Abstract
After allogeneic stem cell transplantation, the establishment of the donor’s immune system in an antigenically distinct recipient confers a therapeutic graft-versus-malignancy effect, but also causes graft-versus-host disease (GVHD) and protracted immune dysfunction. In the last decade, a molecular-level description of alloimmune interactions and the process of immune recovery leading to tolerance has emerged. Here, new developments in understanding alloresponses, genetic factors that modify them, and strategies to control immune reconstitution are described.
In Section I, Dr. John Barrett and colleagues describe the cellular and molecular basis of the alloresponse and the mechanisms underlying the three major outcomes of engraftment, GVHD and the graft-versus-leukemia (GVL) effect. Increasing knowledge of leukemia-restricted antigens suggests ways to separate GVHD and GVL. Recent findings highlight a central role of hematopoietic-derived antigen-presenting cells in the initiation of GVHD and distinct properties of natural killer (NK) cell alloreactivity in engraftment and GVL that are of therapeutic importance. Finally, a detailed map of cellular immune recovery post-transplant is emerging which highlights the importance of post-thymic lymphocytes in determining outcome in the critical first few months following stem cell transplantation. Factors that modify immune reconstitution include immunosuppression, GVHD, the cytokine milieu and poorly-defined homeostatic mechanisms which encourage irregular T cell expansions driven by immunodominant T cell–antigen interactions.
In Section II, Prof. Anne Dickinson and colleagues describe genetic polymorphisms outside the human leukocyte antigen (HLA) system that determine the nature of immune reconstitution after allogeneic stem cell transplantation (SCT) and thereby affect transplant outcomethrough GVHD, GVL, and transplant-related mortality. Polymorphisms in cytokine gene promotors and other less characterized genes affect the cytokine milieu of the recipient and the immune reactivity of the donor. Some cytokine gene polymorphisms are significantly associated with transplant outcome. Other non-HLA genes strongly affecting alloresponses code for minor histocompatibility antigens (mHA). Differences between donor and recipient mHA cause GVHD or GVL reactions or graft rejection. Both cytokine gene polymorphisms (CGP) and mHA differences resulting on donor-recipient incompatibilities can be jointly assessed in the skin explant assay as a functional way to select the most suitable donor or the best transplant approach for the recipient.
In Section III, Dr. Nelson Chao describes non-pharmaceutical techniques to control immune reconstitution post-transplant. T cells stimulated by host alloantigens can be distinguished from resting T cells by the expression of a variety of activation markers (IL-2 receptor, FAS, CD69, CD71) and by an increased photosensitivity to rhodamine dyes. These differences form the basis for eliminating GVHD-reactive T cells in vitro while conserving GVL and anti-viral immunity. Other attempts to control immune reactions post-transplant include the insertion of suicide genes into the transplanted T cells for effective termination of GVHD reactions, the removal of CD62 ligand expressing cells, and the modulation of T cell reactivity by favoring Th2, Tc2 lymphocyte subset expansion. These technologies could eliminate GVHD while preserving T cell responses to leukemia and reactivating viruses.
I. The Alloimmune Response
A. John Barrett, MD,* Katayoun Rezvani, MD, and Scott Solomon, MD
Hematology Branch, National Heart, Lung, and Blood Institute, National Institutes of Health, 9000 Rockville Pike, Bldg. 10, Room 7C103, Bethesda MD 20892-0003
The Molecular and Cellular Basis of Alloimmune Responses
Alloimmunity is a complex process involving donor T cells and natural killer (NK) cells interacting with specific cells of the recipient. The immune response is mediated both by direct lymphocyte–target cell interaction and by cytokines.
T cells
The alloresponse segregates into induction, expansion, and effector phases. In the induction phase, donor CD8 and CD4 T cells interact with peptide antigens complexed with major histocompatibility complex (MHC) class I and II molecules, respectively, on antigen-presenting cells (APCs) of the recipient. The signal given by the MHC through the T-cell receptor (TCR) and CD3, CD4, or CD8 provides the first signal for T-cell activation. A full T-cell response, leading to proliferation of effector cells, requires a second signal delivered by interaction by costimulatory molecules such as CD80 and CD86 on the APCs and CD28 on the lymphocyte surface. During the expansion phase, T cells proliferate, particularly under the influence of growth factors interleukin (IL)-2 and IL-12. The milieu in which T cells expand determines whether they assume the characteristics of T helper (Th) or T cytotoxic (Tc) type 1, or Th/Tc type 2 cells, which have distinct pro- or antiinflammatory functional properties, respectively.1 In the effector phase, these alloactivated T cells interact with their cognate antigen on target cells of the host, causing cell damage by direct cytotoxicity from perforin and granzyme release or by production of inflammatory cytokines. In the context of allogeneic stem cell transplantation (SCT), these effector responses are involved in engraftment, graft-versus-host disease (GVHD), and graft-versus-leukemia (GVL) effects.
NK cells
Whereas T-cell alloresponses require priming and expansion, NK cell interaction with other cells gives a positive signal for the NK cell to immediately kill the target through perforin/granzyme release.2 NK cells are normally prevented from exerting such random cytotoxicity on healthy tissues by receiving a dominant negative signal through their killer immunoglobulin-like receptors (KIR) interacting with target cell MHC class I molecules. When they engage cells that have lost MHC class I or express a class I molecule not recognized as self (as may occur in HLA-mismatched transplants), the negative signal is not delivered and perforin/granzyme release follows.3 In recent years, diverse systems of inhibitory and facilitatory molecules on NK cells have been mapped, together with the rules that determine whether a class I molecule is seen as self or foreign. The fundamental differences between T-cell and NK-cell alloreactions are summarized in Table 1 . These distinct differences in T-cell and NK-cell behavior implicate T cells as the major effectors in GVHD, and NK cells as major effectors of GVL and engraftment in HLA-mismatched transplants.
Clinical Consequences of Alloreactions
Alloimmune reactions are responsible for 3 major transplant events that determine success or failure of the transplant engraftment, GVHD, and GVL effects. These events involve T cells, NK cells, and hematopoietic stem cells. Recent work better defines some of the mechanisms involved in these critical events and the nature of the targets of the alloimmune response.
Engraftment/rejection
The processes involved in engraftment of the donor’s hematopoietic and immune system are becoming increasingly well understood. Both donor and recipient T cells and NK cells and donor CD34+ stem cells are involved in engraftment. Significant immunosuppression of the recipient is needed to allow the graft to gain a foothold. In HLA-identical transplants, engraftment is the result of donor T cells mounting a successful alloresponse against residual recipient T cells, causing the elimination of the recipient immune system. The process is dynamically counterbalanced by destructive graft rejection mechanisms of residual recipient T cells and NK cells on the transplant.5 Purine analogs, such as fludarabine, are being used increasingly for their powerful and long-lasting immunosuppressive effect on recipient T cells, which buys time for incoming donor immune cells to predominate. Experience with highly immunosuppressive but nonmyeloablative fludarabine and cyclophosphamide transplant regimens illustrates the importance of T-cell engraftment in establishing long-term hematopoiesis. In the first few weeks after such transplants, 100% of the T cells are of donor origin, while recovering hematopoiesis is predominantly recipient. Weeks to months later, recipient hematopoiesis is replaced by that of the donor, following what is believed to be a graft-versus-marrow effect of the transplant. In contrast, recipients who do not achieve early donor T-cell engraftment are at risk for graft rejection from residual host immune cells.6
Although murine studies have demonstrated the importance of radioresistant host NK cells in resisting bone marrow engraftment, evidence that host NK cells mediate resistance in clinical SCT is limited. Both immunosuppressive conditioning regimens and large stem cell inoculums may override resistance mechanisms by host NK cells. NK cells from the donor (either contained in the allograft or newly derived from incoming CD34+ stem cells) may have an important role in promoting engraftment. When HLA mismatching leads to NK cell alloreactivity against the recipient, donor NK cells powerfully favor engraftment through their recognition and killing of residual recipient lymphocytes and hematopoietic cells.7
In recent years it has become clear that CD34+ stem cells influence engraftment in several ways. First, CD34+ cells and their immediate progeny can block residual host T-cell function through a “veto” effect, leading to apoptosis of T cells reactive to the host.8 Second, in T-cell-depleted mismatched transplants, donor CD34+ cells are the main source of the NK cells that enhance engraftment. Some experimental and human transplant data indicate that high CD34+ cell doses favor engraftment in both HLA-matched and HLA-mismatched transplants.8,9
GVHD
GVHD is primarily a T-cell-mediated event. The process begins when donor T cells accompanying the SCT encounter recipient antigens. The subsequent expansion of donor T-cell clones recognizing recipient antigens leads to tissue damage either directly through T cells encountering recipient MHC-bearing cells in target tissues or indirectly through cytokine production. Transplant conditioning regimens, especially radiation-based regimens, initiate a cascade of proinflammatory cytokine release, including tumor necrosis factor-alpha (TNF-α). This “cytokine storm” amplifies donor T-cell reactivity to alloantigens and promotes GVHD.10 The absence of a cytokine storm when donor lymphocytes are transfused at later time points after transplant may explain the milder nature of GVHD occurring after delayed T-cell addition. The critical first step in the initiation of GVHD is antigen presentation to donor T cells by recipient (not donor) APCs. The central importance of this direct antigen presentation mechanism was elucidated by Shlomchik and colleagues in a series of experiments. Class I (β2 microglobulin) knockout mice were first transplanted with marrow cells from a wild-type donor. After 2 months, when the mice had fully established an MHC class I expressing hematopoietic system, the mice were retransplanted with stem cells and spleen cells mismatched with the recipient.11 Although none of the recipient’s tissues expressed MHC class I, the mice developed severe GVHD. Reciprocal experiments in which transplants were carried out in wild-type mice previously grafted with class I knockout marrow caused no GVHD, confirming that antigen presentation by recipient hematopoietic cells is alone sufficient to initiate GVHD and that GVHD tissue damage does not require class I expression. Experiments using mismatched transplants into recipients where MHC class II differs between hematopoietic cells and other tissues indicate a similar importance for MHC class II expression on host APCs and CD4+ cell-mediated GVHD.12 While these findings highlight the importance of host APCs in the induction of GVHD, the effector arm of the process, which causes the extensive organ damage of GVHD, is more complicated. Tissue damage is multifactorial, involving cytokine release by alloreacting T cells, direct cytotoxicity of T cells against their tissue targets, and bystander effects by T cells attacking tissue APCs such as Langerhans’ cells. GVHD affects primarily the body integument—the skin, the gastrointestinal tract, and its outfolding into the biliary tree and exocrine glands, and also the bone marrow. These tissues are actively proliferating, facultatively express MHC class I and II, and contain hematopoietic-derived APCs. There is no clear explanation why GVHD does not damage the renal tract, nervous tissue, muscle, the vascular tree, or bone and cartilage. The pattern of lymphocyte homing may also restrict the tissue distribution of GVHD.13
Role of NK cells in GVHD
NK cells cannot initiate GVHD. Mouse transplants where the donor is H2-compatible with the recipient but can exert NK alloreactivity against the recipient do not result in GVHD. Furthermore, very large doses of incompatible NK cell clones can be administered to murine transplant recipients without any GVHD.14 Nevertheless, NK cells may add to target cell damage through GVHD-initiated upregulation of surface molecules that activate NK cells through their NKG2D ligand. Conversely, in HLA-mismatched SCT, donor NK cells may reduce GVHD by specifically targeting cells of hematopoietic origin (as defined by lymphocyte function-associated antigen-1 [LFA-1] expression). Recipient lymphocytes, myeloid cells, and APCs are destroyed within hours after transfusing alloreactive NK cells.7 Since APCs are needed for the initiation of GVHD, administration of allo-NK cells should result in less GVHD. Clinical data support this hypothesis: In haploidentical and unrelated SCT, GVHD in NK alloreactive donor-recipient pairs is actually lower than that encountered in the KIR-compatible setting.7,15,16
GVL/graft-versus-malignancy effects
The GVL effect is thought to be the main reason that allogeneic SCT for hematological malignancies results in lower relapse rates than autologous SCT, given identical conditioning regimens. The development of transplants using highly immunosuppressive but low-intensity (nonmyeloablative) conditioning regimens to treat hematological malignancies has largely borne out the supposition that the GVL effect has a curative potential at least as powerful as a myeloablative conditioning regimen.17 Well-defined tumor regressions following allogeneic SCT for renal cell, breast, and ovarian cancer have now extended the concept of the GVL effect to include a graft-versus-malignancy effect.18 Since most available data concern immune responses to hematological malignancy, this discussion will be restricted to GVL effects. The close association of GVHD with the GVL effect found in both experimental and clinical transplantation has led to the working supposition that T cells are the central mediators of the effect. Recently, however, alloreactive NK cells have also emerged as GVL effectors.
T cells and GVL
The mechanism of induction of a GVL response mirrors that of GVHD. Recipient malignant cells presenting antigens to the donor T cells induce leukemia-specific T-cell expansions. Such leukemia-specific T-cell lines have been used successfully to treat leukemia by adoptive transfer.19 A question is whether direct antigen presentation by the malignant cell is the only mechanism whereby the donor can mount an antimalignancy response. This is an important point, because apart from some myeloid leukemias, whose dendritic cells (DC) are part of the leukemic clone,20 leukemia cells can cause apoptosis in the responding T cell if they lack the costimulatory molecules that deliver the second signal.21 While some animal models have demonstrated that indirect antigen presentation can induce T-cell responses, there is no direct evidence in human SCT that donor or host DCs play a part in presenting leukemia antigens to donor T cells. The effector arm of GVL is incompletely understood. In chronic myeloid leukemia (CML), there is evidence that both CD4+ and CD8+ T cells are involved in the GVL effect: both CD4+ and CD8+ cytotoxic T lymphocytes (CTL), inhibitory to leukemic colony formation, can be generated in vitro, and CD8+-depleted SCT appear to retain GVL activity.22,23 The strong GVL effect and ease of measuring residual disease after transplant has made CML the best-studied GVL model in humans. Unfortunately, other malignancies tend to be less susceptible to GVL effects. Two factors appear to determine GVL efficacy: the proliferation rate of the disease (with slowly growing malignancies responding best) and the phenotype (acute lymphocytic leukemia [ALL] being least sensitive). The fact that there are great differences in the susceptibility of hematological malignancies to GVL probably indicates diversity both in the ability to stimulate antileukemia T cells and in target cell resistance to T-cell damage. Studies of donor lymphocyte transfusion for relapsed CML show that time taken to achieve molecular remission can vary from weeks to months, with a median around 3 months.24 The ability to track leukemia antigen-specific T-cell frequencies in vivo using HLA tetramers has further elucidated the kinetics of the GVL response: after donor leukocyte infusion (DLI), there is a variable period before a sharp but short-lived emergence of antigen-specific CD8 T cells that reach frequencies as high as 10% of the circulating T-cell repertoire coincident with leukemic regression. This surge is followed by a decline of leukemia-specific T cells to a higher-than-original baseline.25
NK cells and GVL
Since hematopoietic cells are highly susceptible targets of NK cell attack, it is no surprise that when NK cells interact with KIR-incompatible leukemic cell targets, they exhibit strong cytotoxicity not observed with HLA-matched or autologous leukemic targets. The clinical importance of NK mismatching has been highlighted by the finding of extremely low relapse rates in AML recipients of haploidentical or unrelated T-cell-depleted KIR-mismatched SCT. However, the effect appears to be limited to myeloid leukemias and to T-cell-depleted transplants (Table 2 ).
Antigens driving donor T-cell responses
Both MHC class I and II molecules participate in presenting cellular peptide antigens to the donor T cells. Antigens eliciting T-cell responses in donor cells can be classified as (1) minor histocompatibilty antigens that can be either tissue restricted or widely distributed on many tissues, (2) normal (nonalleleic) protein sequences overexpressed or aberrantly expressed in malignant cells, or (3) antigens representing a unique tumor-specific peptide sequence.27,28 It should also be remembered that donor T cells contain a repertoire of memory cells responding to antigens of herpes group viruses (cytomegalovirus [CMV], Epstein Barr virus [EBV], herpes simplex virus [HSV], varicella zoster virus [VZV]) resident in the recipient (Table 3 ). Depending on their specificity, host antigens can elicit GVHD, GVL, or both. There is accumulating evidence linking T-cell responses to defined antigens with clinical events. Initially, in studies of transplant patients, Goulmy and colleagues found a statistical association between donor and recipient minor antigens HA-1 (human-antigen-1) and HY (Human Y chromosome antigen) incompatibility and the development of acute GVHD.29 Furthermore, GVHD was associated with rises in tetramer-positive cells specific for HA-1 or HY.30 In skin explant cultures, the histological changes of GVHD could be generated using minor antigen– specific T-cell clones in the assay (see Clinical consequences of alloreactions [above]).31
Immune Reconstitution
The establishment of the donor immune system in the recipient takes months to years to complete. It initially involves the expansion of a postthymic donor T-cell repertoire showing many unusual phenotypic and functional features. Normalization of the immune system in the recipient requires the emergence of tolerized T cells processed from precursors through the recipient thymus. This event is delayed and may be incomplete in older recipients. NK cell numbers promptly recover after SCT but also show unusual phenotypic and functional features.
Postthymic donor T-cell expansion
In the first few months following bone marrow or blood SCT, the immune repertoire is dominated by T cells expanding from transplanted T cells derived from the donor’s peripheral blood T-cell compartment.32,33 This consists predominantly of central and effector memory cells with a smaller population of naïve T cells and end-stage effector cells.34 It is these postthymic cells that are largely responsible for the success or failure of the transplant through their impact on engraftment, GVHD, GVL, and reactivating viruses. In thinking about alloreactivity, one must remember that the donor’s postthymic T-cell repertoire has already been shaped by a process of attrition of immature T cells in the thymus, such that T cells of high affinity to self-antigen are depleted by “suicide” (antigen-induced apoptosis), while those encountering low-affinity antigens are lost by “neglect” (lack of stimulation).35 In the recipient, this mature repertoire encounters a new antigenic environment through host APCs. Reactivating viruses such as CMV and EBV elicit new clonal expansions from virus-specific central memory cells. HLA tetramer analysis reveals massive T-cell expansions responding to viruses such as CMV and EBV, minor antigens such as HA-1 and HA-2, and antigens overexpressed by leukemia cells such as PR1, BCR-ABL, and Wilms tumor-1 (WT1).36,37 The early posttransplant period is characterized by these massive and uneven clonal T-cell expansions that produce a “skewed” repertoire of TCR Vβ families.33,38 New techniques have enabled us to study donor T-cell alloresponses to recipient stimulation in vitro. TCR CDR3 regions of donor T-cell clones stimulated in vitro by host lymphocytes can be cloned into bacteria and identified by their unique TCR sequence. Primers made from these TCR sequences are used subsequently to identify and follow in vivo alloreacting T-cell clones posttransplant. These studies have revealed huge expansions of T cells occupying over 90% of the T-cell repertoire in a patient dying from GVHD and have shown that leukemia cells can elicit T-cell clones that are distinct from GVHD reacting T cells, thus providing molecular proof that GVL and GVHD can be separated.39,40 Thus, the irregular T-cell expansion posttransplant is a two-edged sword, responsible for uncontrollable GVHD reactions but also establishing powerful immune responses to malignant cells and reactivating viruses.
What drives T-cell expansion?
The profoundly lymphopenic environment immediately posttransplant provides a favorable milieu for rapid and extensive lymphocyte expansion.41 Direct evidence for a lymphoproliferative stimulus from lymphopenia comes from a recent study by Rosenberg and colleagues, who described massive expansions of in vitro selected autologous antitumor T-cell clones in cancer patients who had been rendered severely lymphopenic with fludarabine and cyclophosphamide.42 This lymphopenic drive coupled with a strong antigenic stimulus explains the inhomogeneous clonal expansions seen after allografts. Such T-cell–antigen interactions are described as immunodominant. Perreault and colleagues have extensively studied the phenomenon of immunodominance in mouse transplant models. They ascribe the process to a high-affinity T-cell–antigen interaction.27 Recent work in our laboratory links strong posttransplant T-cell expansions of PR1 antigen-specific cells to detectable frequencies of memory T cells in the donor.37 Thus, the most powerful immune responses posttransplant may involve antigen-experienced donor T cells. Unchecked, these alloresponses may reach exotic proportions in association with GVHD or GVL reactions.
Thymic tolerance and normalization of the T-cell repertoire
The final phase of immune recovery involves the emergence of a new T-cell repertoire generated from donor prethymic precursors. These cells, processed by recipient thymic tissue, are tolerant of the allo-environment. A new technique tracks recent thymic emigrants by quantitating TCR excision circles (TRECs) in the T-cell population. These circular DNA residues of TCR recombination are evidence of TCR rearrangement, a process that is unique to the thymic stage of T-cell development. As lymphocytes enter the peripheral circulation and undergo postthymic expansion, TRECs become diluted in each cell division. TREC analysis after SCT reveals distinct age differences in the capacity of the host thymus to “educate” donor prethymic precursors. Children and young adults have functioning thymi as evidenced by increasing TREC levels and regeneration of a new T-cell repertoire within 1 to 2 years of transplant. Older adults have significantly lower TREC levels and may never recover full thymic function; instead, immune competence continues to be partly derived from the postthymic T-cell compartment.43
Factors modulating T-cell recovery.
Factors influencing immune recovery are listed in Table 4 . Notably, certain T-cell functional subtypes have emerged as being of potential importance. The differentiation of T cells into mature effectors is regulated by the prevailing cytokine environment. In animal and human experiments, the flavor of the T-cell response can be biased by the presence of IL-4, which favors a T helper 2 and a T cytotoxic 2 subtype, or IL-12, which stimulates more cytotoxic Th1 and Tc1 T-cell subtypes.1 Animal experiments indicate that Th1 and Tc1 T cells are more prone to cause GVHD than the Th2 subset. However, Th2 cells are biased toward causing cGVHD. Another recently described subtype is the CD4/CD25 regulatory T cell. These cells are derived from the thymus. They regulate T-cell proliferation and expansion to antigen and can prevent or control GVHD in animal models.44,45
NK cell recovery.
In the first few weeks after SCT, there is a massive expansion of NK cells.46 In the HLA-identical setting, recovering NK cells express an immature phenotype with low levels of CD16 and KIR molecules and high expression of the inhibitory receptor CD94:NKG2A. After about 6 months, NK cells begin to express KIR in a faithful copy of the donor’s original NK cell repertoire.47 Following HLA haplotype-mismatched SCT, alloreactive NK clones appear shortly after engraftment but are no longer detectable by 4 months. These NK clones express KIR and can recognize and kill KIR-incompatible targets. NK recovery has been associated with a powerful GVL effect in HLA-mismatched SCT, but it has been difficult to identify a role of NK cells in HLA-identical sibling transplants, although clinical observations suggest that NK cytotoxicity does correlate with relapse in CML patients.48
Clinical Implications
A more detailed understanding of alloimmunity has brought with it an increased ability to control posttransplant immune events. Conditioning regimens are now designed with 2 specific purposes: (1) immunoablation of the recipient, and (2) variable additional intensification to control malignancy. The introduction of peripheral blood–mobilized SCT together with sophisticated cell separation techniques allows the transplanter a wide choice in the dose of stem cells and lymphocytes selected for their graft- and immunity-enhancing effects. Furthermore, techniques are being developed to prevent GVHD while conserving useful immunity (discussed in Antigens driving donor T-cell responses [above]). Protective T-cell responses to CMV and EBV antigens are well characterized, making it possible to track the performance of antiviral immunity with peptide, protein, or dendritic cell vaccines or by adoptive transfer of antigen-specific T cells. The manipulation of viral immune function serves as a model for boosting leukemia-specific immunotherapy posttransplant, using a widening range of leukemia-specific antigens. With suitable techniques in place to selectively eliminate GVHD, immune recovery could be safely enhanced by removal of immunosuppression and administration of IL-7 and other lymphopoietic cytokines.49 The availability of immunological methods to measure antigen-specific T cells and functional T-cell subsets should help us track the complicated process of immune recovery in individual patients and make informed interventions with T-cell transfer or immunological boosts to improve results. With the technologies now available, the next few years should bring significant improvements in the field of allotransplantation, allowing us to safely extend allogeneic SCT to a wide range of malignant diseases in a broader age group of individuals receiving matched or mismatched transplants.
II. Beyond HLA Typing: Genetic Polymorphisms Predicting Transplant Outcome
Anne M. Dickinson, PhD,* Xiao N. Wang, PhD, Gail Stark, MD, Hannah Cullup, PhD, Mark Jarvis, BSc, and Peter G. Middleton, PhD
Hematological Sciences School at Clinical and Laboratory Sciences, The Medical School, University of Newcastle upon Tyne, Claremont Road, Newcastle upon Tyne NE1 4LP, UK Acknowledgments: This work was supported by grants from the Tyneside Leukaemia Research Association, the Leukaemia Research Fund, and the European Commission Contract Nos. QLRT-2000-00010 (EUROBANK) and QLK3-CT-2002-01936 (TRANSEUROPE). The authors are indebted to the clinical transplant team at the Royal Victoria Infirmary, Newcastle upon Tyne, for invaluable help with these studies.
Acknowledgments: This work was supported by grants from the Tyneside Leukaemia Research Association, the Leukaemia Research Fund, and the European Commission Contract Nos. QLRT-2000-00010 (EUROBANK) and QLK3-CT-2002-01936 (TRANSEUROPE).
The authors are indebted to the clinical transplant team at the Royal Victoria Infirmary, Newcastle upon Tyne, for invaluable help with these studies.
Non-HLA Immunogenetics and Transplant Outcome
While HLA typing remains the central means of selecting donors and determining SCT outcome, the sequencing of the human genome has brought to light myriads of single nucleotide polymorphisms (SNPs) whose significance in determining an individual’s immunological phenotype and their possible role in influencing the outcome of an allogeneic SCT is only just beginning to be explored. Here we discuss the emerging field of non-HLA immunogenetics and the patient and or donor gene polymorphisms which may influence transplant outcome, including occurrence and severity of GVHD and survival. These inherited variants can be conveniently grouped as polymorphisms of cytokine genes, genes of uncertain function, and minor histocompatibility antigens.
Cytokine Gene Polymorphisms
Cytokine gene polymorphisms occurring within the 5′ or 3′ regulatory sequences of genes may alter the structure of the transcription factor binding sites within gene promoters and therefore alter the amount of cytokine produced, for example, upon allogeneic stimulation or infection. Many of the reported cytokine gene polymorphisms occur within apparent regulatory regions of the gene.1,2 Polymorphism within, or adjacent to, regulatory regions is quite common in cytokine genes, while coding region polymorphism and variation in protein structure has not been widely reported. Within normal populations, therefore, high or low producers of cytokines naturally exist due to the inherited gene polymorphisms controlling such production. Initial studies within the solid organ transplant setting3 demonstrated that patients with particular regulatory cytokine gene polymorphisms for TNF (high producers) and IL-10 (low producers) were more likely to reject their solid organ graft. It was postulated that TNF high producer genotypes gave rise to an increased graft rejection response which could not be adequately down-regulated in patients with the low producer IL-10 genotype. These studies were extended to the HLA matched sibling allogeneic transplant setting and patients who had high TNF producer and low IL-10 producer genotypes were at greater risk of GVHD.4 A number of cytokine gene polymorphisms have now been associated with GVHD and/or transplant outcome (see Tables 5 and 6 ). The majority of the studies have been carried out in single center HLA matched sibling cohorts and these studies will be summarized, together with results from matched unrelated donor (MUD) transplant cohorts where applicable.
Role of Cytokines and Cytokine Gene Polymorphisms in GVHD and Posttransplant Complications
Even in patients undergoing optimal prophylaxis, acute GVHD incidence following allogeneic SCT between HLA identical siblings is 30–80% and can be fatal in up to 50% of cases.5–,8 GVHD post-transplant is induced by release of proinflammatory cytokines IL-1, IL-6, IL-8, TNFα during the “cytokine storm” following radiation and cytotoxic chemotherapy conditioning regimens. This initial cytokine release is amplified by the activation of transplanted donor T cells, reacting to recipient cells upregulating HLA and adhesion molecules.9–,11 Further recipient tissue damage then ensues from activated T cells and NK cells and release of predominantly Th1 type cytokines (IL-2, interferon gamma [IFN-γ], TNFα).8,12,13 Although T cells are central to the initiation of GVHD, it is important to observe that proinflammatory mediators such as IL-1 and TNFα are capable alone of inducing the pathological changes of GVHD. There is growing evidence that inflammatory cytokines are also involved in transplant outcomes other than GVHD and can therefore affect transplant-related mortality in multiple ways: Patients characterized by high spontaneous IL-10 production (an antagonist of TNFα) were protected from GVHD as well as interstitial pneumonitis (IP) and veno-occlusive disease (VOD).14 Analysis of these results suggested a genetic background was associated with protection by high IL-10 production.14–,17 More recent work (described below) has begun to identify an impact of gene polymorphisms on the susceptibility to infection posttransplant.18 Taken together, it is now evident that the genetic make up of the recipient and the donor can strongly influence the success or failure of the procedure.
The cytokine gene polymorphisms studied for association with outcome in allogeneic stem cell transplantation, to date, are summarized in Tables 5 and 6 and include TNFα, IL-10, IL-6, IFN-γ, IL-1 and transforming growth factor beta (TGFβ). As described below in all studies, both the patient and donor genotype is analyzed with respect to transplant outcome.
TNFα gene
The TNFα gene maps within the class III region of the MHC, a region containing lymphotoxin α and β (LTA and LTB). In the HLA matched sibling transplant setting, since TNFα maps close to the HLA region, both patient and donor polymorphism genotype will be identical and may equally or additively affect TNF production and transplant outcome.
Initial studies suggested a role of TNFα−308 in HLA-matched sibling transplants based on TNFα secretion studies, but this was not confirmed in larger cohorts.19 A recent Japanese study involving MUD transplants described an association of the TNF-863 (C/A) polymorphism and −857 (C/T) polymorphism in donors and/or recipients resulting in a higher incidence of GVHD grade III-IV and a lower rate of relapse.20 However, when the effect of HLA linkage disequilibrium with the TNFα gene was taken into account by restricting analysis to HLA-A, -B and -DRβ1 allele matched pairs, only recipient genotype affected GVHD outcome and no significant effect on relapse was observed.
The commonest allele at the TNFd microstellite, TNFd3, associates with higher in vitro TNFα production and cardiac transplant recipients homozygous for d3 (TNFd3/d3) are more susceptible to increased rejection episodes.3 Our initial studies showed an association with recipient TNFd3 homozygosity and increased severity of acute GVHD (grade III-IV) in cyclosporine-alone treated HLA-matched sibling transplants.4 A larger cohort of HLA-matched sibling transplants receiving cyclosporine plus methotrexate prophylaxis demonstrated an association of recipient TNFd/d3 genotype with increased mortality.
TNF receptor polymorphisms have been associated with both systemic lupus erythematosus (SLE21) and BMT outcome.20,22
In 462 cases of matched unrelated bone marrow transplantation, recipients of TNFRII-196R-positive donors had a higher incidence of severe GVHD and a lower rate of relapse than from TNFRII 196M homozygous donors.20 In our study of HLA matched sibling transplants, TNFRII 196RR genotype in the transplant donor was associated with increased incidence of extensive chronic GVHD.22 Although the exact role of the polymorphism on function is not known, TNFRII receptors stimulate T-cell proliferation and alloimmune responses. The TNFRII-196R may therefore increase immune responsiveness compared to the TNFRII-196M allele.
IL-10 gene
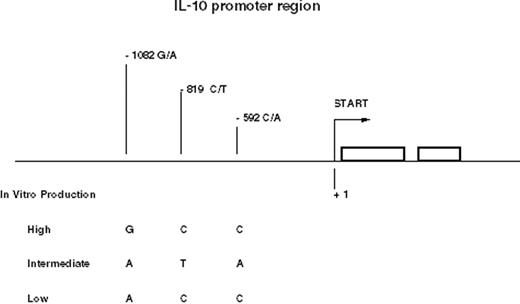
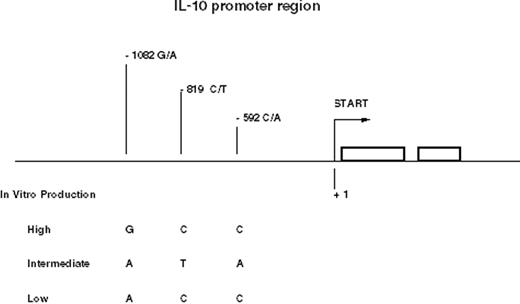
The single nucleotide polymorphisms (SNPs) and microsatellites of the IL-10 gene resolve into several conserved haplotypes. Three common haplotypes of the promoter region between -1082 and -592 represent high, intermediate, and low producer alleles of IL-10 and form the basis of most studies23,24 (Figure 1 ).
IL-6 gene
IFNγ gene
The first intron of the IFNγ gene possesses a microsatellite with a variable number of CA repeats giving rise to 6 alleles. Allele 2 of the microsatellite has been associated with high in vitro IFN-γ production, whereas allele 3 has been linked to lower IFN-γ production by stimulated peripheral blood mononuclear cells. From in vivo IFN-γ knockout mouse experiments, lack of IFN-γ can lead to accelerated acute GVHD. In HLA-matched sibling transplant recipients on cyclosporine monotherapy for GVHD prophylaxis possession of the IFNγ intron-1 allele 3 genotype is associated with development of acute GVHD.25
IL-1 gene family
The IL-1 gene family consists of 10 genes, of which 3 encode for the proteins IL-1α, IL-1β, IL-1Ra (IL-1 receptor antagonist). Initial studies in HLA-matched siblings transplant demonstrated an association of the IL-Ra VNTR (intron 2) where possession of the allele 2 in the donor genotype was associated with less severe acute GVHD, and in the recipient genotype with chronic GVHD.28 This has since been expanded to a European multicentre study and independently reported by another single centre study.18 Our group has recently described an association between carriage of allele 2 in either the VNTR or -889 polymorphisms and GVHD.29 A study of unrelated donor transplants in a heterogeneous pediatric recipient group found that IL-1α-889 in either donor or recipient was associated with improved survival and decreased transplant related mortality, but not with GVHD.30
TGFβ gene
Polymorphism of the TGFβ gene includes promoter region polymorphisms −509 (C/T) and −800 which are associated with variation in plasma concentration of TGFβ (C allele at −509 associated with higher TGFβ production), and amino acid substitutions at codons 10 (leu → pro) and 25 (arg → pro), which alter the structure of the protein.31 A small study on HLA-identical sibling BMT showed no association of the TGFβ −509 polymorphism with either GVHD or outcome.25 However a study in 67 pediatric patients showed an association of TGF-β1 codon leucine/proline polymorphism in the donor genotype with development of acute GvHD. TGF-β1 receptor II polymorphism (1167 C/T) genotype in the recipients in the same study was also associated with the development of GVHD.32
Minor Histocompatibility Antigens and Transplant Outcome
GVHD occurring after HLA matched sibling transplants is initiated by T cells recognizing minor histocompatibility antigens (mHags) (presented by HLA molecules). mHags are polymorphic proteins that differ between patient and donor.33 T cell clones recognizing mHags were initially isolated after BMT in patients developing GVHD or graft rejection.34 Human mHags are peptides derived from intracellular proteins of restricted polymorphisms coded by autosomal or Y chromosome genes (Table 7 ). The male specific mHags encoded by the Y chromosome have been shown to be involved in HLA-matched sex mismatched hematopoietic stem cell transplantation.35,36 The first H-Y gene identified which encoded HLA-B7– and HLA-A2–restricted mHags was SMCY.37,38 A number of other mHags have now been identified. The HLA-A2 restricted mHag HA-1 is a peptide derived from the KIAA0223 gene and the HA-2 from a gene from the class 1 myosin family.39,40 The tissue expression of some mHag (e.g., HA-1 and HA-2) is limited to the hematopoietic system whereas other mHags (e.g., H-Y, HA-3) are ubiquitously expressed on normal tissues.41 The identified mHags derived from polymorphic proteins contain 1–4 amino acid differences compared to their homologous counterparts. Thus in the case of mHag HA-1 a mismatch for HA-1 between patient and donor is due to a histidine/arginine substitution difference between patient and donor. The HLA-1H (Histidine) peptide is antigenic and presentation at the cell surface leads to induction of HLA-A2 restricted CTCs.34,42Table 7 lists recently described mHag, some of which have been associated with clinical transplantation. Mismatches between patient and donor for HA-1, HA-2, 4, and 5 are associated with increased GVHD incidence.33 The precise characterization of the peptide sequence of hematopoietic tissue–restricted or cancer cell–restricted mHa make them ideal targets for immunotherapy.43 The in vitro skin explant assay has been used to predict the ability of mHa specific clones to cause GVHD.44 H-Y-specific T-cell clones cultured within skin sections of male volunteers caused grade III-IV GVHD, whereas HA-1 T-cell clones induced only minimal changes (Figure 2; see Appendix, page618) supporting the clinical use of these latter clones to specifically target hematological malignancies. This approach has given rise to induction of complete remission of relapsed leukemia in 3 patients using antigen-specific donor lymphocyte infusions.45 More recently, HA-1 has been shown to be expressed on epithelial cancer cells and not on normal epithelial cells giving rise to the concept of the use of mHa-specific T cells in cancer therapies.46
CD 31 Polymorphisms—Potential Antigenic and Functional Diversity
The CD31 molecule (platelet–endothelial cell adhesion molecule–1 [PECAM-1]) mediates cell-cell adhesion via either homophilic or heterophilic ligation with other surface molecules. Common polymorphisms of the CD31 gene include amino acid substitutions in exon 3 (codon 125), exon 8 (codon 563) and exon 12 (codon 670). As the different alleles represent different peptide sequences, CD31 has been proposed as a mHa,47 but donor-recipient matching for CD31 alleles has not been reproducibly predictive for GVHD.48 Curiously, the structural variation in CD31 may also confer differences in the adhesive and proliferative behavior of cells expressing different CD31 alleles which could affect GVHD occurrence.49,50 CD31 may therefore exhibit allelic differences which serve both as functional variants and as mHa.
Other Non-HLA-Encoded Genes Implicated in Transplant-Related Complications
Other non-HLA encoded genes have been recently implicated not only in GVHD but also in transplant-related mortality (TRM), infectious episodes and outcome in HLA-matched sibling transplants. The steroid hormone receptor supergene family includes the estrogen receptor and the vitamin D receptor (VDR). Vitamin D and estrogen have marked effects on the development of the immune system and variations in VDR are associated with a wide range of autoimmune and immune dysfunctional diseases. Furthermore, vitamin D analogues can prolong graft survival and prevent GVHD in animal studies.51,52 Polymorphism in the VDR intron 8 region53 and in intron 1 of the estrogen receptor alpha (ERα)54 genes have been associated with both occurrence of GVHD and likelihood of survival following allo-BMT. In HLA-matched sibling BMT53 the Apo1 “a” VDR allele in the patient was associated with severe acute GVHD, but with improved survival if present in the donor. Patients receiving marrow from donors with AA genotype of the VDR gene had an increased rate of infection and relapse.
Non-HLA genes associated with risk of infections and transplant-related mortality
A recent multivariate analysis18 of gene polymorphisms for Fcγ receptors, mannose binding lectin and myeloperoxidase (MPO) showed that first infections of any type posttransplant were increased in patients with the FcγRIIa R-131 genotype while severe infections increased when the MPO donor genotype was AG or AA. TRM was influenced by FcγRIIIb genotype and donor MPO genotype. In this study and those of Mullighan et al,55 donor and recipient gene polymorphisms that regulate the host response to micro-organisms were associated with infections after bone marrow transplant. Rocha et al18 studied 107 donor /recipient DNA pairs for gene polymorphisms of cytokines, adhesion molecules (CD31 and CD54), Fcγ receptors (FcγRIIa, IIIa, IIIb), mannose-binding lectin (MBL) and MPO. First infections were increased in patients with the FCγRIIa genotype and severe bacterial infections were increased when the MPO genotype was AG or AA. Recipient IL-10 genotype and IL-1Ra genotype was associated with chronic GVHD. Six-month transplant-related mortality was influenced by donor FcγRIIIb and MPO genotype. In the study reported by Mullighan et al,55 the MBL gene polymorphism of either donor or recipient was associated with risk of major infection posttransplant (see Table 8 ).55 These studies define further and improve understanding of the mechanisms involved in host defense against infection during BMT.
Association of CGP with transplant outcome—problems of interpretation
The cytokine gene polymorphisms examined in our initial studies were selected on the basis of their association with altered cytokine production in vivo or their demonstrated association with transplant-related pathology or their association with immune dysfunction/autoimmune diseases. Since our first report of associations between GVHD and TNFα and IL-10 polymorphisms in HLA-matched sibling transplants,4 subsequent studies have incorporated a wider array of cytokine genes. Typically, alleles linked with increased expression of pro-inflammatory cytokines such as TNFα, IL-6, and IFN-γ show associations with complications such as GVHD, whereas alleles associating with increased expression of non-inflammatory properties such as IL-10 and IL-1Ra show protection effects (see Table 5 ). However, as more studies are reported this model appears too simple to account for all the findings.
Several problems surround the interpretation of the role of cytokine variations in transplant outcome:
HLA association:
In HLA matched sibling transplants HLA-A3 is associated with a higher and DR1 with a lower risk of GVHD.56 These associations may represent HLA types best able to present specific antigens relevant to disease processes. Alternatively, the effect on GVHD may reflect class III region TNF polymorphisms forming an extended haplotype with the HLA class I and class II region. Determining what precisely occurs at this locus is therefore complex. In the MUD transplant setting the role of HLA will be of paramount importance in interpretation of non-HLA immunogenetic data. High resolution tissue typing and standardization of results will be necessary across BMT centres for comparative studies. GVHD increases proportionally with the degree of HLA disparity between patient and donor, including Class I HLA-A -B and -C and Class II HLA-DP, -DQ and -DR. Mismatching for HLA-C alleles increases the risk of graft failure, GVHD and mortality.57 Disparities between HLA sequence polymorphisms, detected by serology, are termed antigen mismatches; those identified only by DNA-based typing are termed allele mismatches. The risk of mortality after MUD transplants is increased by mismatches at a single allele at HLA-A, -B, -C or -DRBI, with mismatching for antigen rather than the allele increasing the risk.58,59 Any of these types of mismatches must therefore be taken into account prior to further analysis of the role of non-HLA immunogenetics in the MUD transplant setting.
Population differences in gene frequencies:
In vivo disease association may not necessarily correlate with in vitro cytokine expression or production. This could be due, for example, to the fact that genetic differences between populations can also give rise to differences in in vivo or in vitro cytokine production because of genetic variation in cytokine/chemokine gene polymorphisms. These differences, therefore, make comparisons between populations difficult unless local population allele frequency is simultaneously assessed. For example, IL-10 GCC haplotype in Japan is of a very low frequency compared to Europe.60
Different transplant procedures:
Analysis of the complex data emerging on the role of non-HLA immunogenetics on stem cell transplant outcome is compounded by a number of factors including the heterogeneity of the diagnoses and clinical state of the recipients, GVHD prophylaxis regimens, and conditioning strategies. Most of the research has been carried out within the HLA-matched sibling setting. Larger studies are now needed together with multivariate analysis in both HLA-matched siblings and matched unrelated donor transplants.
Future Developments
Skin explant assays are currently used in comparative studies of genotype and cytokine function. Comparison of results from the skin explant model with serial clinical samples and clinical outcomes may also provide further insight into the pathophysiology of GVHD, and identify new predictive factors. Tissue expression of IL-1α, IL-1β, IL-1Ra, IL-6, and heat shock proteins (hsp), measured in skin explants, correlates with graft-versus-host reaction (GVHR) grade. Blocking IL-1Ra activity in the skin explant MLR supernatants for example, results in an increase in GVHR severity.61 Furthermore in the skin explant model, inducible hsp70 expression in the skin directly correlated with GVHD severity and hsp70 was increased in biopsies from GVHD lesions.62
Comparative studies of the association of hsp70 polymorphism with GVHD occurrence are currently under way. These preliminary results suggest a functional role for IL-1Ra and hsp70 in this human in vitro GvHD model.
Clinical studies—developing a risk score and extending the predictive power of genetic polymorphisms:
Large cohorts of HLA-matched and MUD transplants need to be studied to fully investigate the impact of non-HLA immunogenetics on transplant related complications. Combined analysis of TNFd3/d3, IL-10, IFN-γ (intron 1;(3,3), IL-6−174GG, suggest that a risk index for GVHD could be developed.63 This risk analysis would include risk factors such as gender, age, CMV status, and minor histocompatibility mismatches. Risk prediction could aid the tailored prophylaxis and therapy for GVHD. To do this analysis, multifactorial statistical models have been developed.64– 67
In a European cohort of over 200 HLA-matched transplant pairs, a cyclosporine-alone cohort was compared with a prophylaxis regimen that included methotrexate plus cyclosporine and/or Campath or ATG. In multivariant analysis, cytokine gene polymorphisms of recipient and donor including those of IFN-γ, IL-10, and IL-1Ra were significantly associated with acute GVHD in the cyclosporine alone cohort. In the increased prophylaxis cohort cytokine gene polymorphisms of IL-6 and IL-1Ra were significantly associated with GVHD, with IL-6 and IL-10 also associating with chronic GVHD indicating that T-cell depletion strategies obliterate the effect of certain functional genotypes on BMT outcome. Overall, these studies indicate that both patient and donor genotype influence transplant outcome. A study on 87 CML patients transplanted at the same center using the same protocols found in addition to the associations with GVHD an effect on survival, allowing a simple model of survival prediction to be constructed.68 A multicenter European study in CML transplants found that survival was linked to TNFα and IFN-γ genotypes.69
III. Non-Pharmacological Approaches to P reventing Graft-Versus-Host Disease
Nelson J. Chao, MD*
University, 2400 Pratt Street, Suite 1100, Durham NC 27705-3976
Significant progress has been made in decreasing the morbidity and mortality associated with allogeneic SCT. Supportive care has improved over the past decade, notably with better preemptive use of antimicrobials, use of growth factors, and the introduction of peripheral blood SCT. The spectrum of immunosuppressive agents used to prevent and treat GVHD includes drugs with different modes of action, antilymphocyte globulin, and monoclonal antibodies against TNF, CD52, and CD25. Despite these innovations, GVHD remains an important problem following SCT, and steroids are still the most effective treatment. GVHD is in itself life-threatening, and the immunosuppression required to treat or prevent it also leads to serious complications from infection and an increased risk of leukemic relapse. Transplanters have therefore sought to develop techniques to prevent GVHD that avoid the complications incurred by immunosuppression. For this purpose, T-cell depletion was introduced more than 20 years ago. Three to four log depletion of grafted T cells almost completely eliminates GVHD. However, enthusiasm for this procedure was soon tempered by the finding of a significant increase in rejection, relapse, and infectious complications from delayed immune recovery.1 Thus, while T-cell depletion prevented death from GVHD, the approach failed, in particular because the beneficial GVL effect was compromised. At best, disease-free survivals after T-cell depletion are comparable but not clearly superior to those achieved with non T-cell depleted transplants. Clinical data suggest that GVHD and GVL are intricately related: development of GVHD is associated with a lower risk of relapse. Nevertheless, leukemia cure in the absence of GVHD can occur, notably after carefully dosed donor lymphocyte infusions to treat leukemia relapse, supporting animal experiments and in vitro data indicating that there is a basis for separating the two phenomena. The challenge is now to find clinically feasible methods to prevent GVHD while exploiting differences between GVH and other immune reactions so as to retain GVL effects and cell mediated immunity against infectious agents.2,3
Basis of Separating GVHD from Favorable Donor Immune Responses
Improved understanding of the antigenic basis of T-cell responses and the processes involved in T-cell differentiation and maturation have led to the development of methods to manipulate the allograft so as to control donor immune reconstitution. Table 9 lists the features of alloimmune reactions and the clinical techniques under development to selectively prevent GVHD.
Selective Depletion of T Cells—Techniques
Several methods to selectively deplete or tolerize alloreactive cells have been explored. The techniques specifically target alloactivated T cells for destruction, removal, or anergy induction. Antibodies to the CD40 ligand induce anergy by blocking the second signal from the host APC,4,5 antibodies to molecules upregulated upon T-cell activation (CD69,6 CD25,7–,10 Fas11), are used to kill the alloreacting T cell either by internalization of a ricin-based immunotoxin or induction of apoptosis through Fas activation. Alternatively the activated T cell can be removed with immunomagnetic beads6 or flow-sorting.12 We used a photodynamic process to selectively deplete alloreacting T cells using a light-sensitive rhodamine dye TH9042.13 On exposure to visible light the drug releases free radicals causing selective death of activated T cells, which preferentially retain the dye.14
Separating GVHD from GVL by Selective T-Cell Depletion
Numerous studies have shown that when allogeneic T cells are generated against an individual’s leukemic or nonleukemic cells, T-cell lines with different Vβ specificity can be generated.3 These lines may show cytotoxicity uniquely to the leukemic cell, to the nonleukemic stimulator, or to both, suggesting the presence of both lineage-specific and lineage-shared antigens on the stimulators. Recently, molecular proof of the different identity of GVL- and GVH-responding T cells was provided in elegant experiments by Michalek et al who stimulated donor T cells with patient’s leukemia or lymphocytes and identified leukemia-specific clones by their unique TCR sequence.15 The GVL effect may be mediated both by mHag, which, depending on their lineage-specificity, may or may not also cause GVHD, and by tumor-specific antigens, which do not induce GVHD (see Section I). The separation of GVHD and GVL could, therefore, be accomplished by selectively depleting T cells specific for antigens on non-leukemic cells, while sparing antitumor antigen–specific T cells. In order to preserve the GVL effect without causing GVHD, responding T cells must be activated exclusively by host histocompatibility antigens without contamination from tumor-restricted antigens. In transplant models, T cells activated by the host antigens ex vivo have been successfully depleted or tolerized without impairing antileukemia or anti-third-party effect by targeting host-activated T cells.6,11,16 Using the photodynamic purging approach described above, we have demonstrated that host-specific cytotoxic T cells as well as host-specific IFNγ-secreting cells were significantly reduced after exposure of dye-loaded cells to light, while third party–specific cells and tumor cell–specific cells were partly preserved. In a mouse transplant model, photodynamic treatment of primed T cells prevented GVHD while simultaneously preserving the GVL effect and the ability to induce GVHD in a third-party host.13 Clinical trials to prevent GVHD by selective T-cell depletion or anergy induction have now begun. Guinan and colleagues reported promising preliminary results in a clinical trial of GVHD prevention by inducing T-cell anergy through costimulatory blockade.17 Selective depletion using a ricin-A chain immunotoxin targeting CD25 has been evaluated in a clinical trial of parental (haploidentical) transplants in children with non-malignant disorders. Despite the high risk in mismatched SCT, the incidence of GVHD was extremely low. Immune recovery was also superior to historical controls not receiving selectively depleted T cells and despite the relatively small T-cell inocula.18 A similar approach using the same immunotoxin, but with much larger T-cell doses (108 selectively depleted CD3 cells/kg), in HLA matched sibling transplants receiving a low-intensity SCT has been developed at the National Institutes of Health. In this study, the stimulator cells are irradiated and culture-expanded T cells that are more than 99% pure. In this way, the donor responder is deprived of exposure to host myeloid or B-cell antigens with the expectation that T-cell responses to lineage-restricted leukemic and non-leukemic antigens are preserved.19 Current results are encouraging with full donor engraftment and minimal GVHD. Clinical trials using the rhodamine dye to selectively deplete allogeneic T cells at time of SCT or as a safe T-cell source for delayed lymphocyte infusion are now planned.
Removal of Naïve T Cells
Our laboratory has been interested in selecting T cells based on their naïve or memory phenotype. In mice, naïve T cells, which have never encountered antigens specific for their T-cell receptors, express CD62L+. This distinguishes them from antigen-experienced memory/activated T cells, which can be either CD62L− or CD62L+. We hypothesized that since donor T cells have never encountered host alloantigens, GVHD-inducing T cells should reside in the naïve T-cell compartment. Conversely, memory/activated T cells should not induce GVHD since they were not elicited by alloantigens. Mixed lymphocyte cultures showed that the proliferative potential of allogeneic cells resides preferentially in the naïve cell (CD62L+) population. When we separately tested CD62L+ and CD62L− cells in a transplant model, GVHD induction in vivo was retained by the naïve phenotype, whereas memory cells did not cause GVHD. Moreover, memory cells immunized against BCL1 (a leukemia/lymphoma cell) retained the ability to proliferate and protect the animal against a challenge of the tumor cells. These results suggest that it may be possible to endow the recipient with T cells retaining memory for infectious agents but incapable of causing GVHD. Antitumor effects could also be transferred by prior immunization or adoptive transfer of antitumor effector cells or immunization posttransplant.
Selection of Th2/Tc2 Cells
Functional differences in T cells can be induced by selecting the cytokine milieu in which they mature and proliferate to antigen. Four functionally defined T-cell subsets are recognized: CD4+ T helper cells (Th1 or Th2 subsets) and CD8+ T cytotoxic cells (Tc1 or Tc2 subsets). Th1 and Tc1 cells secrete predominantly interleukin-2 and interferon gamma (type I proinflammatory cytokines), while the Th2 and Tc2 cells secrete IL-4, IL-5, and IL-10 (type II anti-inflammatory cytokines). In experimental models, donor Th2 cells do not initiate GVHD and more importantly, they regulate GVHD caused by unmanipulated T cells without adversely affecting engraftment.19–,20 Likewise, alloantigen-specific donor Tc2 cells do not cause significant GVHD, while at the same time mediating GVL effects. Moreover, these Tc2 cells exert a potent veto effect that can prevent SCT rejection. Thus, selection for Th2 and Tc2 cells may allow engraftment to occur with preservation of GVL and prevention of GVHD. Based on these successful mouse transplant experiments, Fowler and colleagues have started a clinical trial using Th2/Tc2 cells in a nonmyeloablative transplant setting for hematological malignancies.20,21
Insertion of Suicide Genes
One means of modifying GVHD reactions in vivo that has attracted continuing interest since its first description by Bordignon et al,22 is to insert a specific marker gene that would allow activated T cells to be selectively depleted should GVHD occur (reviewed in Tiberghien23). The best characterized method involves transducing donor lymphocytes with the herpes simplex virus thymidine kinase (HSV-tk) gene. Transduced cells are then selected and infused into patients, either as part of the initial graft or as a donor lymphocyte infusion. If GVHD should occur and require therapeutic intervention, ganciclovir is given to eliminate transfected cells. Such an approach has been used in relapsing patients and in patients developing Epstein-Barr virus–induced lymphoma after T-cell–depleted BMT.22 Eight patients were treated with donor lymphocytes transduced with the HSV-tk suicide gene. Five patients showed evidence of a GVL effect from the transduced lymphocytes. Three developed GVHD, controlled by treatment with ganciclovir, which led to the elimination of transduced cells. Thus, genetic manipulation of donor lymphocytes could make it possible to halt undesired GVHD effects once a tumor response is observed. Technical difficulties include the problem of ensuring that all transplanted T cells are properly transduced, so as to prevent escape of nontransduced cells from ganciclovir treatment, and the difficulty of conserving a broad T-cell repertoire during in vitro expansion to incorporate the gene.
Addition of Regulatory T Cells
Recent description and improved understanding of regulatory T cells has opened up the possibility of modulating GVHD alloresponses through the addition of regulatory T cells. These regulatory T cells double-mark for CD4+ and CD25+ and are important in the induction and maintenance of self-tolerance.24 Elegant animal studies showed that depletion of these cells led to an increase in autoimmunity, whereas their addition in these models, and in other models of autoimmunity, resulted in the abrogation of autoimmunity. Moreover, CD4+CD25+ T cells inhibited autoimmunity induced by autoantigen-specific T-cell clones. In a murine model, removal of these cells resulted in an increase in GVHD responses; whereas infusion of ex vivo activated and expanded CD4+CD25+ cells resulted in inhibition of GVHD.25 These results suggest a potent immunomodulatory effect for CD4+CD25+ cells and raise the possibility of infusing them to prevent or treat GVHD. One area of concern with the infusion of these cells, however, is that they are not limited to self-tolerance and the prevention of autoimmunity. Depletion of these regulatory cells led to increased tumor-specific immune responses and eradication of tumors in otherwise nonresponding animals. Therefore, the addition of these cells could possibly downmodulate a GVL effect. Clinical trials are clearly necessary to understand these interactions.
Use of Alloreactive NK Cells to Confer GVL Without GVHD
The favorable impact of NK mismatched transplants on relapse and survival in haploidentical transplants (see Section I) have encouraged some transplant teams to deliberately select NK mismatched unrelated and haploidentical family donor transplants. In the future, this approach could be extended to using alloreacting NK cells from third-party donors to enhance the anti-leukemic and myeloablative properties of the preparative regimen without causing GVHD.
Summary
GVHD is a persistent and serious problem for patients undergoing allogeneic stem cell transplantation. Understanding the subpopulation of effector cells that cause GVHD continues to be an active area of research. The ability to selectively remove subpopulations of the cells responsible for GVHD or add cells that could prevent GVHD presents an exciting potential option for modulation of GVHD. While preliminary results from these new approaches are encouraging, it will be important to demonstrate that the important GVL effect is preserved and that survival is improved after these manipulations.
The three SNPs in the promoter region of the IL-10 gene form conserved haplotypes, GCC, ATA, and ACC, which are associated with IL-10 production. Polymorphism at the -1064 microsatellite locus is linked to these three haplotypes.
The three SNPs in the promoter region of the IL-10 gene form conserved haplotypes, GCC, ATA, and ACC, which are associated with IL-10 production. Polymorphism at the -1064 microsatellite locus is linked to these three haplotypes.