Key Points
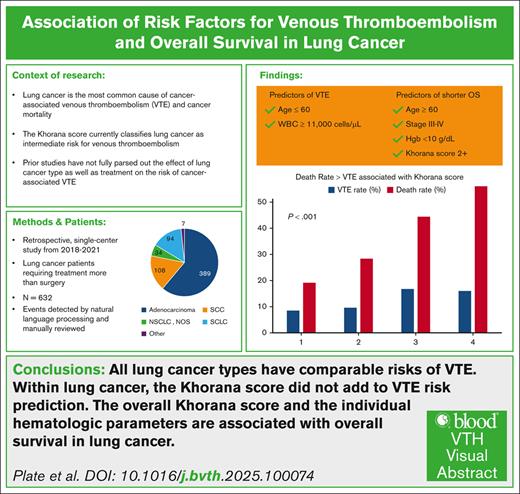
All lung cancer types have comparable risks of VTE, and within lung cancer, the KS did not add to VTE risk prediction.
The overall KS and the individual hematologic parameters are associated with overall survival in lung cancer.
Visual Abstract
Venous thromboembolism (VTE) is a frequent complication in patients with lung cancer, but the risk factors and incidence in different lung cancer subtypes have not been fully characterized. Despite multiple studies supporting the use of VTE prophylaxis in patients with cancer at increased risk of VTE based on the Khorana score (KS), routine use of VTE prophylaxis is uncommon in clinical practice. This study further characterizes the risk factors and incidence of VTE in patients with lung cancer at a university cancer center. Furthermore, we assessed the association of KS and its individual components with overall survival in this same group of patients. Using natural language processing and human review to detect thrombotic events in the electronic medical record, a 12-month incidence of 10.1% was identified in the 632 patients with lung cancer analyzed. Significant risk factors included age <60 years and white blood cell (WBC) count ≥11 × 109/L, but KS itself was not significantly associated with VTE. The median overall survival was 12 months with VTE. The KS, age ≥60 years, stage III to IV, WBC count ≥11 × 109/L, hemoglobin <10 g/dL, body mass index, surgery, and VTE were identified as significant predictors of death. These findings warrant further validation, because the KS and 2 of its individual components in this study of lung cancer were significantly associated with reduced overall survival.
Introduction
Venous thromboembolism (VTE) is a potentially life-threatening complication associated with active malignancy and poses a considerable and increasing burden on patients with lung cancer.1-4 It manifests as deep vein thrombosis (DVT) or pulmonary embolism (PE) and can lead to increased morbidity, compromised treatment outcomes, and mortality.1,5-7 Although the increased risk of VTE in patients with lung cancer has been recognized, a comprehensive understanding of the risk factors, particularly in different lung cancer subtypes, remains elusive.8,9 The complexity of lung cancer etiology and its interplay with thrombosis necessitates further investigation to accurately delineate the determinants of VTE occurrence.
Prophylactic anticoagulation with direct oral anticoagulants has been shown to mitigate VTE risk in patients with cancer at high risk for VTE.10,11 Current National Comprehensive Cancer Network guidelines state, “The National Comprehensive Cancer Network Panel recommends identification of patients at risk for developing VTE and subsequent initiation of VTE prophylaxis based on inpatient/outpatient and medical/surgical oncology status.”12 Current guidelines treat lung cancer as an intermediate-risk entity in the Khorana score (KS) for thrombosis risk prediction.13 Furthermore, grouping all lung cancer subtypes together may obscure subtype-specific associations and hinder the development of tailored thromboprophylaxis strategies.8,9,14 Hence, there is an unmet need to better define the distinct risk profiles of various lung cancer subtypes for VTE.
Furthermore, the impact of different therapy types on VTE risk in patients with lung cancer has not been fully explored. The advent of innovative treatment modalities, including chemoimmunotherapy and targeted therapy, has revolutionized lung cancer management, but the implications of these therapies for VTE risk remain poorly understood.8,9,15,16 Addressing this knowledge gap is crucial to optimizing patient care and potentially preventing VTE-associated complications.
This study endeavors to shed light on the incidence of VTE in patients with lung cancer treated at our university cancer center. Our primary objective is to investigate the risk factors associated with VTE occurrence within distinct lung cancer subtypes. We also explore the impact of various treatment modalities, including chemotherapy, immunotherapy, and targeted therapy, on the risk of thromboembolic events. Lastly, we assessed the impact on overall survival (OS) of the development of VTE and, independently, the impact of the risk factors for VTE on OS. Improved risk stratification for VTE in lung cancer may contribute to evidence-based prophylactic anticoagulation strategies in lung cancer.
Methods
Patient population
This study was approved by the institutional review board of the University of Miami Health System/Sylvester Comprehensive Cancer Center. Patients with a diagnosis of lung cancer between 1 January 2018 and 1 January 2021 were identified through our institutional tumor registry (TR) data. Patients were included in our study if diagnosis was done at our institution and survival outcomes were available. Patients who did not require therapy beyond surgery were excluded. All stages and histologic subtypes of lung cancer were eligible for the study.
Event definition
The start date in all patients was the chemotherapy initiation date of cycle 1 in the first-line setting. Start date for patients who did not receive treatment was considered the diagnosis date. VTE events included all lower extremity DVTs (proximal or distal) and all PEs, including symptomatic and incidental. We included all VTEs detected by an imaging study, including ultrasound of lower extremity veins, computed tomography (CT) chest with contrast, ventilation/perfusion (V/Q) scan, or CT contrast of the lower extremity. VTE events were considered to have occurred if a patient developed VTE between 90 days before and up to 1 year after diagnosis or died from any cause. Data were censored at 1 year from the start date if no event occurred. Thrombosis was defined as developing one of the following: lower extremity DVT and/or PE. Secondary end point was OS.
Data collection
Baseline demographic and laboratory values were extracted from the electronic medical record. Complete blood count data were from the date closest to the start of systemic therapy but before the first day of therapy. Tumor mutation status was assessed by extracting initial tumor gene sequencing data (either tissue-based or circulating tumor DNA, whichever had the earliest result). For event data collection, using automated text search from our electronic medical record (Epic) combined with natural language processing (NLP) review, VTE events were flagged and uploaded to a REDCap (Research Electronic Data Capture) database. Three investigators (A.T., T.E.P., and R.S.K.) reviewed flagged events and adjudicated presence or absence of thrombosis, date, location, and laterality. The senior investigator (G.A.S.) performed a second review to confirm adjudication accuracy. For patient baseline characteristics, treatments, and survival, the University of Miami Health System/Sylvester Comprehensive Cancer Center TR was used. The TR data at our institution include but are not limited to the following: age at diagnosis, body mass index (BMI), dates and types of cancer treatment, surgery, response to treatment, and survival.
Data analysis
Clinical data from the TR and curated event data in REDCap were used.17,18 Descriptive analysis was presented as number and percentage for categorical variables and as mean, standard deviation, median, and range for continuous variable in patients overall. Time to events included 2 end points. The first end point was time to first event (VTE or death), which was defined as the time from the initiation of chemotherapy or diagnosis date, if no chemotherapy was administered, to VTE or date of death. If VTE occurred before diagnosis or initiation of chemotherapy, time to VTE was coded as 0.1. Cumulative incidence rates of VTE were estimated using competing risk methods, with death as the competing risk. Gray test was used to compare VTE incidence curves. Estimated subdistribution hazard ratios (HRs) with corresponding 95% confidence intervals (CIs), and P values were calculated by fitting Fine and Gray proportional subdistribution hazards models to evaluate unadjusted and adjusted effects of variables on the risk of VTE, considering death as the competing risk. The second end point was OS, which was defined as the time from diagnosis to death from any cause. OS was estimated by the Kaplan-Meier method, and its associations with prognostic factors were assessed by log-rank test. Cox proportional hazard analysis was performed to assess the association between the main predictors, lung cancer type, and OS, with unadjusted (univariate)/adjusted (multivariate) analyses for selected prognostic factors, including stage, age, sex, race, ethnicity, white blood cell (WBC) count, hemoglobin (Hb), platelet (Plt) count, BMI, and KS. Results were reported as HRs with 95% CIs. Event-free patients were censored for a maximum period of 12 months in the analysis for both time-to-event outcomes. Statistical significance was set at a threshold of P value <.05. All data analysis was conducted using SAS version 9.4 for Windows (SAS Institute Inc, Cary, NC).
Results
A total of 632 patients with lung cancer were included in the study, with a median age of 65 years (range, 24-96). Of those, 389 had adenocarcinoma, 108 had squamous cell carcinoma, 34 had unspecified non–small cell lung cancer (NSCLC), and 94 had SCLC. Stage I to II disease was present in 102 patients, and stage III to IV disease was present in 490 patients. The median WBC count was 8.8 × 109/L (range, 1.3 × 109 to 71.6 × 109/L), the median Hb was 12.6 g/dL (range, 7.6-17.6), and the median Plt was 286 × 109/L (range, 59 × 109 to 930 × 109/L; Table 1).
Selected characteristics of the patients and associations with VTE and OS
| Characteristic . | Number (632 total) . | % of total . |
|---|---|---|
| Age ≥60 y | 469 | 74.2 |
| Male sex | 326 | 51.6 |
| Race | ||
| White | 561 | 88.8 |
| Black | 54 | 8.5 |
| Other/unknown | 17 | 2.7 |
| Ethnicity | ||
| Non-Hispanic | 361 | 57.1 |
| Hispanic | 252 | 39.9 |
| Unknown | 19 | 3.0 |
| Tobacco history | ||
| None/never used | 138 | 21.8 |
| Any current/prior use | 483 | 76.4 |
| Baseline WBC ≥11 × 109/L | 151 | 23.9 |
| Baseline Hb <10 g/dL | 64 | 10.1 |
| Baseline Plt ≥350 × 109/L | 174 | 27.5 |
| BMI, kg/m2 | ||
| <18.5 | 37 | 5.9 |
| 18.5-24.9 | 240 | 38.0 |
| ≥25-25.9 | 354 | 56.0 |
| KS | ||
| 1-2 | 534 | 84.5 |
| 3-4 | 97 | 15.3 |
| Lung cancer type | ||
| AC | 389 | 61.6 |
| SCC | 108 | 17.1 |
| NSCLC, NOS | 34 | 5.4 |
| SCLC | 94 | 14.9 |
| Unknown/other | 7 | 1.1 |
| AJCC stage | ||
| I-II | 102 | 16.1 |
| III-IV | 490 | 77.5 |
| Unknown | 40 | 6.3 |
| Therapy type | ||
| Chemotherapy | 223 | 35.3 |
| Chemoimmunotherapy | 297 | 47 |
| Targeted therapy | 77 | 12.2 |
| Radiation therapy | 283 | 44.8 |
| Surgery | 158 | 25.0 |
| VTE | ||
| Before therapy (90 d) | 20 | 3.1 |
| After initiating therapy | 44 | 6.9 |
| Yes (total) | 64 | 10.1 |
| Vital status | ||
| Alive at 6 mo | 549 | 86.9 |
| Alive at 12 mo | 466 | 73.7 |
| Characteristic . | Number (632 total) . | % of total . |
|---|---|---|
| Age ≥60 y | 469 | 74.2 |
| Male sex | 326 | 51.6 |
| Race | ||
| White | 561 | 88.8 |
| Black | 54 | 8.5 |
| Other/unknown | 17 | 2.7 |
| Ethnicity | ||
| Non-Hispanic | 361 | 57.1 |
| Hispanic | 252 | 39.9 |
| Unknown | 19 | 3.0 |
| Tobacco history | ||
| None/never used | 138 | 21.8 |
| Any current/prior use | 483 | 76.4 |
| Baseline WBC ≥11 × 109/L | 151 | 23.9 |
| Baseline Hb <10 g/dL | 64 | 10.1 |
| Baseline Plt ≥350 × 109/L | 174 | 27.5 |
| BMI, kg/m2 | ||
| <18.5 | 37 | 5.9 |
| 18.5-24.9 | 240 | 38.0 |
| ≥25-25.9 | 354 | 56.0 |
| KS | ||
| 1-2 | 534 | 84.5 |
| 3-4 | 97 | 15.3 |
| Lung cancer type | ||
| AC | 389 | 61.6 |
| SCC | 108 | 17.1 |
| NSCLC, NOS | 34 | 5.4 |
| SCLC | 94 | 14.9 |
| Unknown/other | 7 | 1.1 |
| AJCC stage | ||
| I-II | 102 | 16.1 |
| III-IV | 490 | 77.5 |
| Unknown | 40 | 6.3 |
| Therapy type | ||
| Chemotherapy | 223 | 35.3 |
| Chemoimmunotherapy | 297 | 47 |
| Targeted therapy | 77 | 12.2 |
| Radiation therapy | 283 | 44.8 |
| Surgery | 158 | 25.0 |
| VTE | ||
| Before therapy (90 d) | 20 | 3.1 |
| After initiating therapy | 44 | 6.9 |
| Yes (total) | 64 | 10.1 |
| Vital status | ||
| Alive at 6 mo | 549 | 86.9 |
| Alive at 12 mo | 466 | 73.7 |
Race and ethnic group were reported by the patient. Patients who received radiation therapy and surgery could have received other therapies. Boldface type indicates significance at P <.05.
AC, adenocarcinoma; AJCC, American Joint Committee on Cancer; NOS, not otherwise specified; SCC, squamous cell carcinoma.
For the 632 patients studied, the NLP workflow iterated >28 615 imaging and encounter notes. Although most medical record numbers had at least 1 NLP-identified concept (606), the volume of notes requiring further review dropped by 55% (12 757 total notes with at least 1 NLP match). There were 74 medical record numbers with at least 1 NLP-identified note and a confirmed event.
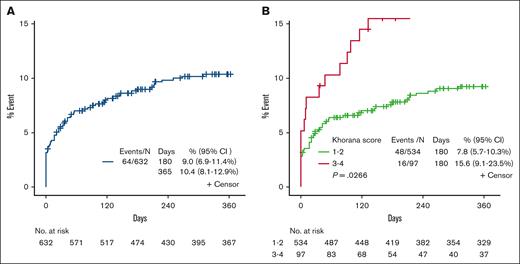
The overall 12-month incidence of VTE (lower extremity DVT and/or PE) was 10.1% (n = 64). Of the 64 VTE events, 20 were identified before the start of therapy (Table 1, Figure 1). For time-to-event analysis, the median follow-up time for patients with VTE and non-VTE was 23 and 365 days, respectively, and for those dead and alive, it was 12 and 6 months, respectively. Univariate cumulative incidence rates of VTE analysis revealed that age <60 years, WBC ≥11 × 109/L, and KS 3 to 4 were associated with lower thrombosis-free survival (TFS; P < .05), all of which remained significant after multivariate analysis, except for KS 3 to 4 (P = .054; Table 2).
Cox regression of risk factors on TFS in lung cancer
| Parameter . | TFS . | |||
|---|---|---|---|---|
| UVA . | MVA . | |||
| VTE HR (95% CI) . | P value . | VTE HR (95% CI) . | P value . | |
| Age ≥60 y | 0.52 (0.32-0.86) | .011 | 0.53 (0.32-0.87) | .012 |
| Male sex | 0.93 (0.57-1.52) | .779 | NA | |
| Race | ||||
| White | Ref | NA | ||
| Black | 0.90 (0.36-2.24) | .818 | NA | |
| Other/unknown | 0.59 (0.08-4.37) | .607 | NA | |
| Ethnicity | ||||
| Non-Hispanic | Ref | NA | ||
| Hispanic | 1.50 (0.91-2.46) | .113 | NA | |
| Unknown | 2.03 (0.62-6.57) | .240 | NA | |
| Tobacco history | ||||
| None/never used | Ref | |||
| Any current/prior use | 0.94 (0.53-1.66) | .827 | ||
| Baseline WBC ≥11 × 109/L | 1.72 (1.04-2.87) | .036 | 1.65 (1.00-2.72) | .050 |
| Baseline Hb <10 g/dL | 0.71 (0.28-1.78) | .463 | NA | |
| Baseline Plt ≥350 × 109/L | 1.24 (0.74-2.08) | .423 | NA | |
| BMI, kg/m2 | ||||
| <18.5 | 0.81 (0.24-2.71) | .737 | ||
| 18.5-24.9 | Ref | |||
| ≥25-25.9 | 1.05 (0.63-1.75) | .849 | NA | |
| KS | ||||
| 1-2 | Ref | |||
| 3-4 | 1.89 (1.08-3.31) | .027 | 1.73 (0.99-3.03) | .054 |
| Lung cancer type | ||||
| AC | Ref | NA | ||
| SCC | 0.73 (0.36-1.48) | .385 | ||
| NSCLC, NOS | 1.37 (0.53-3.50) | .517 | ||
| SCLC | 0.57 (0.24-1.34) | .198 | ||
| Unknown/other | 1.35 (0.18-10.37) | .773 | ||
| AJCC stage | ||||
| I-II | Ref | Ref | ||
| III-IV | 2.09 (0.91-4.81) | .084 | 1.76 (0.51-6.14) | .373 |
| Unknown | NE | NE | ||
| Therapy type | ||||
| Chemotherapy | 0.90 (0.27-2.98) | .868 | NA | |
| Chemoimmunotherapy | 1.18 (0.37-3.78) | .776 | ||
| Targeted therapy | 0.94 (0.25-3.58) | .927 | ||
| Radiation therapy | 1.28 (0.79-2.08) | .324 | ||
| Surgery | 0.54 (0.27-1.05) | .067 | 0.90 (0.34-2.41) | .839 |
| Parameter . | TFS . | |||
|---|---|---|---|---|
| UVA . | MVA . | |||
| VTE HR (95% CI) . | P value . | VTE HR (95% CI) . | P value . | |
| Age ≥60 y | 0.52 (0.32-0.86) | .011 | 0.53 (0.32-0.87) | .012 |
| Male sex | 0.93 (0.57-1.52) | .779 | NA | |
| Race | ||||
| White | Ref | NA | ||
| Black | 0.90 (0.36-2.24) | .818 | NA | |
| Other/unknown | 0.59 (0.08-4.37) | .607 | NA | |
| Ethnicity | ||||
| Non-Hispanic | Ref | NA | ||
| Hispanic | 1.50 (0.91-2.46) | .113 | NA | |
| Unknown | 2.03 (0.62-6.57) | .240 | NA | |
| Tobacco history | ||||
| None/never used | Ref | |||
| Any current/prior use | 0.94 (0.53-1.66) | .827 | ||
| Baseline WBC ≥11 × 109/L | 1.72 (1.04-2.87) | .036 | 1.65 (1.00-2.72) | .050 |
| Baseline Hb <10 g/dL | 0.71 (0.28-1.78) | .463 | NA | |
| Baseline Plt ≥350 × 109/L | 1.24 (0.74-2.08) | .423 | NA | |
| BMI, kg/m2 | ||||
| <18.5 | 0.81 (0.24-2.71) | .737 | ||
| 18.5-24.9 | Ref | |||
| ≥25-25.9 | 1.05 (0.63-1.75) | .849 | NA | |
| KS | ||||
| 1-2 | Ref | |||
| 3-4 | 1.89 (1.08-3.31) | .027 | 1.73 (0.99-3.03) | .054 |
| Lung cancer type | ||||
| AC | Ref | NA | ||
| SCC | 0.73 (0.36-1.48) | .385 | ||
| NSCLC, NOS | 1.37 (0.53-3.50) | .517 | ||
| SCLC | 0.57 (0.24-1.34) | .198 | ||
| Unknown/other | 1.35 (0.18-10.37) | .773 | ||
| AJCC stage | ||||
| I-II | Ref | Ref | ||
| III-IV | 2.09 (0.91-4.81) | .084 | 1.76 (0.51-6.14) | .373 |
| Unknown | NE | NE | ||
| Therapy type | ||||
| Chemotherapy | 0.90 (0.27-2.98) | .868 | NA | |
| Chemoimmunotherapy | 1.18 (0.37-3.78) | .776 | ||
| Targeted therapy | 0.94 (0.25-3.58) | .927 | ||
| Radiation therapy | 1.28 (0.79-2.08) | .324 | ||
| Surgery | 0.54 (0.27-1.05) | .067 | 0.90 (0.34-2.41) | .839 |
A total of 632 patients were included in the analysis, and 64 events occurred.
MVA, multivariate analysis; NA, not applicable; NE, not estimable; Ref, reference population; UVA, univariate analysis.
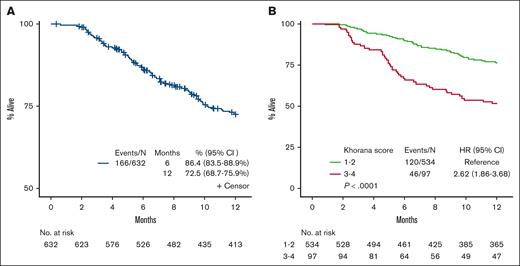
The 6-month and 12-month OS was 86.4% (95% CI, 83.5-88.9) and 72.5% (95% CI, 68.7-75.9), respectively (Figure 2). Multivariate analyses showed that parameters associated with OS included age ≥60, stage III to IV, WBC ≥11 × 109/L, Hb <10 g/dL, BMI <25 kg/m2, surgery, and VTE, all of which remained significant predictors of death. VTE was associated with an increased risk of death (HR, 1.80; 95% CI, 1.20-2.75; P = .005), as was KS of 3 to 4 (HR, 1.87; 95% CI; 1.30-2.70; P < .001; MVA2), after controlling age, sex, stage, lung cancer histology, Hb, Plt, WBC, surgery, therapy type, and VTE (Table 3; Figure 2). We repeated these analyses using an age cutoff of 65 years (the median in our cohort) as well as a KS of 1 vs 2 to 4. These analyses for VTE and OS using these different cutoffs did not change the results described above (data not shown). Analysis by tumor mutational status of epidermal growth factor receptor, ALK receptor tyrosine kinase, ROS proto-oncogene 1, receptor tyrosine kinase, KRAS proto-oncogene, GTPase, MET proto-oncogene, receptor tyrosine kinase, B-Raf proto-oncogene, serine/threonine kinase, neurotrophic receptor tyrosine kinase 1, ret proto-oncogene, erb-b2 receptor tyrosine kinase 2, and tumor protein p53 were not significantly associated with TFS or OS after multivariate analyses (supplemental Table 1).
Kaplan-Meier survival plot on TFS. (A) Rate of VTE for all patients. (B) Rate of VTE for all patients by KS 1 to 2 vs 3 to 4.
Kaplan-Meier survival plot on TFS. (A) Rate of VTE for all patients. (B) Rate of VTE for all patients by KS 1 to 2 vs 3 to 4.
Kaplan-Meier survival plot on OS. (A) OS over time for all patients. (B) OS over time for all patients by KS 1 to 2 vs 3 to 4.
Kaplan-Meier survival plot on OS. (A) OS over time for all patients. (B) OS over time for all patients by KS 1 to 2 vs 3 to 4.
Cox regression of risk factors on OS in lung cancer
| Parameter . | OS . | |||
|---|---|---|---|---|
| UVA . | MVA . | |||
| OS HR (95% CI) . | P value . | OS HR (95% CI) . | P value . | |
| Age ≥60 y | 1.65 (1.12-2.44) | .012 | 1.73 (1.15-2.59) | .008 |
| Male sex | 1.48 (1.09-2.02) | .013 | 1.37 (0.98-1.92) | .066 |
| Race | ||||
| White | Ref | NA | ||
| Black | 0.63 (0.33-1.20) | .160 | ||
| Other/unknown | NE | |||
| Ethnicity | ||||
| Non-Hispanic | Ref | |||
| Hispanic | 0.95 (0.69-1.29) | .725 | NA | |
| Unknown | NE | |||
| Tobacco history | ||||
| None/never used | Ref | |||
| Any current/prior use | 2.26 (1.41-3.60) | <.001 | 1.43 (0.86-2.38) | .171 |
| Baseline WBC ≥11 × 109/L | 2.33 (1.71-3.19) | <.001 | 1.72 (1.22-2.42) | .002 |
| Baseline Hb <10 g/dL | 2.73 (1.87-4.01) | <.01 | 1.84 (1.19-2.83) | .006 |
| Baseline Plt ≥350 × 109/L | 1.53 (1.11-2.11) | .009 | 0.92 (0.64-1.21) | .652 |
| BMI, kg/m2 | ||||
| <18.5 | 1.36 (0.78-2.36) | .282 | NA | |
| 18.5-24.9 | Ref | |||
| ≥25-25.9 | 0.60 (0.44-0.82) | .002 | NA | |
| Unknown | NE | |||
| KS | ||||
| 1-2 | Ref | NA | ||
| 3-4 | 2.62 (1.86-3.82) | <.001 | 1.87 (1.30-2.70) | <.001 |
| Lung cancer type | ||||
| AC | Ref | NA | ||
| SCC | 1.62 (1.10-2.38) | .014 | 0.97 (0.62-1.51) | .896 |
| NSCLC, NOS | 1.77 (0.97-3.23) | .065 | 1.49 (0.78-2.85) | .226 |
| SCLC | 1.56 (1.03-2.36) | .036 | 0.88 (0.53-1.47) | .622 |
| Unknown | NE | NE | ||
| AJCC stage | ||||
| I-II | Ref | Ref | ||
| III-IV | 13.69 (4.37-42.90) | <.001 | 4.17 (1.24-14.07) | .021 |
| Unknown | 1.73 (0.29-10.35) | .536 | 1.02 (0.16-6.46) | .985 |
| Therapy type | ||||
| Chemotherapy | 0.96 (0.44-2.12) | .929 | NA | |
| Chemoimmunotherapy | 1.46 (0.68-3.14) | .333 | ||
| Targeted therapy | 0.40 (0.14-1.09) | .074 | ||
| Radiation therapy | 1.24 (0.92-1.69) | .159 | ||
| Surgery | 0.07 (0.03-0.18) | <.001 | 0.18 (0.07-0.46) | <.001 |
| VTE, yes (total) | 2.05 (1.37-3.06) | <.001 | 1.86 (1.22-2.3) | .004 |
| Parameter . | OS . | |||
|---|---|---|---|---|
| UVA . | MVA . | |||
| OS HR (95% CI) . | P value . | OS HR (95% CI) . | P value . | |
| Age ≥60 y | 1.65 (1.12-2.44) | .012 | 1.73 (1.15-2.59) | .008 |
| Male sex | 1.48 (1.09-2.02) | .013 | 1.37 (0.98-1.92) | .066 |
| Race | ||||
| White | Ref | NA | ||
| Black | 0.63 (0.33-1.20) | .160 | ||
| Other/unknown | NE | |||
| Ethnicity | ||||
| Non-Hispanic | Ref | |||
| Hispanic | 0.95 (0.69-1.29) | .725 | NA | |
| Unknown | NE | |||
| Tobacco history | ||||
| None/never used | Ref | |||
| Any current/prior use | 2.26 (1.41-3.60) | <.001 | 1.43 (0.86-2.38) | .171 |
| Baseline WBC ≥11 × 109/L | 2.33 (1.71-3.19) | <.001 | 1.72 (1.22-2.42) | .002 |
| Baseline Hb <10 g/dL | 2.73 (1.87-4.01) | <.01 | 1.84 (1.19-2.83) | .006 |
| Baseline Plt ≥350 × 109/L | 1.53 (1.11-2.11) | .009 | 0.92 (0.64-1.21) | .652 |
| BMI, kg/m2 | ||||
| <18.5 | 1.36 (0.78-2.36) | .282 | NA | |
| 18.5-24.9 | Ref | |||
| ≥25-25.9 | 0.60 (0.44-0.82) | .002 | NA | |
| Unknown | NE | |||
| KS | ||||
| 1-2 | Ref | NA | ||
| 3-4 | 2.62 (1.86-3.82) | <.001 | 1.87 (1.30-2.70) | <.001 |
| Lung cancer type | ||||
| AC | Ref | NA | ||
| SCC | 1.62 (1.10-2.38) | .014 | 0.97 (0.62-1.51) | .896 |
| NSCLC, NOS | 1.77 (0.97-3.23) | .065 | 1.49 (0.78-2.85) | .226 |
| SCLC | 1.56 (1.03-2.36) | .036 | 0.88 (0.53-1.47) | .622 |
| Unknown | NE | NE | ||
| AJCC stage | ||||
| I-II | Ref | Ref | ||
| III-IV | 13.69 (4.37-42.90) | <.001 | 4.17 (1.24-14.07) | .021 |
| Unknown | 1.73 (0.29-10.35) | .536 | 1.02 (0.16-6.46) | .985 |
| Therapy type | ||||
| Chemotherapy | 0.96 (0.44-2.12) | .929 | NA | |
| Chemoimmunotherapy | 1.46 (0.68-3.14) | .333 | ||
| Targeted therapy | 0.40 (0.14-1.09) | .074 | ||
| Radiation therapy | 1.24 (0.92-1.69) | .159 | ||
| Surgery | 0.07 (0.03-0.18) | <.001 | 0.18 (0.07-0.46) | <.001 |
| VTE, yes (total) | 2.05 (1.37-3.06) | <.001 | 1.86 (1.22-2.3) | .004 |
Discussion
The aim of this study was to define the incidence of VTE in patients with newly diagnosed lung cancer and assess the predictive factors of thrombosis and survival in this population. Our study population was reflective of our local demographics, with 39.7% of patients being Hispanic and 8.5% Black, populations that are underrepresented in other studies.2-4
In this retrospective study of >600 patients with lung cancer, a significant risk of VTE (10.1%) was identified. Moreover, every subgroup of patients analyzed in this study retained a high risk of VTE after multivariate analysis. Interestingly, the only hematologic parameter from the KS significantly associated with VTE after multivariate analysis was WBC, although both WBC and Hb were associated with an increased risk of death. Furthermore, age ≤60 years was associated with an increased risk of VTE, whereas age >60 years was associated with increased mortality. The association between different antineoplastic therapies and thrombosis in lung cancer warrants further investigation, because there were no significant associations between systemic treatments and thrombosis in this study.
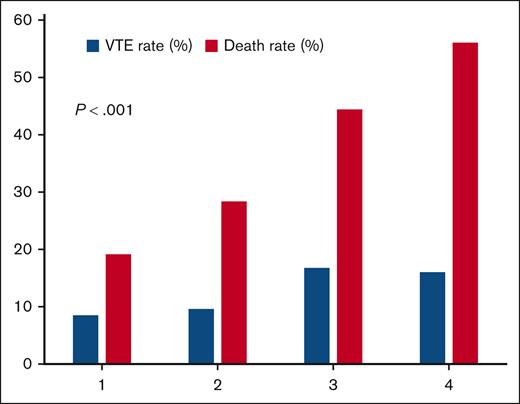
Although an association between a wide number of antineoplastic agents, including chemotherapy, and VTE has been established,3 there are a growing number of studies identifying high rates of VTE in patients receiving immunotherapy.3,15,19 In a recent study, which also examined risk factors for thrombosis in patients with advanced NSCLC, a similar nonsignificant increase in the risk of VTE was seen with patients receiving chemoimmunotherapy compared to chemotherapy alone. Furthermore, this study also identified surgery, radiation therapy, and severe obesity as associated with VTE.16 However, there are some conflicting data including a recent study of patients with NSCLC receiving immunotherapy, in which immune checkpoint inhibitor–containing therapy was associated with a significantly lower risk of VTE than chemotherapy alone, highlighting the need for more rigorous, prospective studies.20 Finally, use of targeted therapy and presence of lung cancer genetic mutations were not significantly associated with TFS or OS. For this current cohort of patients with lung cancer, age ≥60 years, BMI <25 kg/m2, stage III to IV, WBC ≥11 × 109/L, Hb <10 g/dL, and VTE predicted reduced OS in multivariable analysis. Although not significantly associated with reduced TFS after multivariate analyses, a KS of 3 to 4 was also associated with reduced OS. This strong association between KS and OS but not VTE is visualized in Figure 3. The association of KS with lower OS appears to be mediated primarily through high WBC and low Hb in lung cancer, which are presumed to be related to an inflammatory state. This has been observed previously.21 VTE and high KS appear to be independent predictors of survival in lung cancer and may be considered for incorporation into future lung cancer survival prognostic scores in addition to classic parameters such as age and stage at diagnosis.
One strength of our methods was the use of NLP, which reduced the volume of notes required to review by 55%, with the additional benefit of flagging text related to thrombosis to further increase review efficiency. Limitations of this study include its retrospective nature and the single-center data set. Further validation using independent data sets is warranted to confirm these findings. If confirmed, a prospective study reevaluating the risk of VTE and benefits of prophylactic anticoagulation, specifically in contemporary patients with lung cancer receiving systemic therapy regardless of histology, treatment, or other factors described here, may be indicated. We conclude that VTE is prevalent in all subtypes of lung cancer and appears to be an independent risk factor for death. This association should be known, and approaches by clinicians to mitigate its impact are warranted.
Acknowledgments
The authors acknowledge the effort of the Information Technology Department at the University of Miami in extracting data from electronic medical records. The authors also acknowledge the data collection performed by tumor registry personnel.
No funding was used for this study.
Authorship
Contribution: A.T. and T.E.P. collected data, contributed to study design, and wrote the initial manuscript; R.S.K., L.E.C., D.M.-K., and A.J.M. reviewed and edited the manuscript; G.A.S. reviewed, edited, and provided oversight of the project; M.C. developed natural language processing model to extract data from electronic medical record into REDCap; and W.Z. performed statistical analysis of the data in SAS and contributed to the final manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Gerald A. Soff, Division of Hematology, Department of Medicine, University of Miami, 1120 NW 14th St, Suite 610, Soffer Clinical Research Building, Miami, FL 33136; email: gas199@miami.edu.
References
Author notes
T.E.P. and A.T. contributed equally as first authors to this study.
Original data are available on request from the corresponding author, Gerald A. Soff (gas199@miami.edu).
The full-text version of this article contains a data supplement.