Key Points
Elevations in levels of inflammatory cytokines are present in patients with TACO.
A subset of such patients exhibiting nonclassical signs of volume overload may exhibit greater elevations of proinflammatory cytokines.
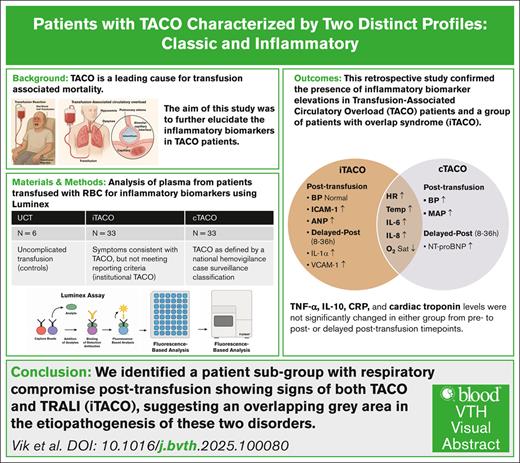
Visual Abstract
Inflammatory Response in TACO
Transfusion-associated circulatory overload (TACO) is a leading cause of transfusion-associated mortality. TACO is thought to result from hydrostatic forces in the vascular space, leading to transudative pulmonary edema. Recent studies suggest that TACO is not solely a volume overload phenomenon, but may involve inflammatory processes. This study aimed to further explore the presence of inflammation in patients with TACO. We conducted a retrospective study with 3 cohorts receiving red blood cell transfusion: (1) patients having TACO as defined by a national hemovigilance case surveillance classification (conventional TACO [cTACO], n = 33); (2) patients having symptoms consistent with TACO but not completely meeting reporting criteria (institutional TACO [iTACO], n = 33); and (3) a patient cohort who experienced uncomplicated transfusions (n = 6). Samples from before transfusion, after transfusion, and 8 to 36 hours after transfusion were examined. Samples were analyzed for levels of tumor necrosis factor α, interleukin-1α (IL-1α), IL-6, IL-8, IL-10, C-reactive protein, intercellular adhesion molecule 1 (ICAM-1), vascular cell adhesion molecule 1, atrial natriuretic peptide, cardiac troponin, and N-terminal pro–B-type natriuretic peptide. Patients with cTACO and iTACO had an elevated body temperature, higher heart rate, and lower oxygen saturation after transfusion, whereas only patients with cTACO had higher blood pressures. Levels of key proinflammatory cytokines, IL-6, and IL-8 were elevated in patients with cTACO and iTACO after transfusion, whereas ICAM-1 was elevated only in patients with iTACO after transfusion. Our results suggest that inflammatory pathways may be invoked in patients with TACO. Patients with iTACO showed a more distinctive inflammatory profile, suggesting a gray area between transfusion-related acute lung injury and TACO.
Introduction
Transfusion-associated circulatory overload (TACO) is the leading cause of transfusion-related morbidity and mortality reported to hemovigilance networks worldwide. Its incidence is reported to range from 0.05% to 8% of all transfused patients, and its mortality can approach 6.5%.1-3 TACO and transfusion-related acute lung injury (TRALI) are the most important pulmonary transfusion complications. Distinctive for TRALI is the exudative edema, due to inflammation and neutrophil migration.4 In contrast, TACO is thought to be the result of hydrostatic forces in the vascular system, leading to transudative pulmonary edema.
Currently, mitigation measures for TACO include restrictive transfusion practices, single-unit transfusions, the use of reduced-volume units, and slow infusion rates.5,6 These strategies all focus on the aspect of fluid overload as the major mechanism in TACO’s pathogenesis. In this regard, the hypothesized mechanism is one in which either or both excessive fluid hydration or rapid infusion of IV fluids results in a pressure increase in the pulmonary capillaries, leading to pulmonary edema and respiratory distress. However, it appears unlikely that TACO is solely a volume overload condition, because >20% of TACO cases occur after transfusion of only 1 unit (approximate volume, 300 mL).7 A similar outcome is not expected in patients receiving an equivalent nonsanguineous fluid challenge.
Previous studies suggest that inflammatory pathways may also have a contributory role in TACO’s pathophysiology. Such mechanisms could be similar in nature to that observed in patients with TRALI, whereby inflammatory mediators are involved in promoting vascular changes, altering capillary permeability, and leading to capillary leakage and subsequent pulmonary edema.8 Several studies exhibit evidence for this theory, reporting fevers and cytokine increases in patients with TACO.9-11 Moreover, TACO incidences were observed to decrease by 52% after implementation of a strategy of leukoreduction of blood products for transfusion.12 This inflammatory process has not yet been thoroughly studied in cohorts of patients with TACO. A better insight into this pathophysiological mechanism leading to the development of TACO is critical for designing effective preventive and therapeutic strategies.
The aim of this study was to further explore the potential contribution of inflammation to TACO’s pathophysiology. To that end, we examined levels of inflammatory cytokines and biomarkers of endothelial activation and injury and searched for differences in these profiles between patients with TACO identified using different diagnostic criteria, as well as in patients experiencing uncomplicated transfusions (UCTs).
Methods
Study design
This was a single-center retrospective study involving patients with a TACO diagnosis judged according to the Centers for Disease Control and Prevention (CDC) National Healthcare Safety Network (NHSN) Hemovigilance (HV) Module criteria (conventional TACO [cTACO]); patients having symptoms and/or signs consistent with TACO, meeting local but not necessarily all CDC criteria (institutional TACO [iTACO]); and a cohort control of patients experiencing UCTs. Patients were included from 1 October 2018 to 31 December 2021. This study was reviewed and approved by the Baystate Medical Center (BMC) Institutional Review Board (BMC-22-010) and adhered to all national and local guidelines.
Study settings
The BMC Blood Bank/Transfusion Medicine Service (Springfield, MA) served as the clinical setting for this study. It issues ∼17 000 blood products and investigates ∼200 suspected transfusion reactions (STRs) annually. The Blood Bank/Transfusion Medicine Service maintains several databases in support of its biovigilance and quality improvement operations, as well as for regulatory and accreditation agencies’ reporting purposes. As part of its routine clinical operations, the transfusion service also maintains a curated residual specimen archive from patients evaluated for STRs reported to it. Samples were selected and retrieved from this archive and, after anonymization, were transported to the University of Massachusetts Amherst through an approved specimen transport protocol and stored at –80°C before analysis. Samples were processed and analyzed in the laboratory of one of our authors (W.W.) as well as in the laboratory of Ann Moorman at the University of Massachusetts Chan Medical School (Worcester, MA).
Participants
Three cohorts were selected: patients with cTACO; patients with iTACO; and UCT controls. All patients had to be adults aged ≥18 years and ≤90 years, admitted to BMC, and transfused with red blood cells during the study interval. Cases were excluded for not having enough sample volume available for the pertinent studies. All reported cTACO or iTACO cases adjudicated to have an “imputability status” of low or indeterminate likelihood that the transfusion was responsible for the patient’s signs/symptoms were also excluded from the study. Cases and their associated specimens were chronologically identified, acquired, and entered into their respective study groups. Additionally, to decrease any temporal/seasonal bias in the data sets, cases were selected as such to ensure a representative sampling throughout the study interval.
All reported STR cases were evaluated for either iTACO or cTACO by the transfusion service medical staff if they fulfilled any of the following criteria: (1) the patient demonstrated an increase in pulse pressure to ≥65 mm Hg or a peritransfusion pulse pressure delta difference ≥8 mm Hg; (2) the patient had a history of a reported STR previously diagnosed as TACO; (3) the patient exhibited signs/symptoms of an adverse pulmonary transfusion complication (eg, dyspnea, tachypnea, decreased oxygen saturation, and rales or wheezing, etc); or (4) at the discretion of the transfusion service medical staff after initial preliminary review of the patient’s electronic medical record chart that indicated the possibility of a fluid overload state of the patient in the peritransfusion interval.
Patients identified as having cTACO were designated as those meeting the TACO case surveillance definition according to CDC’s NHSN HV Module criteria; that is, having acute or worsening respiratory distress and/or acute or worsening pulmonary edema during or up to 12 hours after transfusion, with additional features including cardiovascular system changes not explained by the patient’s underlying medical condition, evidence of fluid overload, or a relevant biomarker.13
Patients with iTACO were defined as those having had a transfusion-associated adverse event characterized as having some but not necessarily all taxonomic features of cTACO, according to evaluations and adjudications performed by the transfusion service medical staff, as previously described.11,14 Such cases were reported to the NHSN HV by the transfusion service’s Transfusion Safety Office using the “best fit” CDC Hemovigilance Case Surveillance definition as determined by the CDC’s reaction classification algorithm.
UCT control patient samples were obtained from patients who experienced an UCT event, defined as having no adverse transfusion problems reported during and up to 24 hours after transfusion completion (posttransfusion [post-tx]), after chart review by transfusion service physicians confirming no occurrence of unrecognized and/or unreported suspected adverse transfusion events.
Clinical data
Clinical and laboratory data were collected from patients’ electronic hospital medical records and their various transfusion service paper/electronic documents. Data collection was restricted to 1 transfusion episode event per patient (ie, issuance of 1 unit of red blood cells to an individual patient) so as not to bias the study groups by including patients with a history of multiple reactions. Clinical data that were collected included baseline patient characteristics, comorbidities, transfusion characteristics, and supportive therapy/management (eg, use of peritransfusion furosemide or supplemental oxygen/ventilatory support requirements). Peritransfusion vital sign value (VSV) data were also extracted, including body temperature, heart rate, mean arterial pressure, pulse pressure, respiratory rate, and peripheral oxygen saturation levels. VSVs were measured in all patients, before transfusion (pretransfusion [pre-tx]), ∼15 minutes after starting hemotherapy, and at its completion. After patient anonymization, collected data were placed into a secure REDCap database. Each patient was assigned a unique code corresponding to the blood samples of that specific patient. All analyses of the samples were performed blinded to all investigators with respect to patient identity and date/time of specimen collection. After all sample testing was completed, sharing of the specimen identity codes with their respective deidentified unique case subject numbers was performed so that pertinent clinical information regarding each patient could be correlated with the individual’s serological testing results. See supplemental Table 1 for the curated clinical case vignettes regarding the patients in this study.
Blood sample collection and analysis
Samples from patients with reported STRs were collected at 3 time points: pre-tx; within 6 hours after termination of the transfusion (post-tx); and 8 to 36 hours after termination of the transfusion (ie, a delayed post-tx interval time point), per BMC hospital policy. For patients with UCTs, samples were collected for the pre-tx and post-tx time points as part of routine clinical testing. All samples were residual specimens collected as part of routine clinical care, obtained as close as possible to the indicated time points. After necessary clinical testing was completed, samples were centrifuged at 2415g (ELITE-H12 centrifuge; Global Focus Marketing & Distribution, Ltd, Dallas, TX) for 8 minutes, and supernatants were frozen at –80°C. Aliquots from selected residual specimens were obtained and shipped to the Amherst laboratory at the University of Massachusetts for further analyses.
Samples were prepared using the recommended dilution following the manufacturer’s protocol and analyzed on Luminex MAGPIX instruments (Luminex, Austin, TX) at the University of Massachusetts Amherst and the University of Massachusetts Chan Medical School. Instruments were calibrated and verified before each run using a MAGPIX Performance Verification Kit (Invitrogen MPXPVERK25) and MAGPIX Calibration Kit (Invitrogen MPXCALK25). Samples were analyzed in duplicate for the following biomarkers: tumor necrosis factor α, interleukin-1α (IL-1α), IL-6, IL-8, IL-10, intercellular adhesion molecule 1 (ICAM-1), vascular cell adhesion molecule 1 (VCAM-1), atrial natriuretic peptide (ANP), cardiac troponin, C-reactive protein, and N-terminal pro–B-type natriuretic peptide (NT-proBNP). Except for NT-proBNP, all biomarkers were assayed using custom Human Magnetic Luminex multiplex kits (LXSAHM; Bio-Techne, Minneapolis, MN), following the manufacturer’s protocol. NT-proBNP analysis was performed using ProcartaPlex Human NT-proBNP Simplex kits following the manufacturer’s protocol (Thermo Fisher, Vienna, Austria).
Statistical analysis
Data were analyzed using R (Rstudio, 4.0.3). Patients’ descriptive characteristics, VSVs, and biomarker data are presented as medians and interquartile ranges, due to the nonnormal distribution of the data. Distribution was assessed using visual inspection of histograms and a Shapiro-Wilk test. A Wilcoxon rank-sum test was used to explore significant differences among all 3 groups. When there was a significant difference, a Mann-Whitney U test was used to explore differences between 2 groups. A Friedman test was used to test for significance within each group over all 3 time points. A Spearman rho test was used to test for significant associations between NT-proBNP and other biomarkers. A 2-sided P value <.05 was considered statistically significant. Biomarker data were also visualized in a heat map using z scores to depict the distribution above and below the mean value.
Results
Patient characteristics
In total, 72 patients were included in this study. There were 33 patients in the cTACO group, 33 patients in the iTACO group, and 6 patients experiencing a UCT. Analyses of aggregated group data revealed no statistically significant differences in patients’ age, sex, or body mass index, or the number of inpatient/outpatient transfusions between groups. Median transfused volume amounts were similar across all groups (Table 1). There was a significant difference in infusion rate, which was slower in patients with cTACO than patients with iTACO and UCTs (P = .04). Furthermore, patients with cTACO were significantly more likely to have received furosemide before or during the transfusion than patients with iTACO and UCTs (P < .01).
Patient characteristics
| Parameter . | UCT (N = 6) . | iTACO (N = 33) . | cTACO (N = 33) . | P value . |
|---|---|---|---|---|
| Age, y | 70 (62-75) | 62 (55-69) | 69 (59-75) | .13 |
| Females, % (n/N) | 33.3 (2/6) | 39.4 (13/33) | 48.5 (16/33) | .67 |
| Body mass index | 25 (23-27) | 28 (24-33) | 27 (26-30) | .50 |
| Hypertension, % (n/N) | 16.7 (1/6) | 30.3 (10/33) | 24.2 (8/33) | .73 |
| Diabetes, % (n/N) | 50 (3/6) | 18.2 (6/33) | 18.2 (6/33) | .18 |
| Renal disease, % (n/N) | 0 (0/6) | 18.2 (6/33) | 30.3 (10/33) | .19 |
| Cardiovascular disease, % (n/N) | 16.7 (1/6) | 39.4 (13/33) | 42.4 (14/33) | .49 |
| Malignancy, % (n/N) | 0 (0/6) | 27.3 (9/33) | 12.1 (4/33) | .14 |
| Inpatient, % (n/N) | 83.3 (5/6) | 87.9 (29/33) | 75.8 (25/33) | .44 |
| Volume transfused, mL | 294 (198-345) | 283 (175-326) | 225 (140-308) | .62 |
| Infusion rate, mL/h | 120 (120-120) | 104 (94-138) | 94 (72-110) | .04 |
| Peritransfusion furosemide, % (n/N) | 0 (0/6) | 21.2 (7/33) | 63.6 (21/33) | <.01 |
| Oxygen requirement, % (n/N) | 0 (0/6) | 3.0 (1/33) | 9.1 (3/33) | .44 |
| Parameter . | UCT (N = 6) . | iTACO (N = 33) . | cTACO (N = 33) . | P value . |
|---|---|---|---|---|
| Age, y | 70 (62-75) | 62 (55-69) | 69 (59-75) | .13 |
| Females, % (n/N) | 33.3 (2/6) | 39.4 (13/33) | 48.5 (16/33) | .67 |
| Body mass index | 25 (23-27) | 28 (24-33) | 27 (26-30) | .50 |
| Hypertension, % (n/N) | 16.7 (1/6) | 30.3 (10/33) | 24.2 (8/33) | .73 |
| Diabetes, % (n/N) | 50 (3/6) | 18.2 (6/33) | 18.2 (6/33) | .18 |
| Renal disease, % (n/N) | 0 (0/6) | 18.2 (6/33) | 30.3 (10/33) | .19 |
| Cardiovascular disease, % (n/N) | 16.7 (1/6) | 39.4 (13/33) | 42.4 (14/33) | .49 |
| Malignancy, % (n/N) | 0 (0/6) | 27.3 (9/33) | 12.1 (4/33) | .14 |
| Inpatient, % (n/N) | 83.3 (5/6) | 87.9 (29/33) | 75.8 (25/33) | .44 |
| Volume transfused, mL | 294 (198-345) | 283 (175-326) | 225 (140-308) | .62 |
| Infusion rate, mL/h | 120 (120-120) | 104 (94-138) | 94 (72-110) | .04 |
| Peritransfusion furosemide, % (n/N) | 0 (0/6) | 21.2 (7/33) | 63.6 (21/33) | <.01 |
| Oxygen requirement, % (n/N) | 0 (0/6) | 3.0 (1/33) | 9.1 (3/33) | .44 |
Median and interquartile range are shown unless specified otherwise. P values in boldface type represent statistical significance (P ≥ .05).
Peritransfusion changes in vital signs
VSVs were similar in all groups before transfusion and did not change significantly after transfusion in the UCT group. In contrast, cTACO and iTACO groups both exhibited statistically significant increases in median body temperature and heart rate at the conclusion of their transfusions. Mean arterial pressure values also increased but only in the cTACO group. Furthermore, oxygen saturation levels decreased in the cTACO and iTACO groups after transfusion. There were significant differences in VSVs between groups after transfusion. Mean arterial pressure and body temperature were higher in cTACO and iTACO groups than the control cohort (P = .03 and P = .02, respectively). Oxygen saturation was lower in both the cTACO and iTACO groups than the UCT group (P = .04). Median peritransfusion VSVs for the different cohorts are displayed in Table 2.
Peritransfusion VSVs
| VSV . | UCT (N = 6) . | iTACO (N = 33) . | cTACO (N = 33) . | |||
|---|---|---|---|---|---|---|
| Pre-tx . | Post-tx . | Pre-tx . | Post-tx . | Pre-tx . | Post-tx . | |
| Temperature, °C | 36.6 (36.4-36.7) | 36.7 (36.7-36.9) | 36.9 (36.7-37.3) | 37.6 (36.8-38.5)∗∗∗ | 36.9 (36.6-37.1) | 37.1 (36.8-37.8)∗∗∗ |
| HR, bpm | 72 (71-83) | 73 (70-87) | 88 (82-95) | 92 (84-110)∗∗ | 85 (75-100) | 99 (79-115)∗∗ |
| MAP, mm Hg | 81 (68-95) | 76.5 (72-89) | 82 (74-89) | 86 (74-93) | 82 (75-91) | 93 (83-101)∗∗∗ |
| PP, mm Hg | 53 (43-56) | 58 (52-66) | 66 (49-72) | 57 (49-80) | 66 (51-79) | 70 (51-89) |
| RR, rpm | 19 (17-20) | 19 (17-20) | 19 (18-22) | 20 (18-23) | 18 (18-21) | 20 (18-25) |
| O2Sat, % | 98 (97-99) | 100 (97-100) | 98 (96-100) | 96 (95-99)∗ | 97 (94-99) | 96 (92-98)∗∗ |
| VSV . | UCT (N = 6) . | iTACO (N = 33) . | cTACO (N = 33) . | |||
|---|---|---|---|---|---|---|
| Pre-tx . | Post-tx . | Pre-tx . | Post-tx . | Pre-tx . | Post-tx . | |
| Temperature, °C | 36.6 (36.4-36.7) | 36.7 (36.7-36.9) | 36.9 (36.7-37.3) | 37.6 (36.8-38.5)∗∗∗ | 36.9 (36.6-37.1) | 37.1 (36.8-37.8)∗∗∗ |
| HR, bpm | 72 (71-83) | 73 (70-87) | 88 (82-95) | 92 (84-110)∗∗ | 85 (75-100) | 99 (79-115)∗∗ |
| MAP, mm Hg | 81 (68-95) | 76.5 (72-89) | 82 (74-89) | 86 (74-93) | 82 (75-91) | 93 (83-101)∗∗∗ |
| PP, mm Hg | 53 (43-56) | 58 (52-66) | 66 (49-72) | 57 (49-80) | 66 (51-79) | 70 (51-89) |
| RR, rpm | 19 (17-20) | 19 (17-20) | 19 (18-22) | 20 (18-23) | 18 (18-21) | 20 (18-25) |
| O2Sat, % | 98 (97-99) | 100 (97-100) | 98 (96-100) | 96 (95-99)∗ | 97 (94-99) | 96 (92-98)∗∗ |
Median and interquartile range are shown. Significance is measured within each group over time: Boldface type indicates statistical significance (∗P < .05; ∗∗P < .01; ∗∗∗P < .001).
bpm, beats per minute; HR, heart rate; MAP, mean arterial pressure; O2Sat, oxygen saturation percentage; PP, pulse pressure; rpm, respirations per minute; RR, respiratory rate.
Cytokines
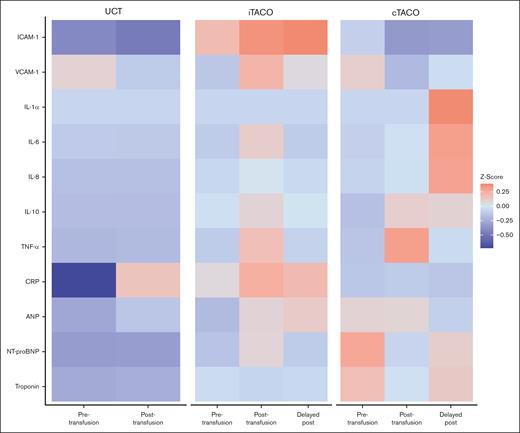
Pre-tx IL-1α levels were elevated in patients with cTACO compared to both patients with iTACO and UCT controls (P = .02 and P = .03, respectively). IL-8 levels were significantly higher in both patients with cTACO and iTACO than in UCT controls (P = .02 and P < .01, respectively) before transfusion. Directly after transfusion, IL-6 levels were significantly higher in patients with cTACO and iTACO than in UCT controls (P < .01 and P = .02, respectively). This was also true for IL-8 levels (P = .04 and P = .01, respectively). ICAM-1 levels were elevated in patients with iTACO compared to patients with cTACO both directly after transfusion and at the delayed post-tx time point (P = .02 and P = .01, respectively). Median IL-6 levels changed significantly during transfusion in both the cTACO and iTACO groups, with levels increasing immediately after transfusion and then decreasing at the delayed post-tx time point (P < .01). A similar pattern was observed for IL-8 and VCAM-1 in patients with iTACO (P = .04 and P < .01, respectively). IL-1α increased at the delayed post-tx time point compared to pre-tx levels in patients with iTACO (P < .01). Median cytokine levels for the various groups are displayed in Table 3, and mean cytokine levels for the various groups are depicted in the heat map in Figure 1.
Levels of biomarkers at selected peritransfusion time intervals
| Marker [reference value] . | UCT (N = 6) . | iTACO (N = 33) . | cTACO (N = 33) . | |||||
|---|---|---|---|---|---|---|---|---|
| Pre-tx . | Post-tx . | Pre-tx . | Post-tx . | Delayed post-tx . | Pre-tx . | Post-tx . | Delayed post-tx . | |
| ICAM-1 [100-200 ng/mL], ng/mL | 320 (221-556) | 386 (263-436) | 486 (313-786) | 516 (358-821)∗ | 536 (311-897)∗ | 461 (336-566) | 423 (281-516) | 362 (280-521) |
| VCAM-1 [449-1103 ng/mL], ng/mL | 1692 (1494-4402) | 2087 (1353-3061) | 2326 (1153-3552) | 3006 (1459-4070) | 2485 (1465-3653) | 2514 (1398-3653) | 2249 (1290-2781) | 2202 (1283-3088) |
| IL-1α [≤3.9 pg/mL], pg/mL | 4.8 (4.8-6.1) | 7.3 (4.7-12.8) | 5.6 (4.8-10.9) | 5.6 (4.8-13.8) | 9.3 (4.8-16.4) | 10.9 (5.0-20.4)† | 8.9 (4.8-15.0) | 8.9 (4.8-14.7) |
| IL-6 [≤2.0 pg/ml], pg/mL | 8.1 (4.0-20.2) | 10.3 (4.0-17.2) | 21.9 (4.0-40.2) | 34.9 (16.6-102.7)‡ | 13.8 (4.0-36.5) | 32.4 (7.9-53.5) | 41.6 (20.4-87.9)‡ | 25.6 (11.3-44.0) |
| IL-8 [≤3.0 pg/mL], pg/mL | 4.7 (3.8-6.9) | 5.0 (3.4-8.1) | 15.8 (7.2-38.6)‡ | 16.8 (6.9-44.4)‡ | 11.2 (4.9-36.2) | 12.0 (7.2-29.6)‡ | 18.1 (6.7-48.0)‡ | 10.6 (5.8-19.4) |
| IL-10 [≤2.8 pg/mL], pg/mL | 4.3 (4.3-7.5) | 4.3 (2.8-4.8) | 4.3 (4.3-7.5) | 4.3 (4.3-9.0) | 4.3 (4.3-4.7) | 4.3 (3.7-7.1) | 4.3 (4.3-14.2) | 4.3 (4.3-10.8) |
| TNF-α [≤7.2 pg/mL], pg/mL | 10.8 (7.9-14.0) | 7.0 (5.5-8.7) | 9.9 (7.2-14.1) | 14.6 (7.7-23.1) | 12.1 (6.5-19.0) | 8.4 (5.5-14.8) | 9.4 (6.2-12.8) | 9.4 (6.4-15.1) |
| CRP [≤5.0 mg/L], mg/L | 5.6 (2.5-7.1) | 6.8 (5.8-7.6) | 7.2 (6.5-8.2) | 7.3 (6.0-9.5) | 7.3 (5.9-11.3) | 7.4 (5.9-7.8) | 8.0 (6.1-9.1) | 7.6 (6.5-7.8) |
| ANP [0.004-0.006 ng/mL], ng/mL | 14.7 (9.6-24.7) | 22.5 (10.0-32.1) | 14.1 (8.7-26.3) | 18.5 (12.7-26.8) | 19.3 (11.9-26.1) | 19.1 (10.7-35.8) | 21.3 (11.3-40.0) | 16.8 (12.3-28.3) |
| NT-proBNP [≤540 pg/mL], pg/mL | 82 (22-779) | 213 (41-635) | 315 (109-977) | 258 (121-779) | 212 (115-548) | 593 (219-1021) | 456 (171-747) | 512 (217-886)§ |
| Troponin [<100 ng/L], ng/L | 52.3 (17.6-81.7) | 43.2 (21.3-69.0) | 50.9 (25.6-100.2) | 56.1 (31.4-109.5) | 56.1 (31.4-109.5) | 66.6 (46.9-99.3) | 58.9 (33.9-84.1) | 50.9 (32.2-106.0) |
| Marker [reference value] . | UCT (N = 6) . | iTACO (N = 33) . | cTACO (N = 33) . | |||||
|---|---|---|---|---|---|---|---|---|
| Pre-tx . | Post-tx . | Pre-tx . | Post-tx . | Delayed post-tx . | Pre-tx . | Post-tx . | Delayed post-tx . | |
| ICAM-1 [100-200 ng/mL], ng/mL | 320 (221-556) | 386 (263-436) | 486 (313-786) | 516 (358-821)∗ | 536 (311-897)∗ | 461 (336-566) | 423 (281-516) | 362 (280-521) |
| VCAM-1 [449-1103 ng/mL], ng/mL | 1692 (1494-4402) | 2087 (1353-3061) | 2326 (1153-3552) | 3006 (1459-4070) | 2485 (1465-3653) | 2514 (1398-3653) | 2249 (1290-2781) | 2202 (1283-3088) |
| IL-1α [≤3.9 pg/mL], pg/mL | 4.8 (4.8-6.1) | 7.3 (4.7-12.8) | 5.6 (4.8-10.9) | 5.6 (4.8-13.8) | 9.3 (4.8-16.4) | 10.9 (5.0-20.4)† | 8.9 (4.8-15.0) | 8.9 (4.8-14.7) |
| IL-6 [≤2.0 pg/ml], pg/mL | 8.1 (4.0-20.2) | 10.3 (4.0-17.2) | 21.9 (4.0-40.2) | 34.9 (16.6-102.7)‡ | 13.8 (4.0-36.5) | 32.4 (7.9-53.5) | 41.6 (20.4-87.9)‡ | 25.6 (11.3-44.0) |
| IL-8 [≤3.0 pg/mL], pg/mL | 4.7 (3.8-6.9) | 5.0 (3.4-8.1) | 15.8 (7.2-38.6)‡ | 16.8 (6.9-44.4)‡ | 11.2 (4.9-36.2) | 12.0 (7.2-29.6)‡ | 18.1 (6.7-48.0)‡ | 10.6 (5.8-19.4) |
| IL-10 [≤2.8 pg/mL], pg/mL | 4.3 (4.3-7.5) | 4.3 (2.8-4.8) | 4.3 (4.3-7.5) | 4.3 (4.3-9.0) | 4.3 (4.3-4.7) | 4.3 (3.7-7.1) | 4.3 (4.3-14.2) | 4.3 (4.3-10.8) |
| TNF-α [≤7.2 pg/mL], pg/mL | 10.8 (7.9-14.0) | 7.0 (5.5-8.7) | 9.9 (7.2-14.1) | 14.6 (7.7-23.1) | 12.1 (6.5-19.0) | 8.4 (5.5-14.8) | 9.4 (6.2-12.8) | 9.4 (6.4-15.1) |
| CRP [≤5.0 mg/L], mg/L | 5.6 (2.5-7.1) | 6.8 (5.8-7.6) | 7.2 (6.5-8.2) | 7.3 (6.0-9.5) | 7.3 (5.9-11.3) | 7.4 (5.9-7.8) | 8.0 (6.1-9.1) | 7.6 (6.5-7.8) |
| ANP [0.004-0.006 ng/mL], ng/mL | 14.7 (9.6-24.7) | 22.5 (10.0-32.1) | 14.1 (8.7-26.3) | 18.5 (12.7-26.8) | 19.3 (11.9-26.1) | 19.1 (10.7-35.8) | 21.3 (11.3-40.0) | 16.8 (12.3-28.3) |
| NT-proBNP [≤540 pg/mL], pg/mL | 82 (22-779) | 213 (41-635) | 315 (109-977) | 258 (121-779) | 212 (115-548) | 593 (219-1021) | 456 (171-747) | 512 (217-886)§ |
| Troponin [<100 ng/L], ng/L | 52.3 (17.6-81.7) | 43.2 (21.3-69.0) | 50.9 (25.6-100.2) | 56.1 (31.4-109.5) | 56.1 (31.4-109.5) | 66.6 (46.9-99.3) | 58.9 (33.9-84.1) | 50.9 (32.2-106.0) |
Mayo Clinic and National Institutes of Health clinical reference ranges are shown in square brackets. Median and interquartile ranges are shown in parentheses. Boldface type indicates statistical significance.
CRP, C-reactive protein; TNF-α, tumor necrosis factor α.
P < .05 compared to cTACO group.
P < .05 compared to iTACO and UCT groups.
P < .05 compared to UCT group.
P < .05 compared to iTACO group.
Heat map of biomarkers. Different colors depict z scores above or below the mean. The concentrations increase as the color changes from blue to orange. CRP, C-reactive protein; TNF-α, tumor necrosis factor α.
Heat map of biomarkers. Different colors depict z scores above or below the mean. The concentrations increase as the color changes from blue to orange. CRP, C-reactive protein; TNF-α, tumor necrosis factor α.
Markers for volume overload
Median NT-proBNP levels were significantly higher in patients with cTACO than patients with iTACO at the delayed post-tx time point (P = .05; Table 3). Median NT-proBNP levels were elevated in patients with TACO before and after transfusion, but these differences were not significant. Median ANP levels increased significantly after transfusion in patients with iTACO (P < .01). NT-proBNP levels were positively associated with ANP levels at all time points (ρ = 0.34, 0.53, and 0.35, respectively; P < .01 [for all time points]). Moreover, elevated NT-proBNP levels correlated with higher cardiac troponin levels before and after transfusion (ρ = 0.33 and 0.24; P < .01 and P = .04, respectively).
Discussion
To the best of our knowledge, this study is the largest cohort of patients to date to examine inflammatory pathways in TACO. In this investigation, we explored whether biomarkers of inflammation or endothelial activation may occur in patients with TACO, with the aim of gaining insights into TACO’s pathophysiology. Our main findings were as follows: (1) we observed inflammation in patients experiencing TACO, as shown by elevations in body temperature and inflammatory biomarkers; (2) patients with iTACO showed a more pronounced inflammatory profile and appear as having a pulmonary transfusion reaction that has features of both TACO and TRALI; and (3) NT-proBNP levels were higher in patients with cTACO than in patients with iTACO.
We found activation of inflammatory pathways in patients with TACO in our biomarker analysis, with patients with TACO showing higher IL-6 and IL-8 levels than UCT controls. Moreover, patients with TACO had higher body temperature after transfusion, which was not seen in UCT controls. Our results confirm that specific inflammatory pathways are activated in patients with TACO. This is in line with previous studies suggesting inflammatory processes in TACO’s pathophysiology.9,11,12 Research by Roubinian et al10 showed a post-tx elevation in the proinflammatory IL-6, similar to our results. In contrast, their results showed elevated levels of the anti-inflammatory cytokine IL-10, whereas we observe elevations in proinflammatory IL-8 and IL-1α in our results. Both IL-6 and IL-8 contribute to the inflammatory process, in which IL-6 is a pleiotropic cytokine that mediates the acute phase response, including inducing the production of IL-8 and monocyte chemoattractant protein-1 (MCP-1) and increasing the expression of endothelial adhesion molecules, which are important for neutrophil migration.15 IL-8 targets neutrophils to migrate toward the site of inflammation. These cytokines are known to be part of the pathogenesis of TRALI, in which they activate and recruit neutrophils into the alveolar space, leading to inflammation, endothelial damage, and ultimately exudative pulmonary edema.16
It did not escape our notice that almost 64% of patients with cTACO received peritransfusion furosemide (Table 1). It is well documented that furosemide exhibits robust anti-inflammatory activity by inhibiting the production and release of cytokines such as IL-6, IL-8, and tumor necrosis factor α.17,18 Our data align well with published findings, showing reduced levels of IL-6 and IL-8 in the delayed post-tx samples, whereas IL-10 and IL-1, which are typically not affected by furosemide, remain relatively stable across sampling timelines.
A similar TRALI-like response could be partially responsible for pulmonary edema and respiratory distress in these patients with TACO. In our cohorts, we observed a more distinctive proinflammatory cytokine profile in both patients with cTACO and iTACO compared to controls than in patients studied by Roubinian et al.10 Such differences could be a result of diversities in the patient populations studied. Our cohorts included patients admitted to general hospital wards, with a smaller percentage of outpatients, whereas their patients were frequently in the intensive care department or operating room, where they were more likely to receive mechanical ventilation and vasopressor therapy. Such interventions could possibly have effects on changing VSVs and inflammatory marker levels, potentially altering or muting their expression in patients receiving such treatments.
As a group, patients with cTACO in this study showed a post-tx increase in heart rate and mean arterial pressure and a decrease in oxygen saturation compared to pre-tx values. These findings strongly suggest that patients with cTACO were pertinently diagnosed according to the TACO definition.13 Additionally, we included an iTACO group comprising patients with a transfusion reaction clinically classified as TACO not meeting all CDC TACO criteria. The iTACO group of patients also had a post-tx increase in heart rate but not in mean arterial pressure. Body temperature increased in both the cTACO and iTACO groups after transfusion; interestingly, this increase was more pronounced in the iTACO group. The iTACO group also displayed elevations in IL-6 and IL-8 compared to UCTs.
Interestingly, the iTACO cohort also exhibited an elevated median ICAM-1 level after transfusion compared to the cTACO and UCT cohorts, as well as a statistically significant rise in median VCAM-1 levels after transfusion compared to pre-tx values. ICAM-1 and VCAM-1 are cell surface proteins that have important roles in inflammatory responses. ICAM-1 facilitates the adhesion of monocytes, lymphocytes, and neutrophils to the endothelium and contributes to the extravasation of these cells into inflammatory tissue.19 VCAM-1 mediates the rolling and firm adhesion of monocytes and lymphocytes to the endothelium.20 These proteins are known to play a vital role in the inflammatory cascade leading to TRALI.16 Overall, the iTACO group appears to have more proinflammatory features. It appears that patients with iTACO display a pulmonary transfusion complication with features of both TACO and TRALI, in which pulmonary edema is a result of both hydrostatic forces and inflammatory endothelial leakage. Previous studies have suggested an overlap between TACO and TRALI. Patients whose TACO and TRALI syndromes could not be distinguished have been observed in previous research, and possibly, these were also patients with features of both pulmonary transfusion complications.21
The occurrence of inflammatory biomarkers in patients with TACO could develop via a “fluidic angioplasty” model, in which the volume, infusion rate, and composition of the IV fluid challenge negatively affect and disrupt the vascular endothelial cells, resulting in the release of various biological response modifiers, leading to a proinflammatory state.22,23 Closely related to this is a second but not mutually exclusive hypothesis, in which a disruption of the endothelial cell glycocalyx layer promotes leukocyte adhesion and inflammation, compromising endothelial barrier function and leading to loss of endothelial cell integrity with subsequent pulmonary edema formation.24 Also contributory to a potential proinflammation state in this regard could be proinflammatory bioactive molecules contained in the transfused blood product, as is similarly thought to occur in some patients with TRALI.
In our patient cohorts, we found higher median NT-proBNP levels in patients with cTACO than in either patients with iTACO or UCTs; however, due to a large spread in data, this was only significant 8 to 36 hours after transfusion. Such a finding supports our hypothesis that hydrostatic forces contribute to a larger degree to the development of pulmonary edema or respiratory distress in patients with cTACO than in patients with iTACO, in whom an initial “inflammatory burst” appears to have a more prominent role. Our data further support the role of NT-proBNP as a diagnostic biomarker for TACO, which has been suggested in previous research.25 Because ANP was also clearly associated with NT-proBNP levels in our cohorts, this marker could also be a potential target of future study as a diagnostic biomarker in TACO. However, it should be noted that some of our study’s findings may, in part, be influenced by the current NHSN HV Module definitions and reaction classification parameters (eg, elevated NT-proBNP and blood pressure levels in the cTACO group), due to the STR case classifications and adjudications used by the BMC Blood Bank in its investigations of STRs.
Limitations of our study are several. One relates to the relatively small number of patients in our control group, which makes meaningful statistical comparisons a challenge. Further limitations of our study are its single-center design and the use of archived residual specimens. We recognize the limitations of a single institutional source for our cases; however, the use of such assets allows for a real-world view regarding the adverse transfusion events that are typically encountered. Such an approach also allows an investigation of this type to be conducted in a logistically and financially cost-effective manner.
The length of time from sample collection to freezing of the specimen may also be a potential limitation; in that, there could be some degradation of cytokines in the samples. We note, however, several points that strengthen our confidence in reporting the results of the levels of these inflammatory markers. First, the data obtained align with previous work demonstrating increases in specific cytokine markers in TACO cases. Second, after reception in the blood bank, specimens were clinically tested per the standard operating procedures of the blood bank as soon as possible by technical staff; and after completion of the required initial serological testing, residual specimen volumes were refrigerated at 4°C. Upon completion of all routine blood bank reflex studies,14 specimens were then frozen at –80°C. It is believed that most cytokines are quite stable at –80°C for very long periods (3 years on average), owing to their small size as peptides rather than complex proteins with side chains that are susceptible to enzyme cleavage. Lastly, we collected multiple separate samples from each patient at different times starting with pre-tx samples. If any degradation was occurring, we would therefore expect similar rates of decline in these various samples at the different time points. The strategy of using specimens from multiple time points and comparing changes in marker levels over these different time points, we believe, is a strength of our study and helps mitigate concerns regarding specimen degradation.
Moreover, this study allowed us to explore measuring cytokine biomarkers to improve our understanding of TACO’s pathophysiology, including a group of patients not necessarily manifesting all of the attributes associated with patients with cTACO (ie, patients with iTACO), offering a broader spectrum of patients for examination. Such an approach thus allowed us to investigate potential differences in biomarker profiles in patients with variant “nonclassical” forms of TACO, whose STR presentations were, nevertheless, compatible with an adverse hemotherapy fluid challenge.
Given the explorative nature of our study, our results should be viewed as hypothesis generating. Nevertheless, our findings suggest that biomarker explorations in TACO may be a fruitful area for further investigations. To determine the benefits of using individual diagnostic biomarkers or biomarker panels in the diagnosis and management of TACO, future studies with larger groups, especially a larger control group, are needed. Such investigations can shed further insights into TACO’s nature and immunopathophysiology and, more importantly, help inform efforts to mitigate the severe pulmonary features not infrequently encountered in patients with it.
Conclusions
In this study, we confirmed that there are signs of inflammation in patients with TACO, as demonstrated by increased IL-6 and IL-8 levels. This work adds to the evidence that inflammation does play a role in the development of certain aspects of TACO. Moreover, we also identified a subgroup of patients with respiratory compromise after transfusion who showed signs of both TACO and TRALI, suggesting an overlapping gray area in the etiopathogenesis of these 2 disorders.
Acknowledgments
The authors recognize and appreciate the efforts and contributions of the Baystate Medical Center (BMC) Blood Bank/Transfusion Medicine Service (BB/TMS) technical staff, BMC Department of Pathology residents, and BMC nursing and physician bedside practitioners who provided patient care pertinent to the conduct of these investigations during the study interval. They extend their special thanks to Lynn Eaton in helping with institutional review board approval processes and to Patcharee Satienpoch for her important role in the maintenance of the BMC BB/TMS specimen archive. They are grateful to Stephen Rich and Patrick Pearson in the University of Massachusetts Amherst Department of Microbiology and Catherine Forconi in the laboratory of Ann Moorman at the University of Massachusetts Chan Medical School for use of their MAGPIX instruments and technical support. Patrick Pearson was especially helpful in facilitating instrumentation training and initial data collection. The authors also gratefully acknowledge Emily Bechtold for her help with R and recognize S. Hassan and L. Stoddard for their thoughtful comments and discussions regarding the initiative.
This research was funded by a Landsteiner Foundation for Blood Research (Amsterdam, The Netherlands) fellowship grant (number 1931F; A.P.J.V.) and the University of Massachusetts Amherst (Amherst, MA) Webley Lab Research Trust Fund.
The funding body was in no way involved in the study design; the collection, analysis, or interpretation of data; or the writing of the manuscript.
Authorship
Contribution: E.B.B., A.P.J.V., C.A. Jr, P.V., and W.C.W. designed the study; R.E.V. designed and performed all laboratory experiments; R.E.V., C.A. Jr, T.S., S.R., P.V., and P.S.M. collected data; E.B.B. and R.E.V. interpreted data and performed data analysis; E.B.B., R.E.V., C.A. Jr, W.C.W., and A.P.J.V. wrote the manuscript; and all authors critically evaluated the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Wilmore C. Webley, Department of Microbiology, University of Massachusetts Amherst, 639 North Pleasant St, N418 Morrill IV, Amherst, MA 01003; email: wilmore@umass.edu.
References
Author notes
R.V. and E.B.B. contributed equally to this study.
C.A. Jr and A.P.J.V. contributed equally to this study.
Original data are available on request from the corresponding author, Wilmore Webley (wilmore@umass.edu).
The full-text version of this article contains a data supplement.