Key Points
Among demographic, clinical, and treatment factors, emicizumab had the greatest effect in decreasing joint bleeding rate in hemophilia A.
Patient and community factors, including age, treatment adherence, insurance, and employment, also influenced joint bleeding rates.
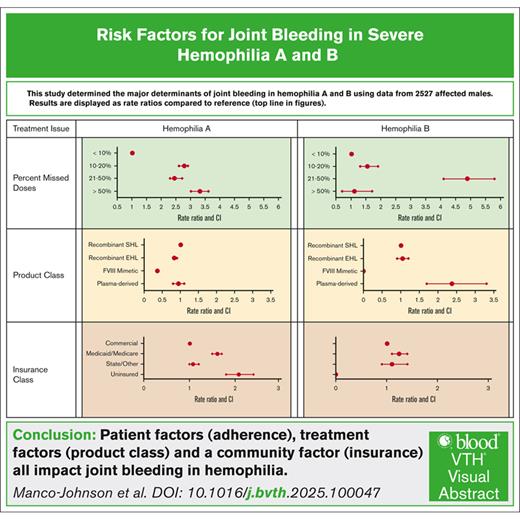
Visual Abstract
Joint bleeding is the primary determinant of end-stage arthropathy in hemophilia; joint bleeding has greatly decreased with the use of prophylaxis and introduction of highly effective therapies. This study aimed to determine current risk factors for joint bleeding in persons with severe hemophilia A or B. Demographic, treatment, and bleeding outcome data from Community Counts, a US national surveillance project, were analyzed. Data were collected at annual visits between 2013 and 2022. Eligibility included factor VIII or IX of <1%, no inhibitor, age of 2 to 44 years, and on treatment with continuous prophylaxis. Annual joint bleeding rate (AJBR) differences across demographic and clinical subgroups were compared via rate ratios and 95% confidence intervals, and with multivariate methods accounting for multiple measurements over time. The analysis included 2527 males with hemophilia, 7211 observation years, and 10 046 joint bleeds. Lower AJBR in hemophilia A was most strongly associated with use of emicizumab. For both hemophilia A and B, patient-associated factors, younger age, fewer missed doses, and full employment were all independently associated with lower AJBR, as was treatment in the Northeast of the United States. The findings of this comprehensive analysis of a large, diverse sample drawn from hemophilia treatment centers across the United States, underscore the important contributions of patient, community, and treatment factors on joint outcomes in severe hemophilia. Use of emicizumab and few missed doses independently predicted low AJBR, highlighting the interplay of access and adherence to care.
Introduction
Hemophilia A and B are inherited bleeding disorders that result in deficiencies of coagulation factors VIII and IX, respectively. These diseases are relatively rare; hemophilia A affects 15.7 per 100 000 males whereas hemophilia B affects 3.7 per 100 000 males.1 Despite the rarity of these diseases, significant progress has been made in understanding the disease phenotype and its management. Historically, bleeding into joints in persons with severe hemophilia, defined as <1% normal plasma clotting factor activity, resulted in chronic arthropathy with pain and decreased joint range of motion that substantially affected physical function and quality of life.2 End-stage joint disease, resulting from recurrent joint hemorrhage and requiring invasive orthopedic interventions such as joint replacement (arthroplasty) or surgical fusion (arthrodesis), has historically been one of the most disabling and costly complications of severe hemophilia.3 With the implementation of continuous prophylaxis treatment regimens with factor VIII/IX concentrates during the past 20 years, there has been significant decline in joint disease.4
Currently, the outlook for severe hemophilia has become much brighter, because of the development of a number of breakthrough therapies that treat and prevent bleeding. These range from extended-half-life (EHL) recombinant replacement factors,5-7 to factor VIII mimetics (emicizumab) that promote the functional activation of factor X in the absence of factor VIII for people with hemophilia A,8 hemostasis rebalancers that increase thrombin generation by reducing physiologic inhibitors of thrombin,9 and gene therapies that restore in vivo production and release of factor VIII or IX into the plasma.10 These therapies have widely different mechanisms of action and potential adverse effects. Because the results of these approaches cannot be compared in laboratory assays or other discrete measurements, clinical trials rely upon episodes of clinical joint bleeding as primary outcome measures. Most clinical trials do not measure contributors to joint bleeding other than treatment product or dose schedule.
Prophylaxis with factor VIII effectively reduces the clinically recognized bleeding episodes (termed annualized bleeding rates [ABR]), that are often trauma induced, from a reported rate of 24.2 ± 17.1 per year to 1.7 ± 4.2 per year.11 Differences in the ABR or joint ABR (AJBR) derived from clinical trials of new agents may reflect differences in underlying joint health of the participants rather than differential effects of the experimental product per se. In addition, sample sizes in pharmaceutical licensure trials are too small and follow-up remains too short to detect meaningful differences in long-term outcome. Because the roots of end-stage joint damage may be sown years before invasive orthopedic interventions are sought, comparison of treatment regimens proximal to joint failure may be misleading. It is unknown whether the remarkable short-term efficacy of the newer therapies will translate to prevention of arthropathy in children who have access to these new approaches from a young age.
In addition to the impact of newer therapies on the reduction of bleeding and subsequent joint disease, it is important to concurrently consider the likely impact of patient-specific risk factors that influence joint outcomes. A previous study using joint range of motion as a surrogate for joint function in individuals with hemophilia aged 2 to 19 year found that older age, non-White race, and elevated body mass index (BMI) were associated with decreased initial range of motion status, whereas elevated BMI increased, and initiation of continuous prophylaxis therapy before the age of 4 years decreased, the rate of range of motion loss over time.4 To address the question of predictors of joint outcomes in hemophilia we sought to determine current predictors of joint bleeding in individuals with severe hemophilia on continuous prophylaxis in the context of newer therapies using data from a national hemophilia surveillance project.
Methods
This study analyzed data from a national surveillance project, Community Counts, funded by the US Centers for Disease Control and Prevention and administered through the American Thrombosis and Hemostasis Network conducted in 147 US hemophilia treatment centers (HTCs). Data were collected either as part of routine surveillance with a waiver of individual consent or with written informed consent, as determined by the ethics committee of each of the participating HTC, as previously described.12 This study was conducted in accordance with the Declaration of Helsinki. Data were collected at the time of annual comprehensive clinical evaluations, between 1 December 2013 and 30 November 2022. Clinical data, including race, ethnicity, the number and location of joint bleeds, and treatment adherence since the last annual visit, were recorded using medical record abstraction and patient report.
Eligibility for this analysis included persons with severe hemophilia aged 2 to 44 years without inhibitory antibodies to the deficient clotting factor and on treatment with continuous prophylactic therapy. Continuous prophylactic therapy was defined as regular infusions of replacement factor or an equivalent nonfactor product given to prevent any and all bleeding events. The primary outcome included AJBR of 10 joints including bilateral shoulders, elbows, hips, knees, and ankles. The secondary outcome focused on patient-specific (demographic and social) and treatment-specific risk factors for AJBR.
In addition to AJBR, data extracted included age; race; ethnicity; BMI; education; employment and insurance status; treatment product class, that is, plasma-derived clotting factor, standard half-life (SHL) recombinant factor, EHL clotting factor, or factor VIII mimetic; percentage of missed doses; and region of the country in which hemophilia care was received. BMI was categorized for adults aged ≥20 years as follows: underweight, <18.5; normal, 18.5 to 24.9; overweight, 25.0 to 29.9; and obese, ≥30. For children aged 2 to 19 years, BMI was characterized using US Centers for Disease Control and Prevention guidelines in which underweight is below the 5th percentile for age, normal is 5th to 84th percentile, overweight is 85th to 94th percentile, and obese is 95th or above percentile (https://www.cdc.gov/bmi/child-teen-calculator/widget.html).
In this study, AJBR differences across demographic and clinical subgroups were compared via rate ratios (RRs) and 95% confidence intervals (CIs). The analytic methods accounted for multiple measurements from the same individual over time.13 Estimates of the difference in AJBR used generalized linear models to adjust for all demographic and clinical variables in multivariate analyses. All analyses were conducted using SAS 9.4 software (SAS Institute, Cary, NC).
Results
Hemophilia A
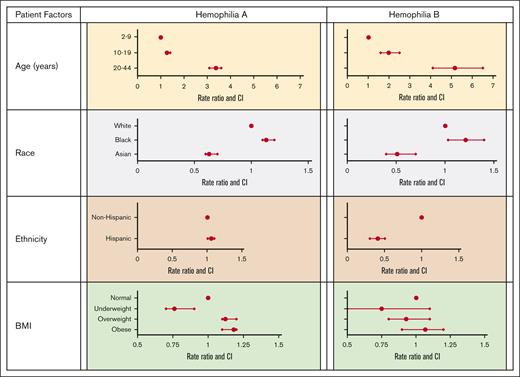
The study cohort included 2140 persons with severe hemophilia A on continuous prophylaxis over 6151 observation years with 8896 recorded joint bleeds, for an overall mean AJBR of 1.45. Table 1 and Figure 1 display the association of patient-specific factors with joint bleeding rate. We identified that, compared with the reference group of children aged 2 to 9 years, adult age significantly increased AJBR, with an RR of 3.38, whereas Asian compared with White race decreased AJBR, with an RR of 0.63. Underweight compared with normal weight had a protective effect on AJBR, with an RR of 0.76.
Relationship of patient-specific factors with JBR by diagnosis
| Patient factors . | Hemophilia A . | Hemophilia B . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n . | % . | Person-years . | Joint bleeds, n . | AJBR . | n . | % . | Person-years . | Joint bleeds, n . | AJBR . | |
| Age, y | ||||||||||
| 2-9 | 477 | 22.3 | 1081 | 747 | 0.69 | 96 | 24.8 | 231 | 86 | 0.37 |
| 10-19 | 767 | 35.8 | 2548 | 2250 | 0.88 | 138 | 35.7 | 448 | 331 | 0.74 |
| 20-44 | 896 | 41.9 | 2522 | 5899 | 2.34 | 153 | 39.5 | 381 | 733 | 1.92 |
| Race | ||||||||||
| White | 1657 | 77.4 | 4898 | 7066 | 1.44 | 296 | 76.5 | 824 | 903 | 1.1 |
| Black | 283 | 13.2 | 718 | 1170 | 1.63 | 49 | 12.7 | 137 | 181 | 1.32 |
| Asian | 119 | 5.6 | 324 | 296 | 0.91 | 28 | 7.2 | 65 | 36 | 0.55 |
| Ethnicity | ||||||||||
| Non-Hispanic | 1792 | 83.8 | 5173 | 7407 | 1.43 | 309 | 79.9 | 851 | 948 | 1.11 |
| Hispanic | 330 | 15.4 | 932 | 1409 | 1.51 | 74 | 19.1 | 201 | 92 | 0.46 |
| BMI category | ||||||||||
| Normal | 1049 | 49 | 2994 | 4124 | 1.38 | 191 | 49.3 | 485 | 533 | 1.1 |
| Underweight | 73 | 3.4 | 191 | 199 | 1.04 | 12 | 3.1 | 29 | 24 | 0.83 |
| Overweight | 437 | 20.4 | 1246 | 1916 | 1.54 | 77 | 19.9 | 206 | 210 | 1.02 |
| Obese | 500 | 23.4 | 1486 | 2423 | 1.63 | 95 | 24.6 | 304 | 356 | 1.17 |
| Patient factors . | Hemophilia A . | Hemophilia B . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n . | % . | Person-years . | Joint bleeds, n . | AJBR . | n . | % . | Person-years . | Joint bleeds, n . | AJBR . | |
| Age, y | ||||||||||
| 2-9 | 477 | 22.3 | 1081 | 747 | 0.69 | 96 | 24.8 | 231 | 86 | 0.37 |
| 10-19 | 767 | 35.8 | 2548 | 2250 | 0.88 | 138 | 35.7 | 448 | 331 | 0.74 |
| 20-44 | 896 | 41.9 | 2522 | 5899 | 2.34 | 153 | 39.5 | 381 | 733 | 1.92 |
| Race | ||||||||||
| White | 1657 | 77.4 | 4898 | 7066 | 1.44 | 296 | 76.5 | 824 | 903 | 1.1 |
| Black | 283 | 13.2 | 718 | 1170 | 1.63 | 49 | 12.7 | 137 | 181 | 1.32 |
| Asian | 119 | 5.6 | 324 | 296 | 0.91 | 28 | 7.2 | 65 | 36 | 0.55 |
| Ethnicity | ||||||||||
| Non-Hispanic | 1792 | 83.8 | 5173 | 7407 | 1.43 | 309 | 79.9 | 851 | 948 | 1.11 |
| Hispanic | 330 | 15.4 | 932 | 1409 | 1.51 | 74 | 19.1 | 201 | 92 | 0.46 |
| BMI category | ||||||||||
| Normal | 1049 | 49 | 2994 | 4124 | 1.38 | 191 | 49.3 | 485 | 533 | 1.1 |
| Underweight | 73 | 3.4 | 191 | 199 | 1.04 | 12 | 3.1 | 29 | 24 | 0.83 |
| Overweight | 437 | 20.4 | 1246 | 1916 | 1.54 | 77 | 19.9 | 206 | 210 | 1.02 |
| Obese | 500 | 23.4 | 1486 | 2423 | 1.63 | 95 | 24.6 | 304 | 356 | 1.17 |
Association of patient-specific factors with JBR by diagnosis.
Association of patient-specific factors with JBR by diagnosis.
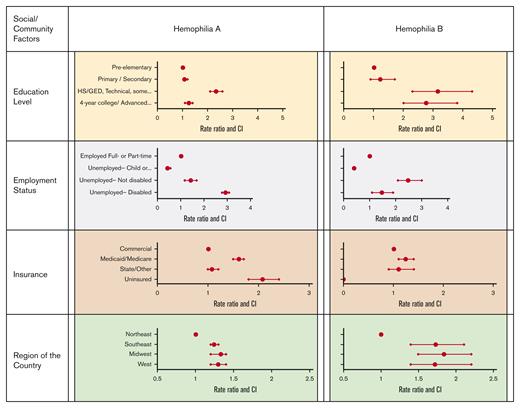
Table 2 and Figure 2 display the association of social and community factors with AJBR. Compared with pre–elementary school children, adults holding a 4-year college or advanced degree showed a smaller increase in AJBR than adults with a 2-year degree or less (1.26 vs 2.34). Similarly, unemployed participants had increased AJBR, which was most dramatic in unemployed participants with disability with severe hemophilia A. Insurance source was also associated with AJBR. Uninsured individuals and individuals insured by Medicaid or Medicare both had increased AJBR in comparison with individuals holding commercial insurance. Surprisingly, the region of the country in which care was provided affected AJBR. Care in the Northeast of the United States was associated with lower AJBR, whereas all other areas of the country were similar.
Relationship of social/community factors with JBR by diagnosis
| Social/community factor . | Hemophilia A . | Hemophilia B . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n . | % . | Person-years . | Joint bleeds, n . | AJBR . | n . | % . | Person-years . | Joint bleeds, n . | AJBR . | |
| Education level | ||||||||||
| Preelementary | 198 | 9.2 | 389 | 408 | 1.05 | 30 | 7.8 | 76 | 46 | 0.61 |
| Primary/secondary | 1050 | 49.1 | 3405 | 3854 | 1.13 | 189 | 49 | 582 | 433 | 0.74 |
| HS/GED, technical, some college, 2-y degree | 491 | 22.9 | 1369 | 3353 | 2.45 | 94 | 24 | 238 | 455 | 1.91 |
| 4-y college/advanced degree | 267 | 12.5 | 764 | 1013 | 1.33 | 44 | 11 | 119 | 199 | 1.67 |
| Employment status | ||||||||||
| Employed, full- or part-time | 644 | 30.1 | 1945 | 3854 | 1.98 | 121 | 31 | 307 | 486 | 1.58 |
| Unemployed, child or student | 1303 | 60.9 | 3737 | 3167 | 0.85 | 236 | 61 | 679 | 432 | 0.64 |
| Unemployed, without disability | 81 | 3.8 | 223 | 624 | 2.8 | 15 | 3.9 | 35 | 138 | 3.94 |
| Unemployed, with disability | 93 | 4.3 | 213 | 1235 | 5.8 | 9 | 2.3 | 28 | 65 | 2.32 |
| Insurance | ||||||||||
| Commercial | 1119 | 52.3 | 3382 | 3965 | 1.17 | 234 | 61 | 663 | 668 | 1.01 |
| Medicaid/Medicare | 793 | 37.1 | 2161 | 4074 | 1.89 | 122 | 32 | 318 | 397 | 1.25 |
| State/other | 183 | 8.6 | 514 | 649 | 1.26 | 27 | 7 | 72 | 80 | 1.11 |
| Uninsured | 32 | 1.5 | 70 | 171 | 2.44 | 1 | 0.2 | 2 | 0 | 0 |
| Region of the United States | ||||||||||
| Northeast | 407 | 19 | 1148 | 1344 | 1.17 | 70 | 18 | 209 | 140 | 0.67 |
| Southeast | 690 | 32.2 | 1971 | 2871 | 1.46 | 123 | 32 | 351 | 406 | 1.16 |
| Midwest | 628 | 29.4 | 2035 | 3165 | 1.56 | 124 | 32 | 356 | 438 | 1.23 |
| West | 415 | 19.4 | 997 | 1519 | 1.52 | 70 | 18 | 144 | 166 | 1.15 |
| Social/community factor . | Hemophilia A . | Hemophilia B . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n . | % . | Person-years . | Joint bleeds, n . | AJBR . | n . | % . | Person-years . | Joint bleeds, n . | AJBR . | |
| Education level | ||||||||||
| Preelementary | 198 | 9.2 | 389 | 408 | 1.05 | 30 | 7.8 | 76 | 46 | 0.61 |
| Primary/secondary | 1050 | 49.1 | 3405 | 3854 | 1.13 | 189 | 49 | 582 | 433 | 0.74 |
| HS/GED, technical, some college, 2-y degree | 491 | 22.9 | 1369 | 3353 | 2.45 | 94 | 24 | 238 | 455 | 1.91 |
| 4-y college/advanced degree | 267 | 12.5 | 764 | 1013 | 1.33 | 44 | 11 | 119 | 199 | 1.67 |
| Employment status | ||||||||||
| Employed, full- or part-time | 644 | 30.1 | 1945 | 3854 | 1.98 | 121 | 31 | 307 | 486 | 1.58 |
| Unemployed, child or student | 1303 | 60.9 | 3737 | 3167 | 0.85 | 236 | 61 | 679 | 432 | 0.64 |
| Unemployed, without disability | 81 | 3.8 | 223 | 624 | 2.8 | 15 | 3.9 | 35 | 138 | 3.94 |
| Unemployed, with disability | 93 | 4.3 | 213 | 1235 | 5.8 | 9 | 2.3 | 28 | 65 | 2.32 |
| Insurance | ||||||||||
| Commercial | 1119 | 52.3 | 3382 | 3965 | 1.17 | 234 | 61 | 663 | 668 | 1.01 |
| Medicaid/Medicare | 793 | 37.1 | 2161 | 4074 | 1.89 | 122 | 32 | 318 | 397 | 1.25 |
| State/other | 183 | 8.6 | 514 | 649 | 1.26 | 27 | 7 | 72 | 80 | 1.11 |
| Uninsured | 32 | 1.5 | 70 | 171 | 2.44 | 1 | 0.2 | 2 | 0 | 0 |
| Region of the United States | ||||||||||
| Northeast | 407 | 19 | 1148 | 1344 | 1.17 | 70 | 18 | 209 | 140 | 0.67 |
| Southeast | 690 | 32.2 | 1971 | 2871 | 1.46 | 123 | 32 | 351 | 406 | 1.16 |
| Midwest | 628 | 29.4 | 2035 | 3165 | 1.56 | 124 | 32 | 356 | 438 | 1.23 |
| West | 415 | 19.4 | 997 | 1519 | 1.52 | 70 | 18 | 144 | 166 | 1.15 |
GED, General Educational Development; HS, high school.
Association of social/community factors with JBR by diagnosis.
Association of social/community factors with JBR by diagnosis.
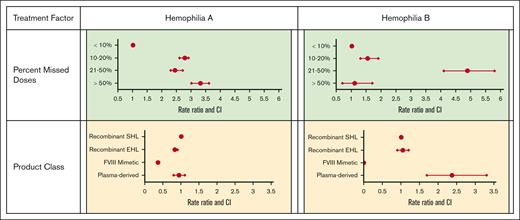
Table 3 and Figure 3 display the association of treatment factors with AJBR. Adherence with prophylactic regimen was important; any percent of missed doses increased the AJBR compared with <10% doses missed. The use of recombinant EHL factor VIII was associated with a modest decrease in AJBR. Use of the factor VIII mimetic emicizumab was associated with the lowest AJBR (0.65 vs 1.80 for recombinant SHL factor treatment), with an RR of 0.36 (95% CI, 0.3-0.4).
Relationship of treatment factors with JBR by diagnosis
| Treatment issue . | Hemophilia A . | Hemophilia B . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n . | % . | Person-years . | Joint bleeds, n . | AJBR . | n . | % . | Person-years . | Joint bleeds, n . | AJBR . | |
| Percent missed doses | ||||||||||
| <10% | 1503 | 70.2 | 4556 | 5041 | 1.11 | 260 | 67.2 | 780 | 710 | 0.91 |
| 10%-20% | 231 | 10.8 | 568 | 1746 | 3.07 | 30 | 7.7 | 84 | 118 | 1.4 |
| 21%-50% | 93 | 4.4 | 241 | 652 | 2.71 | 17 | 4.4 | 34 | 151 | 4.44 |
| >50% | 49 | 2.3 | 128 | 470 | 3.67 | 13 | 3.4 | 24 | 24 | 1 |
| Product class | ||||||||||
| Recombinant SHL | 1265 | 59.1 | 2955 | 5319 | 1.8 | 143 | 37 | 330 | 339 | 1.03 |
| Recombinant EHL | 552 | 25.8 | 1621 | 2435 | 1.5 | 234 | 60.5 | 714 | 772 | 1.08 |
| Factor VIII mimetic | 275 | 12.8 | 1458 | 953 | 0.65 | |||||
| Plasma-derived | 42 | 2 | 109 | 185 | 1.7 | 10 | 2.6 | 16 | 39 | 2.44 |
| Treatment issue . | Hemophilia A . | Hemophilia B . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n . | % . | Person-years . | Joint bleeds, n . | AJBR . | n . | % . | Person-years . | Joint bleeds, n . | AJBR . | |
| Percent missed doses | ||||||||||
| <10% | 1503 | 70.2 | 4556 | 5041 | 1.11 | 260 | 67.2 | 780 | 710 | 0.91 |
| 10%-20% | 231 | 10.8 | 568 | 1746 | 3.07 | 30 | 7.7 | 84 | 118 | 1.4 |
| 21%-50% | 93 | 4.4 | 241 | 652 | 2.71 | 17 | 4.4 | 34 | 151 | 4.44 |
| >50% | 49 | 2.3 | 128 | 470 | 3.67 | 13 | 3.4 | 24 | 24 | 1 |
| Product class | ||||||||||
| Recombinant SHL | 1265 | 59.1 | 2955 | 5319 | 1.8 | 143 | 37 | 330 | 339 | 1.03 |
| Recombinant EHL | 552 | 25.8 | 1621 | 2435 | 1.5 | 234 | 60.5 | 714 | 772 | 1.08 |
| Factor VIII mimetic | 275 | 12.8 | 1458 | 953 | 0.65 | |||||
| Plasma-derived | 42 | 2 | 109 | 185 | 1.7 | 10 | 2.6 | 16 | 39 | 2.44 |
Hemophilia B
The study cohort included 387 persons with severe hemophilia B on continuous prophylaxis over 1060 observation years with 1150 recorded joint bleeds, for an overall mean AJBR of 1.08, which was significantly lower than that for hemophilia A (P < .001).
Table 1 and Figure 1 display the association of patient-specific factors with AJBR in hemophilia B. Adult age conveyed a dramatic increase in AJBR (RR, 5.17) and, again, Asian race was protective (RR, 0.51). In this cohort, Hispanic ethnicity was also associated with a reduced RR (0.41). No weight category was associated with an effect on AJBR.
Table 2 and Figure 2 display the association of social and community factors with AJBR in hemophilia B. As with hemophilia A, the attainment of a 4-year college or advanced degree was associated with a lower AJBR than not having a higher education. Unemployment was again associated with increased AJBR. Because of small sample sizes, the differences between RRs for unemployed individuals with disability and those who are unemployed but without disability were imprecise, as evidenced by wide CIs. Individuals with Medicaid or Medicare insurance also experienced higher AJBR. Finally, individuals receiving hemophilia care in the Northeast of the United States had the lowest AJBR, whereas all other regions were similarly higher.
Table 3 and Figure 3 display the association with treatment variables with AJBR in hemophilia B. Missed doses in the 10% to 50% range were similarly associated with worse AJBR. Plasma-derived factor IX product use was associated with a higher AJBR, whereas the use of an EHL product did not affect the AJBR. As with hemophilia A, the region of country in which care was received affected AJBR outcome. Compared with the Northeast, all other regions of the country showed similar and higher AJBR.
Finally, multivariate analysis was used to identify independent risk factors for increased AJBR. Table 4 displays results for the factors that were found to be independently associated with AJBR in hemophilia A and B in multivariable models. In hemophilia A, factor VIII mimetic use had the strongest effect on lower AJBR (>1 bleed fewer per year on average) with a lesser effect of recombinant EHL FVIII. Specifically, zero bleeding during the previous year was reported in 60% of visits by participants using SHL recombinant factor, 62% of visits by patients on recombinant EHL, and 76% of visits by participants using the factor VIII mimetic. In hemophilia B, only a small increased AJBR with plasma-derived factor IX products was detected. Higher percentages of missed doses were strongly and independently associated with higher AJBR in hemophilia A and in all but the highest percentage of missed doses in hemophilia B.
Results of multivariate analysis of independent risk factors for AJBR in a cohort of males with severe hemophilia
| Characteristic . | Hemophilia A . | Hemophilia B . | ||
|---|---|---|---|---|
| AJBR difference . | P value . | AJBR difference . | P value . | |
| Treatment product | ||||
| Recombinant SHL | Ref | Ref | ||
| FVIII mimetics | −1.0037 | <.0001 | ||
| Plasma-derived | −0.2084 | .2808 | 0.8864 | .0064 |
| Recombinant EHL | −0.258 | .0037 | −0.0811 | .7122 |
| Missed doses | ||||
| <10% | Ref | Ref | ||
| 10%-20% | 0.6757 | <.0001 | 0.2881 | .1122 |
| 21%-50% | 0.6395 | <.0001 | 1.2397 | <.0001 |
| >50% | 0.638 | .0003 | −0.0503 | .9198 |
| Health insurance | ||||
| Commercial | Ref | Ref | ||
| Government | 0.3983 | .0001 | −0.0763 | .6805 |
| Other | 0.1835 | .2759 | 0.4594 | .1209 |
| Employment status | ||||
| Employed, full or part-time | Ref | Ref | ||
| Not employed, with disability | 0.5204 | .011 | 0.2546 | .5533 |
| Not employed, without disability | 0.2902 | .2057 | 0.9796 | .0386 |
| Educational level | ||||
| Preelementary | Ref | Ref | ||
| Primary/secondary | −0.3904 | .1125 | −0.7259 | .0243 |
| 4-y/advanced college degree | −0.4933 | .01 | 0.0722 | .8264 |
| High school/technical/some college | −0.0778 | .6239 | 0.0895 | .6784 |
| Age, y | ||||
| 2-9 | Ref | Ref | ||
| 10-19 | 0.3548 | .0131 | 0.7533 | .0015 |
| 20-44 | 1.0105 | <.0001 | 1.7186 | <.0001 |
| Region | ||||
| Northeast | Ref | Ref | ||
| Midwest | 0.3377 | .0067 | 0.7266 | .0039 |
| Southeast | 0.3335 | .0113 | 0.5389 | .04 |
| West | 0.4779 | .0049 | 0.6144 | .0441 |
| Characteristic . | Hemophilia A . | Hemophilia B . | ||
|---|---|---|---|---|
| AJBR difference . | P value . | AJBR difference . | P value . | |
| Treatment product | ||||
| Recombinant SHL | Ref | Ref | ||
| FVIII mimetics | −1.0037 | <.0001 | ||
| Plasma-derived | −0.2084 | .2808 | 0.8864 | .0064 |
| Recombinant EHL | −0.258 | .0037 | −0.0811 | .7122 |
| Missed doses | ||||
| <10% | Ref | Ref | ||
| 10%-20% | 0.6757 | <.0001 | 0.2881 | .1122 |
| 21%-50% | 0.6395 | <.0001 | 1.2397 | <.0001 |
| >50% | 0.638 | .0003 | −0.0503 | .9198 |
| Health insurance | ||||
| Commercial | Ref | Ref | ||
| Government | 0.3983 | .0001 | −0.0763 | .6805 |
| Other | 0.1835 | .2759 | 0.4594 | .1209 |
| Employment status | ||||
| Employed, full or part-time | Ref | Ref | ||
| Not employed, with disability | 0.5204 | .011 | 0.2546 | .5533 |
| Not employed, without disability | 0.2902 | .2057 | 0.9796 | .0386 |
| Educational level | ||||
| Preelementary | Ref | Ref | ||
| Primary/secondary | −0.3904 | .1125 | −0.7259 | .0243 |
| 4-y/advanced college degree | −0.4933 | .01 | 0.0722 | .8264 |
| High school/technical/some college | −0.0778 | .6239 | 0.0895 | .6784 |
| Age, y | ||||
| 2-9 | Ref | Ref | ||
| 10-19 | 0.3548 | .0131 | 0.7533 | .0015 |
| 20-44 | 1.0105 | <.0001 | 1.7186 | <.0001 |
| Region | ||||
| Northeast | Ref | Ref | ||
| Midwest | 0.3377 | .0067 | 0.7266 | .0039 |
| Southeast | 0.3335 | .0113 | 0.5389 | .04 |
| West | 0.4779 | .0049 | 0.6144 | .0441 |
The findings presented in the table are for those factors that were independently associated with AJBR in hemophilia A and B in multivariable models. Race, ethnicity, and BMI were included in the models but are not shown in the table because the associations between these factors and AJBR were not statistically significant.
AJBR difference is the average number of annual bleeds less (negative value) or more (positive value) than the reference group.
FVIII, factor VIII; Ref, reference group.
Younger age and receipt of care in the Northeast of the United States were independently associated with lower AJBR for both hemophilia A and B. In order to exclude the possibility that data from the Northeast could have resulted from a sampling bias, the number of individuals with severe hemophilia A and B in the Community Counts registry was expressed as a proportion of all individuals with severe hemophilia A or B treated in HTCs in each region from the Community Counts population profile. Table 5 displays results excluding this possibility, with the Northeast enrolling a slightly higher percentage of persons with severe hemophilia A.
Representativeness of Community Counts registry participants as a proportion of all patients treated at HTCs
| Diagnosis . | Severity . | Midwest . | Northeast . | Southeast . | West . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Registry CC patient count . | HTC PP patient count . | % . | Registry CC patient count . | HTC PP patient count . | % . | Registry CC patient count . | HTC PP patient count . | % . | Registry CC patient count . | HTC PP patient count . | % . | ||
| Hem A | Severe | 6530 | 16138 | 40.5 | 4219 | 11453 | 36.8 | 7726 | 21793 | 35.5 | 3786 | 15202 | 24.9 |
| Moderate | 1585 | 4709 | 33.7 | 1271 | 3970 | 32.0 | 2205 | 6250 | 35.3 | 1090 | 5361 | 20.3 | |
| Mild | 2492 | 9915 | 25.1 | 1722 | 7286 | 23.6 | 2245 | 9107 | 24.7 | 1405 | 9403 | 14.9 | |
| Total all Hem A | 10607 | 30762 | 34.5 | 7212 | 22709 | 31.8 | 12176 | 37150 | 32.8 | 6281 | 29966 | 21.0 | |
| Hem B | Severe | 1216 | 2944 | 41.3 | 735 | 2089 | 35.2 | 1236 | 3517 | 35.1 | 578 | 2815 | 20.5 |
| Moderate | 1565 | 6453 | 24.3 | 503 | 2281 | 22.1 | 876 | 2853 | 30.7 | 442 | 2110 | 20.9 | |
| Mild | 798 | 4574 | 17.4 | 358 | 2105 | 17.0 | 765 | 3099 | 24.7 | 263 | 1939 | 13.6 | |
| Total all Hem B | 3579 | 13971 | 25.6 | 1596 | 6475 | 24.6 | 2877 | 9469 | 30.4 | 1283 | 6864 | 18.7 | |
| Diagnosis . | Severity . | Midwest . | Northeast . | Southeast . | West . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Registry CC patient count . | HTC PP patient count . | % . | Registry CC patient count . | HTC PP patient count . | % . | Registry CC patient count . | HTC PP patient count . | % . | Registry CC patient count . | HTC PP patient count . | % . | ||
| Hem A | Severe | 6530 | 16138 | 40.5 | 4219 | 11453 | 36.8 | 7726 | 21793 | 35.5 | 3786 | 15202 | 24.9 |
| Moderate | 1585 | 4709 | 33.7 | 1271 | 3970 | 32.0 | 2205 | 6250 | 35.3 | 1090 | 5361 | 20.3 | |
| Mild | 2492 | 9915 | 25.1 | 1722 | 7286 | 23.6 | 2245 | 9107 | 24.7 | 1405 | 9403 | 14.9 | |
| Total all Hem A | 10607 | 30762 | 34.5 | 7212 | 22709 | 31.8 | 12176 | 37150 | 32.8 | 6281 | 29966 | 21.0 | |
| Hem B | Severe | 1216 | 2944 | 41.3 | 735 | 2089 | 35.2 | 1236 | 3517 | 35.1 | 578 | 2815 | 20.5 |
| Moderate | 1565 | 6453 | 24.3 | 503 | 2281 | 22.1 | 876 | 2853 | 30.7 | 442 | 2110 | 20.9 | |
| Mild | 798 | 4574 | 17.4 | 358 | 2105 | 17.0 | 765 | 3099 | 24.7 | 263 | 1939 | 13.6 | |
| Total all Hem B | 3579 | 13971 | 25.6 | 1596 | 6475 | 24.6 | 2877 | 9469 | 30.4 | 1283 | 6864 | 18.7 | |
The table displays all patients with hemophilia aged ≥2 years when enrolling into the CC surveillance system from 2013 to 2023
CC, Community Counts, a more detailed prospective registry; Hem, hemophilia; HTC/PP, population profile, all participants treated at the HTC.
Boldface numbers indicate cumulative counts and percentages for Hem A and Hem B participants, respectively, in each of the 4 regions.
There were discrepancies in risk factors between hemophilia A and B. A higher education and commercial insurance were significantly associated with lower AJBR for hemophilia A but not hemophilia B, whereas unemployment with disability was associated with higher AJBR for hemophilia A but not hemophilia B.
Discussion
This analysis of associations of patient, social/community, and treatment factors with AJBR is critical to our understanding of joint bleeding in an era in which the differences between efficacy of certain products may be smaller than the differences between patient groups. This study found that product, specifically factor VIII mimetic and, to a lesser extent, EHL factor VIII; rigorous adherence with few to no missed doses; younger age; commercial health insurance; higher education; and treatment at an HTC in the Northeast of the United States convey independent outcome advantages for lower AJBR for hemophilia A whereas only younger age and treatment at an HTC in the Northeast conveyed independent outcome advantages for lower AJBR for hemophilia B. The power of this study derives from the large sample size and inclusion of a population from 147 HTCs that are distributed across a wide geographic area of the United States. The study determined that patient-specific factors, social/community factors, and treatment factors must all be considered in evaluation of joint outcomes in severe hemophilia. The nonsignificant association between disability and AJBR in severe hemophilia B was most likely because of the very small number of patients with hemophilia B and disability. The overall lower AJBR observed in the hemophilia B study population supports previous data that severe hemophilia B is associated with less joint bleeding relative to severe hemophilia A, making it more difficult to distinguish treatment effects in hemophilia B.
It is widely accepted that emicizumab has markedly decreased AJBR in patients with hemophilia A with and without inhibitors.14,15 This study, which excluded patients with inhibitor, showed that emicizumab had the greatest effect on AJBR of any predictive factor examined. These data suggest that, in a more global context, the impact of emicizumab is even greater than demonstrated here.
The study has some limitations. We found a modest effect of EHL factor VIII products on lowering the AJBR; however, this study was conducted before the commercial availability of Fc-VWF-XTEN fusion protein-ehtl (efanesoctocog alfa [Altuviiio]; Sanofi) that has a substantially extended factor VIII activity half-life, and thus no comment can be made on the potential advantages of that product.7 It was surprising that a stronger effect of EHL factor IX products on AJBR was not found, despite their use for continuous prophylaxis by most participants with hemophilia B in the study. Similarly, no hemostasis rebalancers or gene therapies were commercially available during the period of data collection. Nonetheless, this study provides critical comparison data that can be applied to future real-world studies of those newer products.
The study showed a strong association between use of the factor VIII mimetic, emicizumab, and decreased AJBR. Multivariate analysis determined that this effect is independent of prophylaxis adherence. However, the subcutaneous route and infrequent treatment schedule (1-4 times monthly) may have contributed to improved adherence and the favorable outcome. In addition, the very long half-life of emicizumab likely diminishes adverse effects of decreased adherence. The effect of emicizumab was not because of an interaction with age because the proportion of visits in which participants were using this product were similar across all 3 age groups and statistical testing revealed no significant interaction.
The association of greater joint bleeding with increasing age was somewhat unexpected, given that school-aged boys are likely more active in sports and otherwise traumatic activities than adults. This may reflect earlier joint damage suffered by current adults as joint bleeding may be more difficult to control in joints with synovitis and arthritis. Alternatively, because all joint bleeding is self-reported, the adults may have incorrectly identified the pain of arthropathy as acute joint bleeding. It is of great interest to know whether the current generation of children raised without frequent joint bleeding will manifest lower AJBR as adults, but that remains to be seen. These data are very encouraging in support of the recent increase in sports and activities for children with severe hemophilia on highly effective prophylactic regimens because the study did not find a disadvantage of increased joint bleeding in children aged 2 to 19 years. Although data were not available to compare boys engaging vs not engaging in team sports, published literature as well as anecdotal evidence support that sports participation is increasing among boys with hemophilia on highly effective prophylaxis regimens without increased bleeding.16-19 Finally, the study counted clinical events of joint bleeding as reported by persons with severe hemophilia. The contribution of small, unrecognized bleeding into joints to joint damage is as yet unclear and was not detected in this study.
Underlying contributions to the association of higher education with lower AJBR are not clear. Certainly, it has been a principle in hemophilia care for many decades that affected individuals pursue higher education and avoid careers involving heavy labor with risk of injury. However, the effects of higher education, commercial insurance, and employment in multivariate analysis among those with hemophilia A suggests that the contributions of better insurance, better access to health care, and better access to newer treatment products for persons with higher education could also be involved.
The association of lower AJBR with receipt of hemophilia care in the US Northeast is not easily explained. Potentially, that area of the country, being more densely populated, is associated with shorter distances to the HTCs and consequently more timely or more frequent care. The multivariate analysis excluded a confounding effect of younger age, higher education, better insurance, employment, treatment product, adherence, or race in the association. In addition, the proportion of patients attending HTCs who were enrolled in the Community Counts registry was not different between the Northeast and the rest of the country, ruling against enrollment bias.
Finally, the study outcome, AJBR, was used as a surrogate for end-stage joint damage. Although patient-reported number of bleeding events (AJBR) is an imprecise outcome, it is currently the best prognosticator of end-stage joint damage in hemophilia. In the future, application of inexpensive, widely available objective measurements of joint structure and function, such as point-of-care ultrasound and the validated hemophilia joint health score physical examination may supersede or complement AJBR.2,20
In conclusion, the findings of this comprehensive analysis of a large, diverse sample drawn from HTCs across the United States underscore the important contributions of patient, community, and treatment factors to joint outcome in severe hemophilia. Use of emicizumab among persons with hemophilia A, commercial insurance, and few missed doses for persons with hemophilia A or B independently predicted low AJBR, highlighting the interplay between access and adherence to care. Our study provides valuable data for comparing established and emerging hemophilia therapies.
Acknowledgments
Community Counts is a project supported by cooperative agreement NU27DD000020 awarded to the American Thrombosis and Hemostasis Network (ATHN) in partnership with the US Hemophilia Treatment Center Network (USHTCN). The cooperative agreement is an annual financial assistance award totaling $4 300 000, which is 100% funded by the Centers for Disease Control and Prevention (CDC) and the US Department of Health and Human Services. Much of the work of data collection and submission for this project was supported by a grant from the Maternal and Child Health Bureau, H30MC24049, through the 340B Program.
The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of the CDC, the Department of Health and Human Services, ATHN, or the USHTCN. Data reported in this publication were collected through Community Counts: CDC Public Health Surveillance Project for Bleeding Disorders.
Authorship
Contribution: M.J.M.-J. designed and developed the project, interpreted data, wrote the initial manuscript, and finalized the manuscript; B.L. analyzed all study data and contributed to project design; S. Acharya, S. Ahuja, and S.A.F. interpreted data and edited the manuscript; M.C., D.C.-S., D.I., R.K., L.A.S., and A.S. contributed to project design, interpreted data, and edited the manuscript; and J.M.S. contributed to project design, analyzed data, interpreted data, and edited the manuscript.
Conflict-of-interest disclosure: M.J.M.-J. has received honoraria from BioMarin, CSL Behring, Genentech/Roche, Spark, and Novo Nordisk. S. Acharya has received honoraria from Pfizer and Bayer. S. Ahuja has received research funding from XA Tech Inc, Novo Nordisk, Sanofi-Genzyme, and Genentech; and honoraria from CSL Behring, Novo Nordisk, Sanofi-Genzyme, BioMarin, and Genentech. M.C. has received research funding from Genentech, Novartis, and Agios Pharmaceuticals; and honoraria from Novo Nordisk, Genzyme Corp, Hoffman-LaRoche Inc, CSL Behring, and BPL Inc. R.K. has received research funding from Novo Nordisk, Sanofi-Genzyme, and Pfizer; has received consultancy fees from CSL Behring, Novo Nordisk, Sanofi-Genzyme, and Pfizer; has received honoraria from BioMarin; and serves as a speaker for CSL Behring and Sanofi-Genzyme. A.S. is an institutional principal investigator for a Pfizer study (marstacimab [PF-06741086]) and X4 Pharma (institutional principal investigator for a phase 2/phase 3 study of mavorixafor). The remaining authors declare no competing financial interests.
Correspondence: Marilyn J. Manco-Johnson, Department of Pediatrics, Hemophilia and Thrombosis Center, University of Colorado Anschutz Medical Campus and Children's Hospital Colorado, 13199 E Montview Blvd, Suite 100, Aurora, CO 80045; email: marilyn.manco-johnson@cuanschutz.edu.
References
Author notes
Data are available on request from Brandi Dupervil (inm4@cdc.gov).