Key Points
ISCs are 20 times stiffer than nonsickled cells and 6 times less stiff than reversibly sickled cells.
ISC fractions have a negative correlation patient HbF fraction.
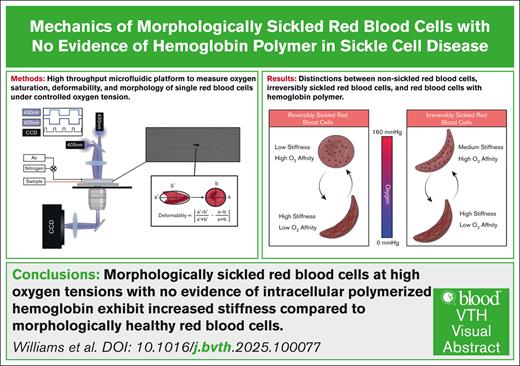
Visual Abstract
We developed a platform to measure the oxygen-dependent mechanical properties and oxygen saturation of individual irreversibly sickled cells (ISCs). We identified and measured ISCs from a cohort of 10 individuals with sickle cell disease. ISCs were found to have an average shear surface modulus 20 times that of nonsickled cells and a sixth that of red blood cells (RBCs) with detectable hemoglobin polymer. We found that the number of ISCs was significantly reduced at 53 mm Hg oxygen compared with ≥91 mm Hg oxygen, suggesting that these RBCs can still form polymer under hypoxia. We also found that the fraction of ISCs present in a blood sample had a negative correlation with donor fetal hemoglobin (HbF) fraction, suggesting that HbF could play a role in mitigating occurrence of ISCs.
Introduction
Mechanical alteration in red blood cells (RBCs) has been recognized as a major source of pathophysiology in sickle cell disease (SCD).1-3 The polymerization of deoxygenated hemoglobin S (HbS) into ordered fibers has been shown to dramatically increase RBC stiffness and alter cellular morphology.4-8 Formation of polymer also decreases Hb oxygen affinity by locking the Hb within the polymer in the low-oxygen affinity T conformation.9 Even in the absence of Hb polymer, SCD RBCs can have reduced deformability.10 Irreversibly sickled cells (ISCs) are RBCs that maintain altered morphology with no intracellular Hb polymer.11 ISCs are thought to occur because of cellular dehydration and membrane damage from repeated cycles of sickling.12 Circulating ISCs have been suggested to contribute to vaso-occlusion and increased hemolysis1,13; however, the properties of these cells are largely unknown, and their impact remains poorly understood.
ISCs have previously been identified using morphological classification of RBCs in blood smears as well as density fractionation.10,14 Studies using ektacytometry have shown ISCs to be a less deformable subpopulation of RBCs in SCD blood samples,15 and micropipette aspiration has detected increased stiffness and more variance in cell mechanics in ISCs.10,16,17 Recently, microfluidic and optical techniques have accurately measured mechanical changes in the membranes and bulk stiffness of ISCs.18,19 Although these previous studies confirmed altered mechanics of ISCs, these methods either do not provide quantification of cellular properties or are too low throughput to provide distributions of mechanical properties that could be used to assess variance and compare with other cell populations. In this study, we present a microfluidic assay designed to rapidly measure mechanical properties and oxygen saturation of individual RBCs under well-defined oxygen tension. These measurements are coupled with a convolutional neural net trained to classify cell morphology. From this assay we are able to determine the relative mechanics of ISCs compared with nonsickled cells and RBCs with detectable Hb polymer, as well as the percentage of RBCs in a blood sample that is irreversibly sickled. We are also able to compare ISC fraction with other clinical biomarkers such as fetal Hb (HbF) levels.
Methods
Blood handling and preparation
Blood samples from healthy donors and donors with SCD were collected from University of Minnesota and Children’s Hospital and Clinics Minnesota. This study was approved by the University of Minnesota institutional review board and the Children’s Hospital and Clinics Minnesota institutional review board. All human patients gave informed consent before participating in this study. Complete blood count was measured using a Sysmex XN-9000, and Hb variants were measured using capillary electrophoresis. These data are included in supplemental Table 1. Samples were collected in vacuum-sealed citrate tubes and all samples were run within 24 hours of draw. Whole blood was rinsed with 1× phosphate buffered saline solution and spun at 400g for 2 minutes to remove plasma. Then, 2.7 μL of packed RBCs were added to 200 μL of 25% human serum albumin solution (Gemini Bio).
Microfluidic assay
A microfluidic device similar to that used in our previous work6 was used to subject RBCs to controlled oxygen tensions and measure single-cell oxygen saturation, deformability, and morphology. The microfluidic device comprised 2 polydimethylsiloxane (PDMS) layers separated by a 100-μm PDMS membrane allowing for gas diffusion. The top layer controlled oxygen tension on the device and the bottom layer was used to subject RBCs to hydrodynamic shearing through a constriction. A detailed schematic of the microfluidic device is provided in supplemental Figure 1. The microfluidic device was placed in an environmentally controlled chamber set at 37°C. The constriction on the device was imaged under 2 alternating light-emitting diodes centered at the molecular absorption peaks of oxygenated and deoxygenated Hb (410 nm and 430 nm, respectively), which were synchronized to the trigger of the high-speed camera (Phantom Miro C110, Vision Research Inc) at a frame rate of 1000 frames per second with an exposure time of 300 μs. The RBC suspension was perfused through the microfluidic device using a pressure controller (Flow EZ, Fluigent). Oxygen tension on the microfluidic device was controlled using previously published methods.20 Briefly, a controlled combination of air and nitrogen was delivered to the gas channels of the device, which diffuses through the PDMS membrane to equilibrate the entirety of the RBC channel to a specific partial pressure of oxygen. On-chip oxygen was verified with an oxygen sensor (NeoFox Oxygen Sensing System). For our assay, we sampled 8 different oxygen tensions between 0 and 160 mm Hg for each blood sample. At each oxygen tension, ∼1000 RBCs were recorded passing through the constriction.
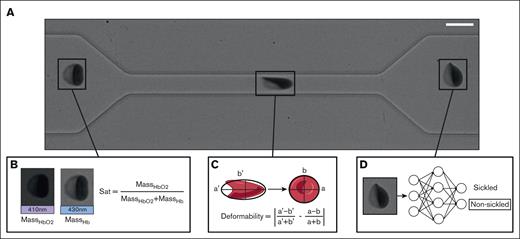
Images of the RBCs passing through the microfluidic constriction were used to measure single-cell oxygen saturation, calculate RBC deformability, and classify RBCs as morphologically sickled or nonsickled (Figure 1A). The oxygen saturation (Figure 1B) and single RBC deformability (Figure 1C) were calculated using methods described in our previous work.6,21 The deformability metric is a unitless shape parameter that quantifies the difference in elongation of the RBC under tension and at rest that can be used to separate RBCs into low and high deformability populations. The shear surface modulus of the RBC can be estimated by modeling the cell as a Kelvin-Voight material and measuring change in elongation due to velocity change as the RBC exits the constriction. A more detailed overview of deformability metric and our method of estimating shear surface modulus can be found in our previous work.6 RBCs were classified as morphologically sickled or nonsickled using a trained ResNet-50 convolution neural net (CNN) on an image of the cell after it had exited the constriction (Figure 1D). Details on the training set for the CNN are included in supplemental Figure 2.
Experimental methods. (A) Imaging of a single RBC traversing the microfluidic constriction. Scale bar, 10 μm. (B) Calculation of single-cell oxygen saturation uses absorption from a 410-nm light-emitting diode (LED) to calculate mass of oxygenated Hb, and absorption from a 430-nm LED to calculate mass of deoxygenated Hb. (C) The single-cell deformation is measured by calculating the absolute difference in cell Taylor deformation within the constriction and outside the constriction. (D) RBCs are classified as either sickled or nonsickled using a ResNet-50 CNN.
Experimental methods. (A) Imaging of a single RBC traversing the microfluidic constriction. Scale bar, 10 μm. (B) Calculation of single-cell oxygen saturation uses absorption from a 410-nm light-emitting diode (LED) to calculate mass of oxygenated Hb, and absorption from a 430-nm LED to calculate mass of deoxygenated Hb. (C) The single-cell deformation is measured by calculating the absolute difference in cell Taylor deformation within the constriction and outside the constriction. (D) RBCs are classified as either sickled or nonsickled using a ResNet-50 CNN.
Selection of ISCs
Following previous literature in the field,11,15 this work defines ISCs as RBCs maintaining a sickled morphology with no evidence of detectable Hb polymer. Under this definition, ISCs present an altered morphology even under fully oxygenated conditions. Similar to previous work, we identify ISCs from fully oxygenated RBCs by their sickled morphology.13,17 To ensure a lack of polymerized Hb, because this has been suggested to be possible even at high oxygen tensions,22 we calculate single RBC oxygen saturation. Polymerized Hb can be detected at intermediate and high oxygen tensions (defined as an oxygen partial pressure of >50 mm Hg in this work) by a decrease in oxygen affinity due to Hb in the polymer fibers being locked in the T conformation unable to shift to the high oxygen affinity R conformation.8 We assume RBCs classified as nonsickled by the CNN contain a negligible amount of polymer. Therefore, any RBCs with an oxygen saturation within 1 standard deviation of the nonsickled population mean also contain negligible Hb polymer. Figure 2A shows the 3 classifications of RBCs. The nonsickled cells are RBCs that were classified as nonsickled by the CNN. ISCs are RBCs that were morphologically sickled, as classified by the CNN but that had no evidence of Hb polymer. RBCs with detectable Hb polymer were morphologically sickled and had evidence of Hb polymer, demonstrated by the single-cell oxygen saturation being more than a standard deviation and less than the mean of the nonsickled population at the same oxygen tension. Although traditionally ISCs have only been considered at atmospheric oxygen tension, the measurement of single-cell oxygen saturation allows for the identification of ISCs at lower oxygen. If a RBC is morphologically sickled but maintains an oxygen saturation within 1 standard deviation of the nonsickled population, then that cell contains no Hb polymer and is an ISC. It is important to note that in complete hypoxia, the oxygen saturation of RBCs with Hb polymer and nonsickled RBCs are the same. As a result, ISCs could not be determined at 0 mm Hg. Representative data from a single donor depicting classification of the 3 subpopulations are included in supplemental Figure 3.
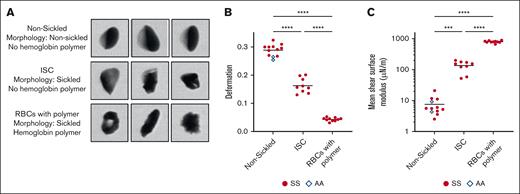
Mechanical properties of nonsickled, irreversibly sickled, and RBCs with detectable Hb polymer. (A) Representative images of nonsickled RBCs and cells classified as sickled by the CNN that have been separated into ISCs and RBCs containing polymerized Hb based on the oxygen tension and cell saturation. (B) Deformation parameter of the 3 cell types for 10 HbSS blood samples and 2 HbAA blood samples. (C) Mean shear surface modulus of the 10 HbSS blood samples and HbAA blood samples calculated from the corresponding deformation parameter. A 1-way analysis of variance was used to identify significant differences, and a Dunnett test was used to compare the subpopulations.
Mechanical properties of nonsickled, irreversibly sickled, and RBCs with detectable Hb polymer. (A) Representative images of nonsickled RBCs and cells classified as sickled by the CNN that have been separated into ISCs and RBCs containing polymerized Hb based on the oxygen tension and cell saturation. (B) Deformation parameter of the 3 cell types for 10 HbSS blood samples and 2 HbAA blood samples. (C) Mean shear surface modulus of the 10 HbSS blood samples and HbAA blood samples calculated from the corresponding deformation parameter. A 1-way analysis of variance was used to identify significant differences, and a Dunnett test was used to compare the subpopulations.
Results
ISCs are more deformable than RBCs with detectable Hb polymer
We measured single-cell mechanical properties of RBCs from 10 individuals who were homozygous for the sickle mutation (genotype HbSS) and from 2 individuals with normal blood genotype (HbAA). The microfluidic assay allows for analysis of the subpopulations of nonsickled, irreversibly sickled, and RBCs with detectable Hb polymer within a blood sample. Figure 2A shows representative images of the 3 cell types and classification criteria. In Figure 2B-C, the deformability parameter and estimated shear surface moduli for each of the 3 RBC subpopulations in the 12 donor samples are shown. Our previous work has shown that oxygen saturation does not affect the deformability of nonsickled RBCs or RBCs with detectable Hb polymer.6 Thus, the deformability metrics for the nonsickled RBCs and RBCs with detectable Hb polymer are pooled across the 8 oxygen tensions. ISC subpopulation metrics, however, are from RBCs only measured at atmospheric oxygen tension. Most previous work has studied ISC mechanics at atmospheric oxygen tension and only considered environmental oxygen rather than RBC saturation. For that reason, we chose to report the mechanical properties of ISCs only at atmospheric oxygen to ensure our results were comparable with previous studies. The oxygen saturation measurement of these cells confirms full oxygenation. The ISCs were found to deform less than nonsickled cells and more than RBCs with detectable Hb polymer (Figure 2B). The mean shear surface moduli of nonsickled RBCs, ISCs, and RBCs with detectable Hb polymer were 7.52, 135, and 801 μN/m, respectively (Figure 2C).
Fraction of ISCs decreases with decreasing oxygen tension
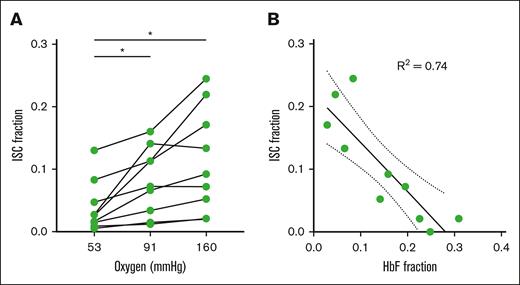
Previous works have only considered ISCs under fully oxygenated conditions; however, the implementation of a saturation measurement allows for ISC detection at high and intermediate oxygen tensions because the formation of Hb polymer will decrease the oxygen saturation to a detectable amount. We estimated a fraction of ISCs at 53 and 91 mm Hg oxygen by looking for RBCs that were classified as morphologically sickled (determined by CNN classification) and were within 1 standard deviation of the oxygen saturation of the nonsickled population. The mechanics of these ISCs at lower oxygen did not change significantly from ISCs at atmospheric oxygen (supplemental Figure 4) offering further evidence that they do not contain HbS polymer. Figure 3A shows the fraction of ISCs at 160, 91, and 53 mm Hg oxygen. There was a significant decrease in fraction of ISCs at 53 mm Hg compared with both 160 and 91 mm Hg for the blood samples analyzed, suggesting that HbS can polymerize within ISCs contributing to an increase in RBCs with Hb polymer at relatively high oxygen tensions.
Fraction of RBCs irreversibly sickled related to measured oxygen tension and donor HbF. (A) ISC percentage of the HbSS blood samples with >1% ISCs at 160 mm Hg samples measured at 3 oxygen tensions. A 1-way analysis of variance was used to identify significant differences, and a Holm-Šídák test was used to compare each oxygen tension. (B) ISC percentage of the 10 HbSS blood samples vs corresponding HbF fraction measured by capillary electrophoresis. Data are fit with a linear regression with the corresponding R2 value shown.
Fraction of RBCs irreversibly sickled related to measured oxygen tension and donor HbF. (A) ISC percentage of the HbSS blood samples with >1% ISCs at 160 mm Hg samples measured at 3 oxygen tensions. A 1-way analysis of variance was used to identify significant differences, and a Holm-Šídák test was used to compare each oxygen tension. (B) ISC percentage of the 10 HbSS blood samples vs corresponding HbF fraction measured by capillary electrophoresis. Data are fit with a linear regression with the corresponding R2 value shown.
Fraction of ISCs negatively correlates with HbF fraction
The high-throughput single-cell measurements from this assay allow us to quantify the fraction of donor RBCs that is irreversibly sickled. The ISC fraction can be compared with common clinical metrics. Figure 3B shows the ISC fraction vs HbF fraction with a linear regression fit and the R2 value. We found a significant negative correlation between HbF and ISC fraction (P = .0015; R2 = 0.74).
Discussion
In this study, we combined measurements of single-cell oxygen saturation and morphological analysis to rigorously define nonsickled RBCs, ISCs, and RBCs containing Hb polymer. We then used high-throughput measurements of single RBC mechanics to reveal population-level mechanics and donor variability of ISCs and RBCs with detectable Hb polymer. ISCs in SS blood have been hypothesized to lead to vascular dysfunction and pain crisis because of their increased rigidity,3,23 yet the change in stiffness of these cells relative to nonsickled RBCs remains understudied, and distinction between ISCs and RBCs containing Hb polymer is poorly defined. The measurements in this study of shear surface modulus of nonsickled RBCs, ISCs, and RBCs with detectable Hb polymer provides a framework to understand the mechanical differences between these 3 populations as well as potential impacts these cells can have on the vasculature.
ISCs were found to be an order of magnitude stiffer than nonsickled RBCs and exhibit increased mechanical variability. The increase in stiffness of ISCs compared with nonsickled cells matches results from previous studies,15,16 and the larger spread of shear surface moduli in the ISC population compared with nonsickled cells (ISC standard deviation, 51.4 μN/m; nonsickled standard deviation, 5.14 μN/m) agrees with findings of Smith et al of ISCs having increased mechanical heterogeneity compared with discoid RBCs.10 Although ISCs can make up a relatively small fraction of total RBCs, computational and experimental studies have suggested even a small amount of stiffened cells can produce pathological flow conditions due to RBC margination.24 Zhang et al developed robust simulations of binary mixtures of healthy and stiffened RBCs.25 This study showed that RBCs with a capillary number a quarter of that of healthy RBCs, corresponding to an order of magnitude stiffness increase, will marginate to the edges of a channel, decreasing the cell-free layer and increasing wall shear stress. The relative stiffness increase and fraction of stiff RBCs used in these simulations are similar to the values we experimentally identified for ISCs in donor blood samples, highlighting the pathological potential of these RBCs. The formation of Hb polymer increases the stiffness of RBCs by 2 orders of magnitude compared with nonsickled cells, distinguishing these RBCs as mechanically different from ISCs. Not only is the stiffness of polymer-containing RBCs much greater than that of ISCs, but their relative fraction is dependent on oxygen tensions and highly variable amongst donors.6,8 Both RBC stiffness and the stiff cell fraction have been shown to significantly alter flow behavior24,26; consequently, ISCs and RBCs containing polymer may create differing pathological behaviors in the body, requiring carefully designed studies to uncover disease mechanisms.
One technical advance in this this study is the ability to detect ISCs under conditions in which they would not be fully oxygenated by combining morphological analysis and oxygen saturation. Because polymer formation decreases oxygen affinity, its presence can be detected at intermediate and high oxygen tensions by a decrease in RBC oxygen saturation compared with RBCs without polymer. Thus, if an RBC is morphologically sickled but has an oxygen saturation within a standard deviation of the mean of nonsickled cells, we assume it does not contain Hb polymer. RBCs measured at intermediate oxygen that display sickled morphology with no relative saturation decrease compared with nonsickled RBCs can be considered ISCs. Using this method, we identified a significant decrease in fraction of ISCs at 53 mm Hg compared with both 160 and 91 mm Hg for the blood samples analyzed. This suggests that HbS can polymerize within ISCs. Additionally, these cells appear to form polymer at relatively high oxygen tension, suggesting that they be among the first cells to polymerize in the body and contribute to vascular pathologies even in relatively high oxygen regions of the vasculature.
We also showed that increased HbF correlates with a decrease ISCs. The negative correlation between HbF fraction and ISC fraction agrees with previous studies using blood smear counting.14,27 HbF has been shown in multiple studies to have a protective effect against Hb polymer formation28 and is the basis for numerous therapies including hydroxyurea and gene therapy.29-31 Our result lends credence to the hypothesis of repeated cycles of RBC sickling being partially responsible for the formation of ISCs as suggested by Ohnishi et al.12 Notably, the method used here has a significantly higher throughput than blood smear counting, analyzing thousands of cells, and the analysis is automated thus introducing no user bias. The ISC fractions found in this work are of similar magnitude to that of previous studies.13,14,27
Acknowledgments
The authors thank the sickle care team at Children’s Hospital and Clinics of Minnesota, including Steve Nelson, Ashley Kinsella, Pauline Mitby, Ali Kolste, Rachel Hinsch, and Emily Olson. The authors thank the sickle care team at M Health Fairview, Minneapolis, MN, including Yvonne Datta. Portions of this work were conducted in the Minnesota Nano Center, which is supported by the National Science Foundation through the National Nano Coordinated Infrastructure Network under award no. ECCS-1542202.
D.C.W., J.M.H., and D.K.W. were supported by the National Institutes of Health (HL132906). D.C.W. was supported by the University of Minnesota on a doctoral dissertation fellowship.
Authorship
Contribution: D.C.W. performed the research, contributed new reagents/analytic tools, and analyzed data; and all authors designed the research and wrote the article.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: David K. Wood, Department of Biomedical Engineering, University of Minnesota, 7-122 Nils Hasselmo Hall, 312 Church St SE, Minneapolis, MN 55455; email: dkwood@umn.edu.
References
Author notes
All code, convolutional neural net model, and training data are available at the GitHub (https://doi.org/10.5281/zenodo.14080807). Raw data files are too large to store on GitHub. These files are available on request from author Dillon C. Williams (will6605@umn.edu).
The full-text version of this article contains a data supplement.