TO THE EDITOR:
Use of IV immunoglobulin (IVIG), derived from pooled plasma, has continued to increase. Side effects can include anaphylaxis, hemolytic reactions, and thromboses, both venous and arterial.1-4
IVIG contains anti-A and anti-B antibodies. ABO antigens are expressed in multiple tissues, and antigen-antibody interactions are proven to cause damage in vitro through multiple mechanisms, including platelet dysfunction, endothelial injury, and varying degrees of hemolysis, which is correlated with thrombotic risk.5-7
There are no studies examining the incidence of thrombotic complications, other morbidity, and mortality after IVIG treatment based on ABO type. Our goal was to determine whether thrombotic and other adverse events were greater in recipients of non-O type after at least 1 dose of IVIG. Secondary goals were to study mortality and number of hospitalizations for O-blood group patients compared to non–O-blood group patients after at least 1 dose of IVIG.
We performed a 10-year retrospective single-center review of patients receiving at least 1 dose of IVIG. Patients were divided using International Classification of Diseases, 9th Revision (ICD-9) or ICD-10 codes for primary immune deficiencies or autoimmune diseases. Inclusion criteria were at least 1 dose of administered IVIG, available ABO data, data about the IVIG administration, and at least 1 recorded follow-up event. Approval was obtained from our institutional review board.
Details on the total doses of IVIG administered, as well as dates of first and last doses, were collected, as well as number of hospitalizations. Mortality events were recorded, and, if not found, time of last follow-up was measured by their last office visit.
We employed χ2 to determine if rates of thrombotic events differed by blood groups. We fit Cox proportional hazard models to the survival of patients from the date of IVIG administration with blood group (O vs non-O) as a predictor. This analysis was repeated on a subset of patients with blood group O vs A, given the known greater biologic and clinical potency of anti-A antibodies. We graphically evaluated the assumption of proportional odds over time. We fit Poisson regression models to determine if the rates of hospital admissions differed between blood groups, using time since IVIG as an exposure variable, restricted to patients with at least 1 year of follow-up.
We identified 1077 patients receiving IVIG between June 2013 and June 2023 with available ABO data; 488 patients (45%) had diagnoses classified as autoimmune disorders and 589 (54%) had diagnoses classified as immunodeficiencies. A consort chart of exclusions and numbers for analysis are provided in the supplemental Material.
The diagnoses of hypogammaglobulinemia and osteoarthritis were excluded as most had a secondary cause, mainly treatment-related immunodeficiency associated with chemotherapy, stem cell transplants, or other comorbidities. After these exclusions, there were 763 patients. Within this group, 54 (7.1%) patients had documented thromboembolic-events, 47 of which had recorded completed IVIG doses.
For 763 eligible patients, the rate of thromboembolic events was 6.2% in the O blood group, 7.9% in the A blood group, 7.7% in the B blood group, and 6.5% in the AB blood group. Rates of thromboembolic events were not significantly different between the blood groups (χ2 = 0.82; P = .845).
Blood group was not found to be a significant factor for predicting number of hospital admissions per year after receiving IVIG when compared between O group and non-O groups (P > .05; relative rate = 0.978 [0.885-1.080]). However, blood group A patients had a higher rate of hospital admissions per year than blood group O patients (P = .039, relative rate = 1.118 [1.005- 1.242]).
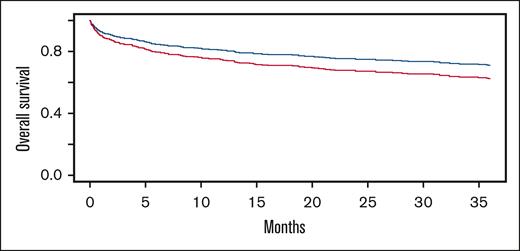
For n = 672 patients with at least 1 day of follow-up after IVIG, there were 101 (33%), 107 (41%), 28 (35%), and 8 (31%) deaths observed for O, A, B, and AB blood group patients, respectively. Patients with a non-O blood group had a higher hazard ratio (HR) for death over time than O blood group patients (Figure 1) (HR, 1.294; 95% confidence interval, 1.003-1.669; P = .0478). Blood group A recipients had a higher HR for death than blood group O (Figure 2) (HR, 1.376; 95% confidence interval, 1.048-1.807; P = .0215).
Cox proportional hazard modeled survival curves over 36 months from IVIG administration for O (blue) vs non-O (red) blood groups. Non-O blood recipients had a significantly greater hazard of death (HR, 1.294, 95% confidence interval [CI], 1.003-1.669; P = .0478).
Cox proportional hazard modeled survival curves over 36 months from IVIG administration for O (blue) vs non-O (red) blood groups. Non-O blood recipients had a significantly greater hazard of death (HR, 1.294, 95% confidence interval [CI], 1.003-1.669; P = .0478).
Cox proportional hazard modeled survival curves over 36 months from IVIG administration for O (blue) vs A (red) blood groups. Recipients of blood group A had a significantly higher hazard of death (HR, 1.376; 95% CI, 1.048-1.807; P = .0215).
Cox proportional hazard modeled survival curves over 36 months from IVIG administration for O (blue) vs A (red) blood groups. Recipients of blood group A had a significantly higher hazard of death (HR, 1.376; 95% CI, 1.048-1.807; P = .0215).
There was no statistically significant difference in the incidence of thromboembolic events between patients with O and non-O blood groups after IVIG. There was a statistically significant difference in mortality between patients with O and non-O blood group after IVIG administration, with non-O blood groups having a higher mortality. This increased mortality was quantitatively and statistically greater for group A recipients. There was also a significant difference in hospitalizations for O blood group patients compared to non-O group patients, with O blood group being a significant predictor for fewer hospital admissions than A blood group.
Previous studies examining ABO-specific mortality have in 1 case found an association between non-O blood groups and higher overall mortality, but this was not true for patients who received transfusion in other studies.8-11 Decreased mortality was seen in patients as a longer-term event compared with our 3 year follow-up analysis. Cardiovascular mortality has been speculated as an explanation for the difference in mortality, with the lower concentrations of von Willebrand factor and factor VIII in persons with the O blood group acting as a protective factor. It is possible that patients with blood group O had improved survival in our cohort due to combined reduced vascular mortality overall and lack of toxic effects of infusion of anti-A antibodies.
ABO antigens are expressed in multiple tissues. IVIG, which contains anti-A and anti-B antibodies and ABO immune complexes, might increase the risk for antibody mediated organ injury and clinical complications. If this association with increased near-term hospitalizations and mortality in non-O recipients of IVIG is a causal one, we speculate that low level, clinically silent hemolysis with ensuing inflammation and organ injury is a potential mechanism.12-18 There is a potential analogy to diseases with ongoing levels of common, clinically inapparent hemolysis, such as sickle cell anemia and paroxysmal nocturnal hemoglobinuria.
Our study has a number of limitations. It is a retrospective study using data from a single center and depends on ICD codes and electronic medical record data, which entails the possibility of missed cases due to incomplete or erroneous data input. The electronic medical record data also does not allow for precise follow-up times.
These results suggest that non-O, particularly group A, patients may experience harm, including increased mortality from infusion of IVIG. We cannot rule out a primary effect of ABO blood group on hospitalizations and mortality unrelated to IVIG administration, but these have not been reported in the literature yet. Mortality might be particularly increased due to anti-A antibodies present in IVIG, which is consonant with the known more potent biologic and clinical toxicity of anti-A antibodies. Our data suggest that the mechanism for this increased mortality is not related to thrombosis but might be secondary to the well-established pathophysiologic effects of anti-A antibodies in causing varying degrees of hemolysis. Clinically inapparent hemolysis is linked to increased organ injury in many studies.19 If a true association exists, efforts to reduce ABO isoagglutinins in IVIG may reduce the risk of side effects of this therapy. Future studies with detailed clinical information and quantitation of hemolysis will be necessary to evaluate our hypothesis that clinically inapparent hemolysis harms blood group A patients who receive IVIG.
Contribution: L.S.D. performed research, analyzed data, and wrote the manuscript; T.L. and J.M. performed data analysis; N.B., M.A.R., and A.A. generated the initial research hypothesis and designed research; L.S.D., R.J.L., S.A.N., and J.R.A. provided clinical expertise in their area of practice; K.H. performed research; and all authors edited and approved the final manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Neil Blumberg, Department of Pathology and Laboratory Medicine, University of Rochester Medical Center, Box 608, 601 Elmwood Ave, Rochester, NY 14642; email: neil_blumberg@urmc.rochester.edu.
References
Author notes
Data are available on request from the corresponding author, Neil Blumberg (neil_blumberg@urmc.rochester.edu).
The full-text version of this article contains a data supplement.

![Cox proportional hazard modeled survival curves over 36 months from IVIG administration for O (blue) vs non-O (red) blood groups. Non-O blood recipients had a significantly greater hazard of death (HR, 1.294, 95% confidence interval [CI], 1.003-1.669; P = .0478).](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodvth/2/3/10.1016_j.bvth.2025.100075/2/m_bvth_vth-2024-000303-gr1.jpeg?Expires=1765896945&Signature=1xlGocAzenI23uMQsjl9ThgMLKJDhTD1IZV8M7Eu5fA9m-G~VPMHVh4hpJvvVSK3KlmC1X~dBgOCsTTJ7HbMoQ3nScGd8uSbYQ~hB3uLll0Mr5StJ3xLGVhqL76V7xM3Jejtzk4Az-edf701~B1WKo2GcQRzlcBHAf5tu8isgSXwsNjkcxJzMjwYbuxY3Fsc-FRg15AVZH4ru5t905ZJFTF77IXsH98~0wMVHsWp-SniPXh9K7E0DjwwQTYLBHTqETzEFZbqpAO3yPGm3WuvImmHwNS5IFEKEeFKanN81q79pRUwHb0YmhGObPYWy7i6a2-KYoBQ27bSb7hjhFg5yQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)