Key Points
Data from this cohort study demonstrate that inhibitor development remains a significant CFC complication.
Inhibitor surveillance is critical to identify clinically significant inhibitors in young children with severe HA.
Visual Abstract
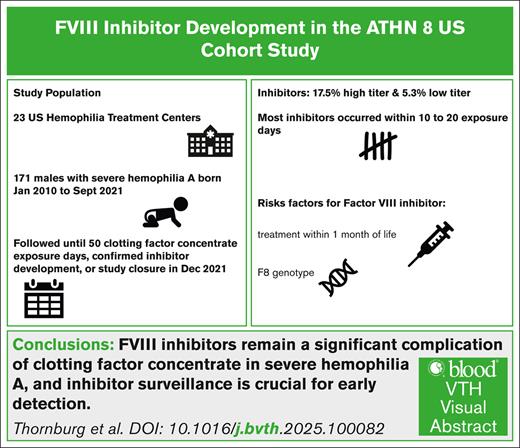
Clotting factor concentrate (CFC), used to treat and prevent bleeding in hemophilia, is rendered ineffective if clotting factor neutralizing antibodies (inhibitors) develop. Inhibitors occur most often in children, early in treatment. The American Thrombosis and Hemostasis Network (ATHN) 8: US Cohort Study of Previously Untreated Patients (PUPs) with Congenital Hemophilia, conducted in children born in 2010 to 2020 with severe or moderate hemophilia, was designed to determine the percentage of participants who developed a confirmed, clinically significant inhibitor within the first 50 CFC exposure days (EDs). Cox proportional hazards models were used to evaluate risk factors for inhibitor development in PUPs with severe hemophilia A (HA). A total of 171 males with severe HA enrolled: 39 (22.8%) developed an inhibitor, 30 (17.5%) developed a high-titer inhibitor, and 9 (5.3%) developed a low-titer inhibitor; 82.1% within 20 EDs. Product exposure at <1 month (hazard ratio [HR], 2.57; 95% confidence interval [CI], 1.22-5.44), large structural changes (HR,16.59; 95% CI, 1.94-142.20), and nonsense variants (HR, 12.53; 95% CI, 1.41-111.49) were associated with inhibitor development. Overall, inhibitor development remains a significant CFC complication especially in the first 10 to 20 EDs. Further study should evaluate the impact of new treatments on inhibitor rates and age at inhibitor development and identify strategies to reduce inhibitor development.
Introduction
Clotting factor concentrate (CFC) is a key part of treatment and prevention strategies for children with hemophilia. The primary risk of CFC is the development of neutralizing antibodies (inhibitors) rendering CFCs ineffective and increasing morbidity and mortality. Inhibitor data have been collected through clinical trials and registries with variation in inhibitor definitions and study populations.1-14 These studies demonstrate high-titer inhibitor rates of 10.0% to 28.6% and overall inhibitor rates of 26.7% to 52.2% in severe hemophilia A (HA). Ongoing evaluation is critical,15,16 particularly in the changing treatment landscape as new treatment options, including factor VIII (FVIII) mimetics and rebalancing agents,17,18 which may delay or reduce CFC exposure, are implemented in clinical practice.
In the United States, the American Thrombosis and Hemostasis Network (ATHN) focuses on research and public health surveillance for individuals with bleeding disorders.19,20 ATHN 8: US Cohort Study of Previously Untreated Patients (PUPs) with Congenital Hemophilia (ATHN 8; ClinicalTrials.gov identifier: NCT03818529) was an ATHN-sponsored project evaluating current treatment patterns in young children and documenting rates of, and risk factors for, inhibitor formation. The primary objective of ATHN 8 was to determine the percentage of participants with a confirmed, clinically significant inhibitor within the first 50 exposure days (EDs). In addition, the study aimed to evaluate variables contributing to inhibitor development in the highest risk population with severe HA and to serve as a platform for future PUP studies in the United States.
Patients and methods
Study cohort and enrollment
ATHN 8, conducted at 23 ATHN affiliates within the US Hemophilia Treatment Center Network was a longitudinal, observational cohort study of children with moderate or severe hemophilia born on, or after, 1 January 2010 and before 30 September 2021. This report includes those with congenital HA and FVIII activity of <1% and with care established at one of the participating sites. ATHN 8 was modeled after the long-standing PedNet Registry (ClinicalTrials.gov identifier: NCT02979119).21 Enrollment was from October 2018 through September 2021. Participants were eligible if there was no previous history of bleeding or CFC treatment or if there was a history of previous bleeding and/or treatment and there was a report of bleeds and CFC use in the medical record. Participant data were collected until 50 EDs, development of a confirmed inhibitor, or end of data collection on 31 December 2021, whichever occurred first. Treatment was not directed by the study protocol. Sites were encouraged to enroll all eligible patients to avoid selection bias.
Objectives and end points
The primary objective was to determine the percentage of participants with a confirmed inhibitor (primary end point) within the first 50 EDs. To capture clinically relevant inhibitors, a confirmed inhibitor was defined as 2 consecutive positive inhibitor titers on different blood specimens and a change in bleed management or prophylaxis or addition of immune tolerance induction. Low-titer inhibitors were ≤5.0 Nijmegen Bethesda units (BU) and high-titer inhibitors were >5 BU. The lower cutoff for a positive inhibitor specimen was based on the performing laboratory values and ranged from 0.3 to 0.6 BU.
Clinical data
If a participant met a study end point criterion at enrollment, all clinical data were collected based on historical reporting (ie, retrospective data collection). If a participant had not met a study end point at enrollment, clinical data were collected until a study end point was met or until study closure. Data were abstracted from the medical record per a study-specific data dictionary (available upon request). Data included sociodemographics, reason for diagnostic testing, F8 genotype, hemophilia diagnosis and severity, medical history, and inhibitor results completed as part of clinical care. Bleed (date, location, and reason) and treatment (treatment regimen and class, dose, and duration) data were collected from patient reports, patient infusion records, medical records, and research records if the participant was on a therapeutic trial. Treatment regimens were categorized as continuous prophylaxis or on-demand. Continuous prophylaxis was defined as use of any treatment product administered on a regular basis to prevent bleeding with the intention to treat for 52 weeks per year. Treatment classes included blood bank products (cryoprecipitate, fresh frozen plasma, and packed red blood cells), activated prothrombin complex concentrate, recombinant activated factor VII (rFVIIa), CFC, plasma-derived (pd) FVIII, standard half-life rFVIII, extended half-life (EHL) rFVIII, FVIII mimetic, other hemostatic agent, and unknown. For participants who were followed up prospectively, study personnel conducted quarterly follow-up in person or by telephone to supplement data in the medical record.
To assign treatment regimen and treatment class at the end of study, the study team assigned the most regular treatment before the end of study. For example, if a participant was prescribed FVIII mimetic prophylaxis and received 1 dose of rFVIII EHL for a bleed before study exit, the treatment regimen would be prophylaxis and treatment class would be FVIII mimetic prophylaxis.
Laboratory assays
For participants enrolled before study end point development, inhibitor testing was performed at local and/or Centers for Disease Control and Prevention laboratories. Per the study protocol, inhibitor testing was to be completed at baseline, annually, if there was clinical suspicion for inhibitor development, and within 10 days of inhibitor detection at the Centers for Disease Control and Prevention. Additional inhibitor testing was recommended per Medical and Scientific Advisory Council (MASAC) document 236: recommendations on standardized testing and surveillance for inhibitors in patients with HA and hemophilia B.22 For participants who developed an inhibitor after study enrollment, the principal investigators confirmed that participants met the criteria for confirmed inhibitor before study exit.
For participants without documented F8 genotyping, genotyping was performed at Versiti Blood Diagnostic Laboratories using whole-blood samples. Gene variants were classified uniformly based on Human Genome Variation Society nomenclature.
Sample size
The enrollment population was defined as all eligible children at participating sites, estimated as an average of 5 participants per site. Sample size calculation for the primary end point was based on participants with severe HA, the population with highest inhibitor rates. At the time of study design, ∼15% of all participants with HA in the ATHN data set had documented inhibitors. Based on clinical experience and reports in the literature, the estimated inhibitor rate of participants with severe HA was ∼30%. Power analysis based on a z test for asymptotic normal approximation showed that 53 participants with severe HA will result in 80% power to detect the expected 15% difference in inhibitor rate between the ATHN data set and the study population, with a type-1 error rate set as 0.05.
Statistical analysis
Summary statistics of demographic, clinical, and laboratory data were performed for all participants with severe HA. For birth cohort comparisons, birth cohorts were defined by availability of new treatments included EHL CFC and FVIII mimetic (2010-2012; 2013-2018; and after 2018).
We used Cox proportional hazards (CoxPH) models to evaluate the risk factors associated with inhibitor development in the severe HA cohort. Participants who exited the study early were excluded from the CoxPH analysis. Statistical significance was assessed using an α level of .05. Our multivariable CoxPH model was adjusted for 5 covariables: race, ethnicity, F8 gene variants, age at initial exposure, and the administration of the first product for a duration of ≥5 days. For each of these 5 covariables, we calculated the hazard ratio (HR) estimates, 95% confidence intervals (CIs), and P values to compare different categories within each variable against the reference group. Data analyses were performed using Python version 3.12.3, R version 4.4.0 (ucrt, released on 24 April 2024), and SAS version 9.4 (SAS Institute Inc, Cary, NC).
Data management
All data collected were entered into electronic case report forms within the secure ATHN Study Manager system. Missing data were not imputed. The study was approved by participating sites’ institutional review boards, and permission was obtained from a parent or legal guardian for enrollment in ATHN 8 as well as the ATHN data set.19
Results
The study enrolled 171 male participants with severe HA. A total of 120 (70.2%) were White and non-Hispanic. Of 171 participants, 98 (57.3%) exited the study because they reached 50 EDs, 39 (22.8%) exited the study because they had a confirmed inhibitor, and 34 (19.9%) exited the study before reaching a study end point when the study was closed in December 2021. The mean age at study exit was 3.85 years and cumulative person-years of follow-up from birth to study exit was 659. Table 1 shows the demographic, clinical characteristics, and reason for study exit. Table 2 shows genotype data.
Demographics and clinical characteristics of participants with severe HA
| . | Total cohort N = 171 . | 50 EDs end point n = 98 . | Confirmed inhibitor end point n = 39 . | Early study exit∗ n = 34 . |
|---|---|---|---|---|
| Birth year cohort, n (%) | ||||
| 1 Jan 2010 to 31 Dec 2012 | 34 (19.88) | 25 (25.51) | 8 (20.51) | 1 (2.94) |
| 1 Jan 2013 to 3 Oct 2018 | 106 (61.99) | 66 (67.35) | 29 (74.36) | 11 (32.35) |
| After 3 Oct 2018 | 31 (18.13) | 7 (7.14) | 2 (5.13) | 22 (64.71) |
| Ethnicity, n (%) | ||||
| Hispanic, Latino/a, or Spanish origin | 34 (19.88) | 21 (21.43) | 6 (15.38) | 7 (20.59) |
| Not Hispanic, Latino/a, or Spanish origin | 137 (80.12) | 77 (78.57) | 33 (84.62) | 27 (79.41) |
| Unknown | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Race, n (%) | ||||
| White | 120 (70.18) | 69 (70.41) | 27 (69.23) | 24 (70.59) |
| Black or African American | 25 (14.62) | 13 (13.27) | 8 (20.51) | 4 (11.76) |
| Asian | 7 (4.09) | 4 (4.08) | 1 (2.56) | 2 (5.88) |
| Native Hawaiian or other Pacific Islander | 1 (0.58) | 0 (0.0) | 0 (0.0) | 1 (2.94) |
| American Indian or Alaska Native | 2 (1.17) | 1 (1.02) | 0 (0.0) | 1 (2.94) |
| Multiple races | 9 (5.26) | 7 (7.14) | 1 (2.56) | 1 (2.94) |
| Unknown | 7 (4.09) | 4 (4.08) | 2 (5.13) | 1 (2.94) |
| Ever CFC exposure, n (%) | ||||
| Yes | 164 (95.91) | 98 (100.0) | 39 (100.0) | 27 (79.41) |
| No‡ | 7 (4.09) | 0 (0.0) | 0 (0.0) | 7 (20.59) |
| Ever prophylaxis, n (%) | ||||
| Yes | 123 (71.93) | 86 (87.76) | 24 (61.54) | 13 (38.24) |
| No | 48 (28.07) | 12 (12.24) | 15 (38.46) | 21 (61.76) |
| Age at first CFC exposure, n (%) | ||||
| <1 mo | 55 (32.16) | 26 (26.53) | 16 (41.03) | 13 (38.24) |
| 1-12 mo | 78 (45.61) | 56 (57.14) | 12 (30.77) | 10 (29.41) |
| 1-10 y | 27 (15.79) | 16 (16.33) | 7 (17.95) | 4 (11.76) |
| Unknown | 4 (2.34) | 0 (0.0) | 4 (10.26) | 0 (0.0) |
| Never had CFC exposure | 7 (4.09) | 0 (0.0) | 0 (0.0) | 7 (20.59) |
| Treatment regimen before study exit, n (%) | ||||
| On-demand | 54 (31.58) | 17 (17.35) | 19 (48.72) | 18 (52.94) |
| Prophylaxis | 110 (64.33) | 81 (82.65) | 17 (43.59) | 12 (35.29) |
| No product usages | 4 (2.34) | 0 (0.0) | 0 (0.0) | 4 (11.76) |
| Unknown | 3 (1.75) | 0 (0.0) | 3 (7.69) | 0 (0.0) |
| Treatment class before study exit, n (%) | ||||
| Plasma derived | 29 (16.96) | 13 (13.27) | 8 (20.51) | 8 (23.53) |
| SHL recombinant | 95 (55.56) | 61 (62.24) | 25 (64.1) | 9 (26.47) |
| EHL recombinant | 25 (14.62) | 22 (22.45) | 2 (5.13) | 1 (2.94) |
| FVIII mimetic | 15 (8.77) | 2 (2.04) | 1 (2.56) | 12 (35.29) |
| No product usages | 4 (2.34) | 0 (0.0) | 0 (0.0) | 4 (11.76) |
| Missing data§ | 3 (1.75) | 0 (0.0) | 3 (7.69) | 0 (0.0) |
| Known CFC EDs | ||||
| N | 167 | 98 | 35 | 34 |
| Mean (standard deviation) | 33.2 (21.01) | 50 (0) | 12.09 (8.87) | 6.53 (9.81) |
| Median | 50 | 50 | 11 | 2 |
| IQR | 41 (9-50) | N/A | 12 (5-17) | 6 (1-7) |
| Range | 0-50 | 50 | 1-38 | 0-41 |
| . | Total cohort N = 171 . | 50 EDs end point n = 98 . | Confirmed inhibitor end point n = 39 . | Early study exit∗ n = 34 . |
|---|---|---|---|---|
| Birth year cohort, n (%) | ||||
| 1 Jan 2010 to 31 Dec 2012 | 34 (19.88) | 25 (25.51) | 8 (20.51) | 1 (2.94) |
| 1 Jan 2013 to 3 Oct 2018 | 106 (61.99) | 66 (67.35) | 29 (74.36) | 11 (32.35) |
| After 3 Oct 2018 | 31 (18.13) | 7 (7.14) | 2 (5.13) | 22 (64.71) |
| Ethnicity, n (%) | ||||
| Hispanic, Latino/a, or Spanish origin | 34 (19.88) | 21 (21.43) | 6 (15.38) | 7 (20.59) |
| Not Hispanic, Latino/a, or Spanish origin | 137 (80.12) | 77 (78.57) | 33 (84.62) | 27 (79.41) |
| Unknown | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Race, n (%) | ||||
| White | 120 (70.18) | 69 (70.41) | 27 (69.23) | 24 (70.59) |
| Black or African American | 25 (14.62) | 13 (13.27) | 8 (20.51) | 4 (11.76) |
| Asian | 7 (4.09) | 4 (4.08) | 1 (2.56) | 2 (5.88) |
| Native Hawaiian or other Pacific Islander | 1 (0.58) | 0 (0.0) | 0 (0.0) | 1 (2.94) |
| American Indian or Alaska Native | 2 (1.17) | 1 (1.02) | 0 (0.0) | 1 (2.94) |
| Multiple races | 9 (5.26) | 7 (7.14) | 1 (2.56) | 1 (2.94) |
| Unknown | 7 (4.09) | 4 (4.08) | 2 (5.13) | 1 (2.94) |
| Ever CFC exposure, n (%) | ||||
| Yes | 164 (95.91) | 98 (100.0) | 39 (100.0) | 27 (79.41) |
| No‡ | 7 (4.09) | 0 (0.0) | 0 (0.0) | 7 (20.59) |
| Ever prophylaxis, n (%) | ||||
| Yes | 123 (71.93) | 86 (87.76) | 24 (61.54) | 13 (38.24) |
| No | 48 (28.07) | 12 (12.24) | 15 (38.46) | 21 (61.76) |
| Age at first CFC exposure, n (%) | ||||
| <1 mo | 55 (32.16) | 26 (26.53) | 16 (41.03) | 13 (38.24) |
| 1-12 mo | 78 (45.61) | 56 (57.14) | 12 (30.77) | 10 (29.41) |
| 1-10 y | 27 (15.79) | 16 (16.33) | 7 (17.95) | 4 (11.76) |
| Unknown | 4 (2.34) | 0 (0.0) | 4 (10.26) | 0 (0.0) |
| Never had CFC exposure | 7 (4.09) | 0 (0.0) | 0 (0.0) | 7 (20.59) |
| Treatment regimen before study exit, n (%) | ||||
| On-demand | 54 (31.58) | 17 (17.35) | 19 (48.72) | 18 (52.94) |
| Prophylaxis | 110 (64.33) | 81 (82.65) | 17 (43.59) | 12 (35.29) |
| No product usages | 4 (2.34) | 0 (0.0) | 0 (0.0) | 4 (11.76) |
| Unknown | 3 (1.75) | 0 (0.0) | 3 (7.69) | 0 (0.0) |
| Treatment class before study exit, n (%) | ||||
| Plasma derived | 29 (16.96) | 13 (13.27) | 8 (20.51) | 8 (23.53) |
| SHL recombinant | 95 (55.56) | 61 (62.24) | 25 (64.1) | 9 (26.47) |
| EHL recombinant | 25 (14.62) | 22 (22.45) | 2 (5.13) | 1 (2.94) |
| FVIII mimetic | 15 (8.77) | 2 (2.04) | 1 (2.56) | 12 (35.29) |
| No product usages | 4 (2.34) | 0 (0.0) | 0 (0.0) | 4 (11.76) |
| Missing data§ | 3 (1.75) | 0 (0.0) | 3 (7.69) | 0 (0.0) |
| Known CFC EDs | ||||
| N | 167 | 98 | 35 | 34 |
| Mean (standard deviation) | 33.2 (21.01) | 50 (0) | 12.09 (8.87) | 6.53 (9.81) |
| Median | 50 | 50 | 11 | 2 |
| IQR | 41 (9-50) | N/A | 12 (5-17) | 6 (1-7) |
| Range | 0-50 | 50 | 1-38 | 0-41 |
Birth cohorts chosen based on approval dates for new products.
Dec, December; Jan, January; IQR, interquartile range; SHL, standard half-life.
Severe HA: 1 Jan 2010 to 31 Dec 2012: 1 of 34 exited early and had 1 ED at study exit. 1 Jan 2013 to 3 Oct 2018: 11 of 106 participants exited early with mean 8.45 ± 12.47 EDs; median, 2 EDs; range, 0-41 EDs; IQR, (Q1, Q3) 6 (1.5, 7.5) EDs. After 3 Oct 2018: 22 of 31 participants with mean 5.82 ± 8.56 EDs; median, 2 EDs; range, 0-29 EDs; IQR, (Q1, Q3) 7 (0, 7) EDs.
Includes participants on prophylaxis taking FVIII mimetic.
Three participants with confirmed inhibitor did not have any recorded factor products.
Genotyping results for participants with severe HA
| F8 genotype . | n (%) . |
|---|---|
| Genotyping not completed | 47 (27.49) |
| Genotyping completed | 124 (72.51) |
| Missense | 16 (12.90) |
| Splice site change | 7 (5.65) |
| Inversion intron 22 | 34 (27.42) |
| Inversion, other | 4 (3.23) |
| Frameshift | 26 (20.97) |
| Large structural change of >50 base pairs | 14 (11.29) |
| Nonsense | 18 (14.52) |
| Promoter | 0 (0.0) |
| Small structural change | 2 (1.61) |
| Synonymous | 0 (0.0) |
| None identified | 3 (2.42) |
| F8 genotype . | n (%) . |
|---|---|
| Genotyping not completed | 47 (27.49) |
| Genotyping completed | 124 (72.51) |
| Missense | 16 (12.90) |
| Splice site change | 7 (5.65) |
| Inversion intron 22 | 34 (27.42) |
| Inversion, other | 4 (3.23) |
| Frameshift | 26 (20.97) |
| Large structural change of >50 base pairs | 14 (11.29) |
| Nonsense | 18 (14.52) |
| Promoter | 0 (0.0) |
| Small structural change | 2 (1.61) |
| Synonymous | 0 (0.0) |
| None identified | 3 (2.42) |
Treatment characteristics
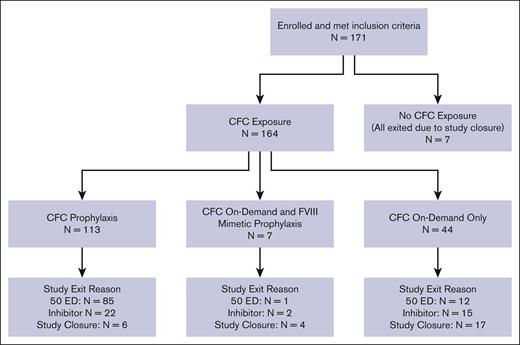
Figure 1A shows the distribution of participants based on CFC exposure during the study and reasons for study exit.
Distribution of participants with severe HA by CFC exposure and reason for study exit. Of 171 patients, 164 (95.9%) had CFC exposure at some time during the study; 113 of 164 (68.9%) had CFC exposure through prophylaxis, including 7 who also had FVIII mimetic prophylaxis; and 51 of 164 (31.1%) only had on-demand CFC exposure, including 7 who also had FVIII mimetic prophylaxis. Of 171 patients, 7 (4.1%) had no CFC exposure. In addition, of 113 patients who were on CFC prophylaxis, 85 exited because of 50 EDs (2 also were prescribed an FVIII mimetic); 22 exited because of FVIII inhibitor (none were also prescribed an FVIII mimetic); and 6 exited because of study closure (5 were also prescribed an FVIII mimetic).
Distribution of participants with severe HA by CFC exposure and reason for study exit. Of 171 patients, 164 (95.9%) had CFC exposure at some time during the study; 113 of 164 (68.9%) had CFC exposure through prophylaxis, including 7 who also had FVIII mimetic prophylaxis; and 51 of 164 (31.1%) only had on-demand CFC exposure, including 7 who also had FVIII mimetic prophylaxis. Of 171 patients, 7 (4.1%) had no CFC exposure. In addition, of 113 patients who were on CFC prophylaxis, 85 exited because of 50 EDs (2 also were prescribed an FVIII mimetic); 22 exited because of FVIII inhibitor (none were also prescribed an FVIII mimetic); and 6 exited because of study closure (5 were also prescribed an FVIII mimetic).
Overall, 123 (71.9%) received prophylactic treatment at some time during the study. The median age at prophylaxis initiation was 1.24 years (range, 0.13-6.86) for birth cohort 1 January 2010 to 31 December 2012; 0.96 years (range, 0.01-4.07) for birth cohort 1 January 2013 to 3 October 2018; and 0.72 years (range, 0.04-1.92) for birth cohort after 3 October 2018. Seventeen were treated with a prophylactic FVIII mimetic during the study; starting in 2018, there was a steady increase in the cumulative number of participants with HA using FVIII mimetic prophylaxis (1 in 2018, 6 in 2019, 13 in 2020, and 17 in 2021).
Primary end point
Of 171 patients, 159 (93.0%) had inhibitor testing (Table 3). Overall, the frequency of inhibitor testing was lower than expected; ∼40% had the recommended inhibitor testing at ED 4 but only 6.3% had recommended inhibitor testing by ED 22.
Adherence with inhibitor testing recommendations from the NBDF MASAC
| ED . | Participants with at least this number of EDs, N . | Expected number of inhibitor tests, N . | Participants with completion of recommended inhibitor testing∗ at this ED, n (%) . |
|---|---|---|---|
| 4 | 137 | 1 | 54 (39.42) |
| 7 | 127 | 2 | 34 (26.77) |
| 10 | 113 | 3 | 15 (13.27) |
| 13 | 110 | 4 | 10 (9.09) |
| 16 | 102 | 5 | 10 (9.80) |
| 19 | 98 | 6 | 8 (8.16) |
| 22 | 96 | 7 | 6 (6.25) |
| ED . | Participants with at least this number of EDs, N . | Expected number of inhibitor tests, N . | Participants with completion of recommended inhibitor testing∗ at this ED, n (%) . |
|---|---|---|---|
| 4 | 137 | 1 | 54 (39.42) |
| 7 | 127 | 2 | 34 (26.77) |
| 10 | 113 | 3 | 15 (13.27) |
| 13 | 110 | 4 | 10 (9.09) |
| 16 | 102 | 5 | 10 (9.80) |
| 19 | 98 | 6 | 8 (8.16) |
| 22 | 96 | 7 | 6 (6.25) |
Expected number of tests at each ED is based on NBDF MASAC document 236: at least every third ED or every 3 months (whichever occurs sooner) until 20 EDs have been reached and then every 3 to 6 months until 150 EDs.
Of 171 participants with severe HA, 39 (22.8%) developed a confirmed inhibitor; 30 of 171 (17.5%) had a high-titer inhibitor and 9 of 171 (5.3%) had a low-titer inhibitor. The median of the first and second of 2 consecutive positive inhibitor titers were 6.0 (range, 0.6-176) and 8.1 (range, 0.7-437.3) respectively.
The mean age of inhibitor development was 1.03 ± 0.57 years; median 1.02 years (interquartile range (Q1, Q3), 0.53 (0.73-1.26); range, 0.08-3.18). The mean EDs before inhibitor development were 10.85 ± 9.18 EDs; median 10.0 EDs (interquartile range (Q1, Q3), 11.5 (3.5-15); range, 0-38). Table 4 shows the percentage of inhibitors in participants by ED. Overall, 82% of inhibitors in participants with severe HA developed by ED 20. Treatment information after inhibitor development was known for 28 patients; 27 started immune tolerance induction and 9 were started on FVIII mimetic.
Inhibitor development in participants with severe HA (N = 171) and previous CFC exposure
| . | Number of EDs reached . | ||||
|---|---|---|---|---|---|
| ≤10 EDs . | ≤20 EDs . | ≤30 EDs . | ≤40 EDs . | ≤50 EDs . | |
| Participants, n (%) | 47 (27.49) | 61 (35.67) | 71 (41.52) | 72 (42.11) | 171 (100.0) |
| Cumulative inhibitor development, n (%) of 39 total inhibitors | 20 (51.28) | 32 (82.05) | 38 (97.44) | 39 (100.0) | 39 (100.0) |
| . | Number of EDs reached . | ||||
|---|---|---|---|---|---|
| ≤10 EDs . | ≤20 EDs . | ≤30 EDs . | ≤40 EDs . | ≤50 EDs . | |
| Participants, n (%) | 47 (27.49) | 61 (35.67) | 71 (41.52) | 72 (42.11) | 171 (100.0) |
| Cumulative inhibitor development, n (%) of 39 total inhibitors | 20 (51.28) | 32 (82.05) | 38 (97.44) | 39 (100.0) | 39 (100.0) |
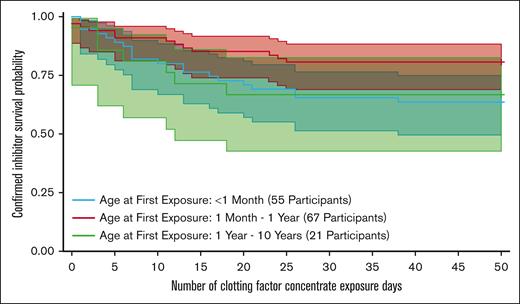
Table 5 includes data from the CoxPH model for inhibitor development in only the 137 participants with severe HA with either 50 EDs (98/137 [71.5%]) or a confirmed inhibitor (39/137 [28.5%]). In this analysis, participants who were aged <1 month at first product exposure had ∼2.6-fold increase (HR, 2.57; 95% CI, 1.22-5.44) in the rate (P = .013 without adjustment) of a confirmed inhibitor compared with those whose first product exposure occurred within the age range of 1 month to 1 year (Figure 2). Treatment reasons within the first month of life for participants with severe HA included bleeding (n = 46), prophylaxis (n = 16), and other (n = 3). Types of bleeds in the first month included soft tissue bleeds, muscle bleeds, genitourinary bleeding, and intracranial and extracranial hemorrhage. Types of treatment included recombinant and pd CFC, blood bank products (cryoprecipitate, fresh frozen plasma, and packed red blood cells), aminocaproic acid, and rFVIIa.
CoxPH ratio analysis of inhibitor risk in participants with severe HA who had either 50 EDs or confirmed inhibitor
| Variable . | Univariate with no adjustment . | With adjustment∗ . | ||||
|---|---|---|---|---|---|---|
| HR . | 95% CI . | P value . | HR . | 95% CI . | P value . | |
| Ethnicity,not Hispanic, Latino, or Spanish origin (reference) | ||||||
| Hispanic, Latino, or Spanish origin | 0.72 | 0.30-1.72 | .460 | 0.89 | 0.27-2.91 | .844 |
| Race, White (reference) | ||||||
| Asian | 0.70 | 0.10-5.17 | .729 | 0.27 | 0.02-4.27 | .351 |
| Black or African American | 1.50 | 0.68-3.31 | .312 | 1.31 | 0.30-5.70 | .721 |
| Others | 0.68 | 0.21-2.24 | .526 | 0.73 | 0.16-3.39 | .690 |
| Variant type (for participants with genotyping), missense and splice site change (reference) | ||||||
| Frameshift | 5.98 | 0.67-53.52 | .110 | 3.86 | 0.39-38.60 | .250 |
| Inversion intron 22 | 7.59 | 0.96-59.96 | .054 | 7.23 | 0.85-61.45 | .070 |
| Large structural change of >50 base pair | 16.59 | 1.94-142.20 | .010 | 14.50 | 1.54-136.92 | .020 |
| Nonsense | 12.47 | 1.53-101.36 | .018 | 12.53 | 1.41-111.49 | .023 |
| Age at first product exposure,† 1-12 mo (reference) | ||||||
| <1 mo | 2.57 | 1.22-5.44 | .013 | 2.27 | 0.89-5.82 | .087 |
| 1-10 y | 2.01 | 0.79-5.10 | .143 | 3.41 | 0.96-12.08 | .057 |
| First product exposure† given for ≥5 days, no (reference) | ||||||
| Yes | 1.22 | 0.50-2.96 | .655 | 1.20 | 0.21-6.93 | .838 |
| Unknown | 1.30 | 0.45-3.71 | .625 | 1.09 | 0.34-3.45 | .885 |
| Variable . | Univariate with no adjustment . | With adjustment∗ . | ||||
|---|---|---|---|---|---|---|
| HR . | 95% CI . | P value . | HR . | 95% CI . | P value . | |
| Ethnicity,not Hispanic, Latino, or Spanish origin (reference) | ||||||
| Hispanic, Latino, or Spanish origin | 0.72 | 0.30-1.72 | .460 | 0.89 | 0.27-2.91 | .844 |
| Race, White (reference) | ||||||
| Asian | 0.70 | 0.10-5.17 | .729 | 0.27 | 0.02-4.27 | .351 |
| Black or African American | 1.50 | 0.68-3.31 | .312 | 1.31 | 0.30-5.70 | .721 |
| Others | 0.68 | 0.21-2.24 | .526 | 0.73 | 0.16-3.39 | .690 |
| Variant type (for participants with genotyping), missense and splice site change (reference) | ||||||
| Frameshift | 5.98 | 0.67-53.52 | .110 | 3.86 | 0.39-38.60 | .250 |
| Inversion intron 22 | 7.59 | 0.96-59.96 | .054 | 7.23 | 0.85-61.45 | .070 |
| Large structural change of >50 base pair | 16.59 | 1.94-142.20 | .010 | 14.50 | 1.54-136.92 | .020 |
| Nonsense | 12.47 | 1.53-101.36 | .018 | 12.53 | 1.41-111.49 | .023 |
| Age at first product exposure,† 1-12 mo (reference) | ||||||
| <1 mo | 2.57 | 1.22-5.44 | .013 | 2.27 | 0.89-5.82 | .087 |
| 1-10 y | 2.01 | 0.79-5.10 | .143 | 3.41 | 0.96-12.08 | .057 |
| First product exposure† given for ≥5 days, no (reference) | ||||||
| Yes | 1.22 | 0.50-2.96 | .655 | 1.20 | 0.21-6.93 | .838 |
| Unknown | 1.30 | 0.45-3.71 | .625 | 1.09 | 0.34-3.45 | .885 |
Results with adjustment are from multivariable analysis with all 5 variables.
Product classes include factor replacement, activated prothrombin complex concentrate, rFVIIa, FVIII mimetic, blood bank products (cryoprecipitate, fresh frozen plasma, and packed red blood cells), other hemostatic agents, and unknown.
Kaplan-Meier analysis of inhibitor development by age of first treatment for participants with severe HA.
Kaplan-Meier analysis of inhibitor development by age of first treatment for participants with severe HA.
In the CoxPH model, adjusted for race, ethnicity, age at first exposure, and first product exposure given for 5+ days, in the 137 participants with either 50 EDs or a confirmed inhibitor and completed genotype, the presence of large structural changes, (HR, 14.50; 95% CI, 1.54-136.92) and nonsense variants, (HR,12.53; 95% CI, 1.41-111.49) were significantly associated with the higher risk of inhibitor development compared with the reference category of missense and splice site variants. Inversion intron 22 and frameshift variants were not statistically associated with an increased risk of inhibitor development. Some types of F8 variants were not prevalent enough within the population to evaluate the risk for inhibitor development. Inhibitor development was not associated with ethnicity, race, or first product exposure given for 5+ days in either univariate or multivariable CoxPH models.
Half of participants experience their first bleeding within 1 month of life. Of 335 reported bleeds, the most frequent bleeding episodes were joint (60/335 [17.9%]), soft tissue (65/335 [19.4%]), and extracranial head bleeds (63/335 [18.1%]). The overall rate of intracranial hemorrhage (ICH) was 6.4% (11/171 participants). The mean ± standard deviation age of ICH was 289.3 ± 354.7 days; median, 174; range 2-1273. Of 11 ICH events, 2 (18.2%) occurred within the first month of life; 8 had state/federal insurance and 3 had commercial insurance. No participants with ICH were on a FVIII mimetic at the time of ICH; 2 were on a FVIII mimetic after the ICH. Only 1 participant with ICH exited because of inhibitor development. Association of ICH with trauma or delivery was not captured.
Discussion
ATHN 8 is, to our knowledge, the first US-based natural history study of PUPs with moderate and severe hemophilia. In ATHN 8, inhibitor rates for PUPs with severe HA were consistent with published data.
The frequency of inhibitor surveillance/testing was less than recommended based on the National Bleeding Disorders Foundation (NBDF) MASAC guidelines.22 Approximately 40% had inhibitor testing by ED 4, but adherence to testing declined with only 6.3% having the suggested number of measured inhibitor titers by ED 22. Reasons for lower inhibitor testing during the study may have been less frequent clinic visits or higher use of telemedicine during the severe acute respiratory syndrome coronavirus 2/COVID-19 pandemic. In addition, clinicians may only send testing at scheduled annual visits or when an inhibitor is clinically suspected, particularly when travel to clinic is a barrier. Early detection of inhibitors through surveillance for individuals with any hemophilia severity is important to guide treatment plan management and avoid bleeding complications. The impact of less frequent inhibitor surveillance is missing low-titer inhibitors and delaying identification of high-titer inhibitors, particularly for those with bleed protection from FVIII mimetic. Clinical teams at hemophilia treatment centers should assess inhibitor screening practices and develop interventions to improve inhibitor surveillance.
Previously reported risk factors for inhibitor development include severe disease, high-risk genotype (null mutations), family history of inhibitor, non-White ethnicity, intensive treatment at high dose within the first 20 EDs, and CFC class (recombinant higher than pd).1-5,10,23-25 In ATHN 8, inhibitor development in participants with severe HA was associated with age of first treatment, with higher risk in participants who had product exposure at an aged of <1 month. There are conflicting reports in the literature related to the association between age of first CFC exposure and inhibitor risk. Gouw et al provided data from the CANAL study (Concerted Action on Neutralizing Antibodies in severe hemophilia A) attributing higher risk of inhibitor with age to be due to higher intensity treatments.25 More recently, in the Guardian 4 trial,8 the incidence of inhibitor increased with age at first infusion of FVIII, although this result was not statistically significant. However, in the NuProtect trial,3 patients aged 1 to 6 months and >6 to 12 months at first treatment had higher incidence of inhibitors compared with patients aged >12 to 24 months and >24 months, and treatment intensity did not affect risk. The investigators suggested this was because of lower immunogenicity with simoctocog alfa compared with other CFCs. However, this result may be biased because industry studies, by nature, have a selected population missing infants with bleeding directly after birth. Since initiation of ATHN 8, HAVEN 7 was initiated and completed. In this phase 3, open-label study of FVIII mimetic prophylaxis in 55 infants with severe HA without FVIII inhibitors, 2 participants developed an inhibitor to CFC, 1 at ED 3, and 1 at ED 10.26 Ongoing risk evaluation is warranted, especially because the age of CFC initiation may be influenced by new treatment options.
As expected, F8 genotype was associated with risk of inhibitor development in participants with severe HA, which is similar to findings from the PedNet Registry.11
Participants in this study had substantial bleeding including joint and soft tissue bleeds and ICH. Although a high proportion of participants were on prophylactic treatment at some point during the study, the rate of joint bleeding was still high. Also notable, bleeding was the most common cause for treatment in those aged <1 month; and, as noted earlier, this group was shown to have a higher rate of inhibitor development. The rate of ICH in this cohort was 6.4%. Kulkarni et al reported a rate of 8.4% in children aged ≤2 years,27 whereas Andersson et al found an incidence of 2% in the newborn period.28 A study using the Universal Data Collection database of children included 1635 participants with severe HA and found rates of ICH of 9.1% in those aged <4 years.29 Association with trauma was not collected in ATHN 8 although spontaneous ICH is only expected in severe HA based on the Universal Data Collection study.29 The persistent risk of ICH in this age group deserves further study to identify measures to mitigate the risk, including earlier initiation of prophylaxis.
Use of FVIII mimetic prophylaxis increased throughout the study and may lead to earlier prophylaxis initiation, and possibly less joint disease over time as demonstrated with CFC prophylaxis.30 With earlier initiation of FVIII mimetic prophylaxis, it is possible that it will be protective against ICH in the newborn period. In HAVEN 7, no infants had ICH or spontaneous bleeding26,31; 12 who were treated with a FVIII mimetic at some point during the study exited the study before reaching study end point of 50 EDs or inhibitor. We suspect that with more patients on FVIII mimetic prophylaxis the time to 50 EDs will be much longer as proposed by Patel et al.32 In addition, the use of efanesoctocog alfa, which has sustained FVIII levels with weekly prophylaxis because of decoupling the FVIII half-life from endogenous von Willebrand factor may decrease the rate of CFC exposure.33 The impact of new therapies on inhibitor surveillance and risk as well as clinical outcomes will be investigated in ATHN Transcends (ClinicalTrials.gov identifier: NCT04398628), a natural history study including PUPs with hemophilia.
Limitations
Too few participants with hemophilia B and moderate HA were enrolled to make valid conclusions and these data are excluded from this report. A limitation of the study was that most of the participants were enrolled after first bleed and/or treatment. If CFC treatment is underreported, then participants may have been followed up for inhibitor development beyond the true ED 50, leading to an underestimation of the number of EDs before inhibitor development. However, between extensive data cleaning and close communication with ATHN, the principal investigators and the study sites minimized missing data and addressed data queries. As noted earlier, lower inhibitor testing could miss low-titer inhibitors and/or delay time to high titer inhibitor detection. Also, 10.4% of participants exited the study without meeting an end point at study closure. This is because of short follow-up for participants born closer to the time of study closure and lower rates of bleeding and prophylaxis for participants with moderate disease and/or treated with FVIII mimetic, thus not reaching the primary end point in the allotted time. Longer follow-up is planned for children enrolled in ATHN Transcends and can better assess long-term inhibitor risk in those who are treated less frequently and reach 50 ED at a later age. The study was conducted at ATHN affiliates and may not be generalizable outside of these sites. The direction and magnitude of the potential selection bias is unknown, although one can hypothesize that the rate of inhibitors may be overestimated because those with more severe disease may be more likely to seek care at specialized centers and have treatment records. To minimize selection bias at individual sites, sites were encouraged to enroll all eligible patients and allow use of translation of consents and short-forms consents depending on institutional practices.
Conclusions
In ATHN 8, inhibitor rates for PUPs with severe HA were consistent with published data. As expected, inhibitor development was associated with F8 genotype in severe HA. The risk was also higher for those with early treatment. For PUPs with severe HA, FVIII mimetic prophylaxis increased over the course of the study period. All institutions that care for individuals with hemophilia should maintain inhibitor testing protocols, including for those treated with FVIII mimetic, and identify methods to increase adherence with testing. Longitudinal follow-up is needed to determine whether new treatment products and implementation practices are associated with changes in inhibitor rates or alteration of the age at inhibitor development.
Acknowledgments
The authors acknowledge the 23 hemophilia treatment centers and participants for their commitment to the American Thrombosis and Hemostasis Network (ATHN) 8 study. The authors also acknowledge the long-standing commitment of the Centers for Disease Control and Prevention to conduct inhibitor surveillance and the testing conducted for this study. Hilary Markoe from the ATHN provided writing support.
This work was supported by grants to the ATHN from Versiti Blood Research Institute, CSL Behring, Octapharma, Hemophilia of Georgia, and Pfizer Hemophilia. C.D.T. and S.L.C. received research funding from ATHN with designated funds from CSL Behring for their role as principal investigators.
Authorship
Contribution: C.D.T., H.M.v.d.B., L.M., M.R., and S.L.C. contributed to the concept and design of the study; C.D.T. and S.L.C. wrote the original draft manuscript; and all authors contributed to the analysis and interpretation of data, critical revisions of the manuscript, and final approval of the version to be published.
Conflict-of-interest disclosure: C.D.T. reports previous research grant support from the ATHN, BioMarin, and Novo Nordisk; has received honoraria for consultancy from CSL Behring, Genentech/Roche, Pfizer, Regeneron, Spark Therapeutics, and Sanofi; and serves as an advisor on the National Bleeding Disorders Foundation Medical and Scientific Advisory Council. L.M. has received honoraria for consultancy from Genentech/Roche, BioMarin, CSL Behring, Novo Nordisk, Sobi, Sanofi, and Pfizer; has received honoraria for speaking from CSL Behring and Sanofi; and serves on the executive board for the Hemostasis and Thrombosis Research Society. M.R. has received research grant support from Bayer, BioMarin, CSL Behring, Genentech/Roche, Grifols, HEMA Biologics, LFB USA, Novo Nordisk, Octapharma, Pfizer, Sanofi, Spark, Takeda, and uniQure; has received honoraria for consultancy from CSL Behring, Genentech/Roche, HEMA Biologics, Novo Nordisk, Pfizer, Sanofi, Takeda, and uniQure; and serves on the board for Partners in Bleeding Disorders. S.L.C. reports research grant support from the ATHN; has received honoraria for consultancy from Genentech/Roche, Kedrion, and Novo Nordisk; and serves on the executive board for the Hemostasis and Thrombosis Research Society. The remaining authors declare no competing financial interests.
The current affiliation for C.D.T. is Division of Blood Diseases and Resources, National Heart, Lung, and Blood Institute, National Institutes of Health, Bethesda, MD.
The current affiliation for M.M. is MD Anderson Cancer Center, Houston, TX.
The current affiliation for C.O. is Dartmouth Health, Hanover, NH.
A complete list of the members of the ATHN 8 Study Group appears in “Appendix.”
Correspondence: Courtney D. Thornburg, Division of Blood Diseases and Resources, National Heart, Lung, and Blood Institute, National Institutes of Health, 6705 Rockledge Dr, Bethesda, MD 20892; email: courtney.thornburg@nih.gov.
Appendix
The members of the ATHN 8 Study Group are Christine Knoll, Phoenix Children’s Hospital; Lynn Malec, Blood Center of Wisconsin; Michael Wang, University of Colorado Hemophilia & Thrombosis Treatment Center; Michael Guerrera, Children’s National Medical Center; Cristina Tarango, Cincinnati Children’s Hospital Medical Center; Robert Sidonio, Comprehensive Bleeding Disorders Center at Emory University and Children’s Healthcare of Atlanta; Nidra Rodriguez, Gulf States Hemophilia & Thrombophilia Center, The University of Texas Health Science Center–McGovern Medical School; Kenneth D. Friedman, Green Bay Wisconsin Hemophilia Outreach Center; Amy Shapiro, Indiana Hemophilia Treatment Center; Hassan Yaish, Intermountain Hemophilia & Thrombosis Center; Shannon Carpenter, Kansas City Regional Hemophilia Center; Maissaa Janbain, Louisiana Center for Bleeding and Clotting Disorders; Eric Larsen, Maine Hemophilia and Thrombosis Center; Eric Grabowski, Massachusetts General Hospital; Rajiv Pruthi, Mayo Comprehensive Hemophilia Center; Kristina Haley, Hemostasis & Thrombosis Center, Oregon Health & Science University; Courtney Thornburg, Rady Children’s Hospital San Diego; Sanjay Ahuja, Rainbow Babies and Children’s University Hospital Health System; Mindy Simpson, Rush University Medical Center; Ulrike Reiss, St. Jude Children’s Research Hospital; Tiffany Lucas, University of California at San Francisco Pediatric Hemophilia Center at Mission Bay; Vinod Balasa, Valley Children’s Hospital; and Allison Wheeler, Vanderbilt Hemostasis/Hemophilia Clinic.
References
Author notes
Presented orally in abstract form at the 64th annual meeting of the American Society of Hematology, New Orleans, LA, 10 to 13 December 2022.
Restrictions apply to the availability of the data, which were used under license for this study. Data inquiries can be made by emailing American Thrombosis and Hemostasis Network at support@athn.org.