Key Points
Pregnant patients homozygous for PAR4-Thr120 have similar response to 81-mg aspirin as measured by PFA-100 epinephrine closure time.
Despite 81-mg aspirin daily, pregnant patients homozygous for PAR4-Thr120 may be at increased risk for placental intervillous thrombosis.
Visual Abstract
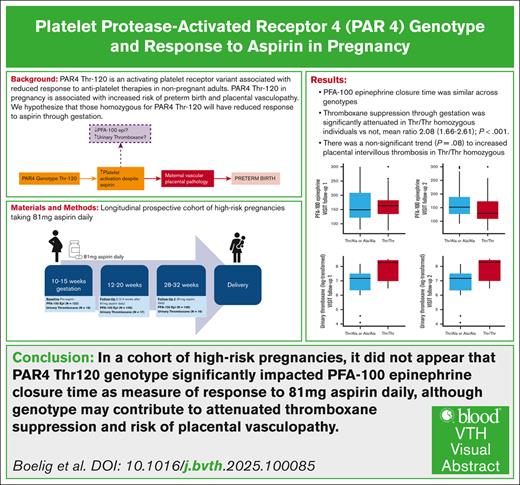
The platelet protease-activated receptor 4 (PAR4) threonine 120 (Thr120) allele is an activating allele associated with reduced aspirin response in vitro. Aspirin is recommended in high-risk pregnancies to prevent preeclampsia and preterm birth. We evaluated the impact of PAR4 genotype on aspirin response in pregnancy, as measured by platelet function assay 100 (PFA-100) epinephrine closure time, and perinatal outcomes. We conducted a prospective cohort study of high-risk pregnant patients who took 81-mg aspirin daily. PFA-100 was assessed at baseline, 2 to 4 weeks after aspirin initiation (follow-up 1), and 28 to 32 weeks’ gestation (follow-up 2). Primary outcome was difference in PFA-100 by genotype. Exposure was defined as PAR4-Thr120 homozygous vs not. Of the 122 participants were included, 24 (19.6%) were PAR4-Thr120 homozygous, and 106 completed follow-up 1 with >75% adherence. Participants homozygous for PAR4-Thr120 had a significantly higher rate of prior preterm birth (50.0% vs 16.1%; P = .004). Genotype was not significantly associated with PFA-100 response in multivariable regression. In the subset with urinary thromboxane data available (n = 18), thromboxane levels were higher in those who were homozygous vs not (geometric mean ratio, 208 [95% confidence interval, 1.66-2.61]; P < .001) in multivariable regression. There was a higher rate of placental intervillous thrombosis, although not statistically significant (16.7% vs 3.9%; P = .08). Patients homozygous for PAR4-Thr120 had a higher incidence of prior preterm birth, a risk factor for poor perinatal outcome. Aspirin response, measured by PFA-100, was similar across genotypes, although Thr120 homozygosity may be associated with reduced thromboxane suppression and a higher rate of placental vasculopathy even with 81-mg aspirin daily.
Introduction
Preeclampsia and preterm birth remain leading causes of maternal and infant morbidity and mortality. Pregnant patients at increased risk of preeclampsia are recommended low-dose aspirin daily to reduce the risk of preeclampsia and preterm birth in the current pregnancy. However, 20% to 30% of high-risk patients taking low-dose aspirin daily will still suffer these complications.1,2
Protease-activated receptor 4 (PAR4) is a G-protein–coupled receptor and a major contributor to platelet activity in hemostasis and thrombus formation. A well-characterized PAR4 variant with a threonine (Thr) substitution for alanine (Ala) at amino acid 120 (PAR4-Thr120) is associated with increased receptor activity. Previous studies have demonstrated that isolated platelets from PAR4-Thr120 donors have enhanced aggregation potential in the presence of aspirin compared to those from PAR4-Ala120 donors.3 We subsequently conducted a cohort study on pregnant patients in an unselected pregnant population and identified significantly increased odds of preterm birth and placental vascular pathology (intervillous thrombosis) in pregnant patients homozygous for PAR4-Thr120, whereas those who were heterozygous or wild type had similar outcomes.4 It is unclear whether this variant affects response to low-dose aspirin and perinatal outcomes in high-risk pregnancies with daily aspirin use. The objective of this study was to evaluate the impact of PAR4-Thr120 homozygosity on response to aspirin therapy and perinatal outcomes in high-risk singleton pregnancies. We hypothesized that those homozygous for Thr120 would have reduced platelet inhibition after aspirin therapy.
Materials and methods
Cohort description
This was a planned secondary analysis of high-risk pregnancies prescribed 81 mg of aspirin daily, enrolled from August 2020 to May 2022 at Thomas Jefferson University in Philadelphia, Pennsylvania. Results of the primary cohort have been published.5 The primary study included singleton pregnancies who were considered high-risk for preeclampsia based on United States Preventative Services Task Force criteria,6 enrolled at <16 weeks of gestation and recommended 81 mg of aspirin daily. The study was approved by the Thomas Jefferson University Institutional Review Board before study initiation, and all participants provided written consent. Participants had blood collected for genotyping at the baseline visit, in addition to baseline laboratory assessments including platelet function assay 100 (PFA-100) epinephrine closure time before aspirin initiation. They subsequently had PFA-100 assessed 2 to 4 weeks after aspirin initiation (follow-up 1) and again at 28 to 32 weeks of gestation (follow-up 2).
At delivery, pregnancy outcomes were collected, and placenta was sent for standard histopathology. Placental pathology was categorized according to Amsterdam criteria.7 Participants were included in this study if they had successful genotyping completed and took 81 mg of aspirin daily. Analysis of PFA-100 response was limited to those with documented >75% adherence to aspirin, assessed by pill counting at each study visit.
Outcomes
The exposure of interest was PAR4 genotype, categorized as Thr120 homozygous (Thr/Thr) vs not (heterozygous [Thr/Ala] or Ala120 homozygous, wild type [Ala/Ala]). The primary outcome of interest was PFA-100 epinephrine closure time, compared as a continuous variable at each visit. Aspirin response was assessed with PFA-100 (Siemens) epinephrine closure time through the Thomas Jefferson University Hospital Hematology Clinical Laboratory; the test was run in duplicate per manufacturer protocol. There are a number of different ways to assess platelet activity in response to aspirin. The gold standard is usually considered to be light aggregometry; however, this was time intensive and laboratory specific, limiting its use as a generalizable assay for clinical use.8 There are commercially available assays to evaluate aspirin response that have been validated against light aggregometry, including PFA-100.8 In a subset of participants who participated in a pharmacokinetic substudy,9 urinary thromboxane data were available, and this was similarly compared across PAR4 genotype.
Secondary outcomes included pregnancy outcomes: hypertensive disorders of pregnancy (preeclampsia or gestational hypertension, per standard clinical criteria and as documented in the medical record); preterm birth (delivery <37 weeks); and placental intervillous thrombosis, because these were findings we previously identified as associated with PAR4 genotype in pregnancy.4
Sample size and power
The initial cohort design was powered based on findings that the presence of Thr120 allele was associated with reduced response to aspirin. Based on an estimated cohort size of 120 and the estimated allelic frequency by race,10 we anticipated 70 participants would have Thr120 allele (heterozygous or homozygous), and 50 would not, which had 80% power to detect a mean difference of 13 seconds in PFA-100 epinephrine closure time with a 2-sided α of 0.05.
Statistical analysis
The relationship between PFA-100 closure time and urinary thromboxane with genotype was assessed with 2-sample t test or Mann-Whitney U test, as appropriate (unadjusted), as well as generalized estimating equation modeling to account for repeat measures and relevant covariates (adjusted). For regression analysis regarding aspirin response, the following covariates were included: PAR4 genotype (Thr120 homozygous vs not), age, hypertension, diabetes, obesity (body mass index ≥30 kg/m2), weight at visit, platelet count at visit, baseline creatinine, and enteric- vs non–enteric-coated aspirin. The relationship between perinatal outcome (binary) and genotype was assessed with χ2 test (unadjusted) and binary logistic regression (adjusted). For regression analysis of pregnancy outcome, the following covariates were included: PAR4 genotype (Thr120 homozygous vs not), age, hypertension, diabetes, obesity (body mass index of ≥30 kg/m2), weight, nulliparity, and aspirin enteric coating vs not. For all regression analyses, covariates were retained in the model if P value was <.20. Two-sided α of 0.05 was considered significant for all analyses. All analyses were completed with R (2025; R Project, Vienna, Austria) as well as the R package glmtoolbox (2024).11
Results
Of the 130 participants in the original cohort, 122 completed PAR4 genotyping and baseline visit blood work. Of those, 106 completed follow-up 1 with >75% adherence; 98 completed follow-up 2 with >75% adherence; delivery outcome was available for 115; and placental pathology data were available for 94 (Figure 1). There was a difference in nulliparity across genotypes (Table 1). Notably, among multiparous patients, there was a significantly higher incidence of prior preterm birth among those homozygous for Thr/Thr allele (Table 1).
Baseline characteristics
| Baseline characteristics . | Thr/Thr n = 24 . | Thr/Ala or Ala/Ala n = 98 . | P value . |
|---|---|---|---|
| Age, y | 31.6 ± 6.0 | 31.7 ± 6.1 | .96 |
| Race | <0.001 | ||
| Black, Non-Hispanic | 50 (50.5%) | 23 (95.8%) | |
| White, Non-Hispanic | 32 (32.3%) | 0 | |
| Asian | 5 (3.9%) | 0 | |
| Hispanic, White | 11 (11.1%) | 1 (4.2%) | |
| Other | 1 (1.0%) | 0 | |
| Weight | 207.8 ± 44.3 | 202.2 ± 53.1 | .59 |
| Obesity, BMI of >30 kg/m2 | 18 (75.0) | 66 (67.3) | .63 |
| Hypertension | 9 (37.5) | 29 (29.6) | .61 |
| Diabetes | 2 (8.3) | 11 (11.2) | 1.0 |
| Nulliparous | 2 (8.3) | 36 (36.7) | .01 |
| Previous preeclampsia | 9/22 (40.9) | 27/62 (43.5) | 1.0 |
| Previous preterm birth | 11/22 (50.0) | 10/62 (16.1) | .004 |
| Previous preterm preeclampsia | 6/22 (27.3) | 6/62 (9.7) | .07 |
| PFA-100 (before aspirin) | 137.1 ± 44.3 | 129.1 ± 37.6 | .42 |
| Baseline characteristics . | Thr/Thr n = 24 . | Thr/Ala or Ala/Ala n = 98 . | P value . |
|---|---|---|---|
| Age, y | 31.6 ± 6.0 | 31.7 ± 6.1 | .96 |
| Race | <0.001 | ||
| Black, Non-Hispanic | 50 (50.5%) | 23 (95.8%) | |
| White, Non-Hispanic | 32 (32.3%) | 0 | |
| Asian | 5 (3.9%) | 0 | |
| Hispanic, White | 11 (11.1%) | 1 (4.2%) | |
| Other | 1 (1.0%) | 0 | |
| Weight | 207.8 ± 44.3 | 202.2 ± 53.1 | .59 |
| Obesity, BMI of >30 kg/m2 | 18 (75.0) | 66 (67.3) | .63 |
| Hypertension | 9 (37.5) | 29 (29.6) | .61 |
| Diabetes | 2 (8.3) | 11 (11.2) | 1.0 |
| Nulliparous | 2 (8.3) | 36 (36.7) | .01 |
| Previous preeclampsia | 9/22 (40.9) | 27/62 (43.5) | 1.0 |
| Previous preterm birth | 11/22 (50.0) | 10/62 (16.1) | .004 |
| Previous preterm preeclampsia | 6/22 (27.3) | 6/62 (9.7) | .07 |
| PFA-100 (before aspirin) | 137.1 ± 44.3 | 129.1 ± 37.6 | .42 |
Thr/Ala or Ala/Ala represents PAR4-Thr120 genotype homozygous for Ala or heterozygous for Ala and Thr at position 120; and Thr/Thr, PAR4-120 variant homozygous for Thr. Data are presented as mean ± standard deviation or n (%) as appropriate.
BMI, body mass index.
There was no significant difference in platelet response to aspirin therapy (PFA-100 epinephrine closure time) by genotype in follow-up 1 (2 to 4 weeks after aspirin initiation): median of 167 seconds (range, 134-202) vs 152 seconds (range, 126-212; P = .90; Table 2; Figure 2A). At follow-up 2 (28-32 weeks gestation), there was reduced PFA-100 epinephrine closure time, although it was not statistically significant (median difference, –19 [range, –43 to 6]; P = .14; Table 2; Figure 2B). In regression analysis, PAR4 genotype was not associated with PFA-100 closure time through gestation (P > .20).
Aspirin response and pregnancy outcome
| . | Thr/Thr . | Thr/Ala or Ala/Ala . | P value . | Adjusted odds ratio or geometric mean ratio (95% CI); P value . |
|---|---|---|---|---|
| Aspirin response | ||||
| PFA-100, follow-up 1 (second tm) | 167 (134-202) n = 19 | 152 (126-212) n = 87 | .90 | NS |
| PFA-100, follow-up 2 (third tm) | 128 (107-175) n = 19 | 151 (126-194) n = 79 | .14 | |
| Urine thromboxane (second tm) | 2761.1 ± 2034.1 n = 6 | 1433.8 ± 1186.5 n = 11 | .18 | mean ratio, 2.08 (1.66-2.61); P < .001 |
| Urine thromboxane (third tm) | 3982.3 ± 1399.8 n = 7 | 1402.9 ± 1019.6 n = 9 | <.001 | |
| Pregnancy outcome | n = 22 | n = 93 | Adjusted Odds ratio | |
| Hypertensive disorder of pregnancy | 4 (18.2) | 28 (30.1) | .39 | NS |
| Preterm birth (<37 weeks) | 4 (18.2) | 13 (14.0) | .74 | NS |
| Intervillous thrombosis∗ | 3/18 (16.7) | 3/76 (3.9) | .08 | NC |
| . | Thr/Thr . | Thr/Ala or Ala/Ala . | P value . | Adjusted odds ratio or geometric mean ratio (95% CI); P value . |
|---|---|---|---|---|
| Aspirin response | ||||
| PFA-100, follow-up 1 (second tm) | 167 (134-202) n = 19 | 152 (126-212) n = 87 | .90 | NS |
| PFA-100, follow-up 2 (third tm) | 128 (107-175) n = 19 | 151 (126-194) n = 79 | .14 | |
| Urine thromboxane (second tm) | 2761.1 ± 2034.1 n = 6 | 1433.8 ± 1186.5 n = 11 | .18 | mean ratio, 2.08 (1.66-2.61); P < .001 |
| Urine thromboxane (third tm) | 3982.3 ± 1399.8 n = 7 | 1402.9 ± 1019.6 n = 9 | <.001 | |
| Pregnancy outcome | n = 22 | n = 93 | Adjusted Odds ratio | |
| Hypertensive disorder of pregnancy | 4 (18.2) | 28 (30.1) | .39 | NS |
| Preterm birth (<37 weeks) | 4 (18.2) | 13 (14.0) | .74 | NS |
| Intervillous thrombosis∗ | 3/18 (16.7) | 3/76 (3.9) | .08 | NC |
PAR4-120 variant with Thr vs Ala. Data are presented as mean ± standard deviation, median (interquartile range), or n (%) as appropriate. Repeat measures (PFA-100 and urinary thromboxane) were compared with generalized estimating equation modeling. The P value for PAR4 genotype is >.20.
NC, not calculable (too few cases); NS, not significant; tm, trimester.
Of those with placental histopathology available.
Aspirin response by PAR4 genotype. Aspirin response as assessed by PAR4 genotype in pregnancy at PFA-100 epinephrine closure time for 106 (A) and 98 (B). (C) Urinary thromboxane (n = 17) with >75% aspirin adherence, visit follow-up 1, 2 to 4 weeks after initiation of 81 mg daily. (D) Urinary thromboxane for 16 patients with >75% aspirin adherence; visit follow-up 2, 28 to 32 weeks gestation. The generalized estimating equation model taking into consideration repeat measures and relevant covariates was not significant for PFA-100 but was significantly different for urinary thromboxane (geometric mean ratio, 2.08 [95% CI, 1.66-2.61]; P < .001).
Aspirin response by PAR4 genotype. Aspirin response as assessed by PAR4 genotype in pregnancy at PFA-100 epinephrine closure time for 106 (A) and 98 (B). (C) Urinary thromboxane (n = 17) with >75% aspirin adherence, visit follow-up 1, 2 to 4 weeks after initiation of 81 mg daily. (D) Urinary thromboxane for 16 patients with >75% aspirin adherence; visit follow-up 2, 28 to 32 weeks gestation. The generalized estimating equation model taking into consideration repeat measures and relevant covariates was not significant for PFA-100 but was significantly different for urinary thromboxane (geometric mean ratio, 2.08 [95% CI, 1.66-2.61]; P < .001).
In a subset of 18 who participated in a pharmacokinetic substudy9 and had PAR4 genotyping available with appropriate adherence at follow-up 1 and/or 2, we found significantly reduced higher geometric mean ratio of urinary thromboxane (reduced thromboxane suppression) in those homozygous for Thr/Thr vs Thr/Ala or Ala/Ala (mean ratio, 2.08 [95% confidence interval (CI), 1.66-2.61]; P < .001; Table 2; Figure 2C-D). In examining individual genotypes, this difference in thromboxane suppression was identified for both Thr/Ala and Ala/Ala compared to Thr/Thr (mean ratio, 0.59 [95% CI, 0.46-0.74; P < .001] and 0.41 [95% CI, 0.25-0.66; P < .001], respectively).
The incidence of preterm birth and hypertensive disorders of pregnancy (preeclampsia and gestational hypertension) were similar across genotypes (Table 2) in both unadjusted and adjusted analyses. Rates of intervillous thrombosis were higher in those homozygous for Thr/Thr than wild type (Table 2); however, there were too few cases to perform a regression analysis, and this did not reach statistical significance (P = .08). Results were similar when evaluating across the 3 genotypes (supplemental Table 1; supplemental Figure 1), and there was a significantly increased rate of placental intervillous thrombosis in those with Thr/Thr vs Thr/Ala (16% vs 0%; P = .04).
Discussion
This is a unique study in pregnancy, evaluating the impact of activating platelet receptor allele PAR4-Thr120 on response to aspirin in pregnancy and perinatal outcomes. We did not find a difference in initial response to aspirin in those homozygous for PAR4-Thr120 vs not; however, there may be a reduced response in the third trimester that we were underpowered to detect. There was no significant difference in rates of preterm birth or hypertensive disorders of pregnancy; however, there was a trend of increased intervillous thrombosis in high-risk pregnancies homozygous for PAR4-Thr120 even with daily low-dose aspirin. Placental intervillous thrombosis are associated with adverse perinatal outcomes12 and are derived from microthromboses of maternal origin,13 thus may be related to increased maternal platelet activation and PAR4 genotype.
Results in the context of what is known
Our hypothesis was based on a platelet aggregation study in which aspirin treatment preceding a challenge with a potent and specific PAR4 activator demonstrated increased platelet aggregation in those with Thr120 allele. In contrast to that study, this study evaluated platelet activity using the PFA-100 epinephrine closure time assay, which is routinely used to measure efficacy of aspirin treatment. Because the PFA-100 epinephrine assay does not directly assess PAR4 activity, it is possible that the impact of PAR4 genotype on platelet activation is not fully captured in this study. Our finding of improved thromboxane suppression in those without the Thr120 allele supports this fact. PAR4 activation is associated with thromboxane production,14,15 thus an activating genotype may mitigate the effect of aspirin. The trend toward reduced response in the third trimester is interesting. In the primary study, we noted an overall decrease in aspirin response in the third vs second trimester.5 Pregnancy-related changes with advancing gestation may result in increased platelet turnover and activation16 and thus reduced response to low-dose aspirin. It is possible that, with advancing gestation, the difference in platelet activation is further accentuated in those homozygous for PAR4-Thr120.
In contrast to our previous unselected delivery cohort, we did not find evidence of increased risk of preterm birth in those homozygous for Thr120. This study focused on high-risk pregnancies taking 81-mg aspirin daily. Our findings are certainly limited by sample size, but they highlight an important consideration: whether aspirin may mitigate the increased preterm birth risk noted in those homozygous for Thr120.
Finally, we found an increased rate of intervillous thrombosis in those homozygous for Thr120 in this population of high-risk pregnancies taking aspirin daily, at a similar rate to what we had previously identified in an unselected population.4 Placental intervillous thromboses are of maternal origin13; thus microthrombi in maternal circulation due to maternal platelet receptor genotype could certainly result in this finding. Placental intervillous thrombosis is associated with adverse perinatal outcomes, including fetal growth restriction and stillbirth.7,12 A previous study identified a lower rate of placental thrombotic vascular pathology in populations taking aspirin daily.17 PAR4 genotype may attenuate this benefit, and on a population level, this may result in increased perinatal complications in those homozygous for PAR4-Thr120, even with daily 81-mg aspirin use.
Implications for research
Our study has a number of implications for research. First, one primary limitation of this study is that we did not evaluate platelet aggregation with PAR4 specific agonists (thrombin) in pregnancies maintained on aspirin; thus our findings represent an indirect assessment of the potential impact of platelet receptor PAR4 genotype on response to aspirin in pregnancy. Additional studies on platelet aggregation, and perhaps specifically on variability in thromboxane suppression with aspirin in response to PAR4 stimulants in pregnancy, would be critical to identify the pathophysiology by which high-risk patients with this genotype may be at increased risk for adverse outcomes even with aspirin use. The similar perinatal outcomes we found in this population taking aspirin daily, in contrast to an earlier study in an unselected population, highlights the potential for PAR4 genotype itself to be a potential indication for aspirin use in pregnancy for preterm birth prevention; this would need to be studied prospectively. Finally, the persistent finding of increased placental vascular pathology highlights the important role PAR4 genotype may have in determining perinatal risk and outcomes, as well as the need for further study into the pathophysiology for PAR4 genotype and placental vascular pathology development.
Strengths and limitations
This study has a number of strengths. It is a unique evaluation of the impact of PAR4 genotype on aspirin response and perinatal outcomes specifically in a high-risk pregnant population. We evaluated translational and clinical outcomes. The cohort represents a diverse patient population and is representative of patients who are at increased risk for, and suffer from increased rates of, adverse perinatal outcomes, including preeclampsia and preterm birth. There are some important limitations to note. First, as previously noted, we did not directly evaluate platelet aggregation in response to PAR4 stimulation, as was done in a previous study in nonpregnant adults.3 Second, although the findings regarding variation in thromboxane suppression by genotype was notable, the sample size was limited (n = 18). Finally, the cohort of those homozygous for PAR4-Thr120 was limited (n = 19); thus, we are underpowered to evaluate clinical outcomes.
Conclusions
In a cohort of high-risk pregnancies, it did not appear that PAR4-Thr120 genotype significantly affected PFA-100 epinephrine closure time as measure of response to 81 mg aspirin daily, although genotype may contribute to attenuated thromboxane suppression in response to aspirin through gestation. Patients homozygous for PAR4-Thr120 had higher rate of prior preterm birth, a significant risk factor for subsequent poor perinatal outcome, but had similar pregnancy outcomes, including rates of preterm birth in the current pregnancy, suggesting daily aspirin may mitigate the increased risk of preterm birth in patients homozygous for PAR4-Thr120. In addition, PAR4-Thr120 homozygous genotype may be associated with higher rate of placental vascular pathology even with daily aspirin use. Our findings support the need for further study to evaluate the role of this genotype in aspirin response through gestation and placental vascular pathology in high-risk pregnancies.
Acknowledgments
This study was funded in part by March of Dimes grant 24-FY20-58; National Institutes of Health, Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) grant R21HD101127; and an AMAG Research Grant for Prematurity and Preeclampsia. Research reported in this publication was supported by the NICHD (award R21HD101127).
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, the March of Dimes, or AMAG.
Authorship
Contribution: R.C.B. conceptualized the study, contributed to methodology, conducted investigation, obtained grant funding, drafted the manuscript, and oversaw the study as a whole; J.V.M., A.T., and S.E.M. contributed to study design, conducting the protease-activated receptor 4 genotyping, data interpretation, and draft revisions; J.S.Y.C. and W.K.K. contributed to study design, interpretation of results, and draft revisions; T.Z. contributed to study design, statistical planning, and statistical analyses; and all authors approved the final version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Rupsa C. Boelig, Division of Maternal Fetal Medicine, Department of Obstetrics and Gynecology, Thomas Jefferson University, 833 Chestnut St, Philadelphia, PA 19107; email: rupsa.boelig@jefferson.edu.
References
Author notes
Presented as an oral presentation at the annual meeting of the American Society of Hematology, San Diego, CA, 7 December 2024.
Data may be requested from the corresponding author, Rupsa C. Boelig (rupsa.boelig@jefferson.edu), for research purposes and will be shared on completion of appropriate data sharing agreements.
The full-text version of this article contains a data supplement.

![Aspirin response by PAR4 genotype. Aspirin response as assessed by PAR4 genotype in pregnancy at PFA-100 epinephrine closure time for 106 (A) and 98 (B). (C) Urinary thromboxane (n = 17) with >75% aspirin adherence, visit follow-up 1, 2 to 4 weeks after initiation of 81 mg daily. (D) Urinary thromboxane for 16 patients with >75% aspirin adherence; visit follow-up 2, 28 to 32 weeks gestation. The generalized estimating equation model taking into consideration repeat measures and relevant covariates was not significant for PFA-100 but was significantly different for urinary thromboxane (geometric mean ratio, 2.08 [95% CI, 1.66-2.61]; P < .001).](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodvth/2/3/10.1016_j.bvth.2025.100085/2/m_bvth_vth-2025-000350-gr2.jpeg?Expires=1769153341&Signature=qnWRQ~UNyaDIY~2kMAZDUUkPQdht8ieUtUsjrQfhd2ZezJ1frYW-tqB2XxgqyDkDPE30xXie2FvzjfYriA7tEadPq6151xIcqyICI8ZZMbTGZnAO0JFjqNS32-mbJeuB1ycozrAM2P~l4pR9eWmj8z9aN4MWk5v3hDOFS7FP2Ua2SPv5kU-QLMBcBjQSK~drpzOzaMTDW9T7Oq976fLHJPpoWiaj5V5Ta-kzsBB8DUGh~Zb~zwyOih~fUci9lKp4ZYUCcK1iUFV7fkLXYdCEs1U2PNGMVU-LwIr8iuCeQnHb0~EH2VLJy-8a~HhGx9VSgBi-si5nkKLCqNc2UdDGsg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)