual in their formation and composition. They result from inadequate enzymatic downregulation of VWF permitting pathological platelet-VWF binding. In addition to ADAMTS13, studies have shown that several neutrophil-derived proteases cleave VWF at or near the ADAMTS13 cleavage site.7-10 An alternative explanation for the phenomenon might therefore be increasing or decreasing concentrations of neutrophils supplying more or less protease for cleavage and downregulation of VWF. Downregulating VWF function would be expected to downregulate platelet-VWF aggregation, resulting in rising PLTs. In this pathogenic model, greater numbers of leukocytes with more enzymatic regulation of VWF (independent of ADAMTS13) lead to resolving thrombocytopenia. Notably, it is not consistent with our data that comorbid events in patients causing rising TLCs should be accompanied by falling PLTs and worsening of TTP disease.
Potentially critical clues to the mechanism of platelet-leukocyte interactions in TTP were recently described by Yada et al.19 These investigators observed that neutrophil extracellular trap formation is markedly upregulated during acute episodes of TTP. In experimental models, both neutrophils and platelets are entangled in thrombi, which are prevented or resolved by the addition of recombinant ADAMTS13 or caplacizumab, a drug that blocks platelet-VWF interaction. This elegant study indicates that both platelets and neutrophils (the major subpopulation of leukocytes) are trapped in TTP microthrombi, and that thrombus formation is abated by enzymatic cleavage of VWF. This mechanism is consistent with our observation that PLTs and TLCs rise concurrently in TTP. Future studies analyzing the relationship of platelet counts with neutrophils and other leukocyte subpopulations may further clarify the complex mechanism of microvascular thrombosis in TTP.
Insofar as our data support that rising TLC portends disease resolution in TTP, it may be noted that in STX-HUS (Shiga toxin–associated hemolytic uremic syndrome), the opposite is true; higher neutrophil counts are a poor prognostic sign.20 This contrast emphasizes the different pathological mechanisms of microvascular thrombosis in TTP and STX-HUS.
Limitations of our study include the absence of data on drugs, intercurrent illnesses, or other physiological or clinical events that may have contributed to changes in TLCs. However, the phenomenon being consistent across 2 hospitals and multiple patients, we hypothesize that the rise in PLT appears to be largely independent of the causes of the rise in TLC. The time span of our study includes a period before ADAMTS13 testing was readily available or at all standardized, and predates therapeutic advances such as caplacizumab. We therefore did not include ADAMTS13 activity as a parameter in our modeling.
In conclusion, our data reveal a statistical association between rising TLCs and rising PLTs in patients recovering from TTP. This relationship is found within a very heterogeneous and potentially noisy data set derived from 2 hospitals over a several-year time span. The 1 commonality among all patients was the use of plasma-exchange therapy. Thus, from a statistical modeling standpoint, observing the signal despite the noise is a sign of strength of the signal, not a methodological weakness. Clinicians may therefore be relieved to understand that a rising TLC in a patient with TTP is not necessarily a worrisome trend but, rather, might be a welcome trend. Indeed, whether deliberately raising the TLC in patients with TTP could be an effective treatment measure in TTP is a matter for consideration.
Authorship
Contribution: N.I.S. and T.J.R. designed the research, performed the research, analyzed the data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Noelle I. Samia, Department of Statistics and Data Science, Northwestern University, 2006 Sheridan Rd, Evanston, IL 60208; email: n-samia@northwestern.edu.
References
Author notes
Because of the data confidentiality, the data cannot be shared.
The full-text version of this article contains a data supplement.