Abstract
In the past seven years numerous genes that influence iron homeostasis have been discovered. Dr. Beutler provides a brief overview of these genes, genes that encode HFE, DMT-1, ferroportin, transferrin receptor 2, hephaestin, and hepcidin to lay the groundwork for a discussion of the various clinical forms of iron storage disease and how they differ from one another.
In Section I, Dr. Beutler also discusses the types of hemochromatosis that exist as acquired and as hereditary forms. Acquired hemochromatosis occurs in patients with marrow failure, particularly when there is active ineffective erythropoiesis. Hereditary hemochromatosis is most commonly due to mutations in the HLA-linked HFE gene, and hemochromatosis clinically indistinguishable from HFE hemochromatosis is the consequence of mutations in three transferrin receptor-2 gene. A more severe, juvenile form of iron storage disease results from mutations of the gene encoding hepcidin or of a not-yet-identified gene on chromosome 1q. Autosomal dominant iron storage disease is a consequence of ferroportin mutations, and a polymorphism in the ferroportin gene appears to be involved in the African iron overload syndrome.
Evidence regarding the biochemical and clinical penetrance of hemochromatosis due to mutations of the HFE gene is rapidly accumulating. These studies, emanating from several centers in Europe and the United States, all agree that the penetrance of hemochromatosis is much lower than had previously been thought. Probably only 1% of homozygotes develop clinical findings. The implications of these new findings for the management of hemochromatosis will be discussed.
In Section II, Dr. Victor Hoffbrand discusses the management of iron storage disease by chelation therapy, treatment that is usually reserved for patients with secondary hemochromatosis such as occurs in the thalassemias and in patients with transfusion requirements due to myelodysplasia and other marrow failure states. Tissue iron can be estimated by determining serum ferritin levels, measuring liver iron, and by measuring cardiac iron using the MRI-T2* technique. The standard form of chelation therapy is the slow intravenous or subcutaneous infusion of desferoxamine. An orally active bidentate iron chelator, deferiprone, is now licensed in 25 countries for treatment of patients with thalassemia major. Possibly because of the ability of this compound to cross membranes, it appears to have superior cardioprotective properties. Agranulocytosis is the most serious complication of deferiprone therapy and occurs in about 1% of treated patients. Deferiprone and desferoxamine can be given together or on alternating schedules. A new orally active chelating agent ICL 670 seems promising in early clinical studies.
In Section III, Dr. James Cook discusses the most common disorder of iron homeostasis, iron deficiency. He will compare some of the standard methods for identifying iron deficiency, the hemoglobin level, transferrin saturation, and mean corpuscular hemoglobin and compare these with some of the newer methods that have been introduced, specifically the percentage of hypochromic erythrocytes and reticulocyte hemoglobin content. The measurement of storage iron is achieved by measuring serum ferritin levels. The soluble transferrin receptor is a truncated form of the cellular transferrin receptor and the possible value of this measurement in the diagnosis of iron deficiency will be discussed. Until recently iron dextran was the only parental iron preparation available in the US. Sodium ferric gluconate, which has been used extensively in Europe for many years, is now available in the United States. It seems to have a distinct advantage over iron dextran in that anaphylactic reactions are much less common with the latter preparation.
I. Hemochromatosis
Ernest Beutler, MD*
The Scripps Research Institute, 10550 North Torrey Pines Road, La Jolla CA 92037
Acknowledgments: This is manuscript number 15873-MEM. Supported by National Institutes of Health grants DK3505-06 and RR00833 and the Stein Endowment Fund.
Definition
Hemochromatosis is generally considered to be a disease in which increased iron storage causes pathologic changes. However, the definition of hemochromatosis has undergone considerable evolution since the disorder was recognized as a distinct clinical syndrome in the late 19th century. Until the performance of plasma iron and ferritin determinations became commonplace, the designation was reserved for patients who had, as a result of iron deposition, frank cirrhosis of the liver and usually diabetes, bronzing of the skin, and cardiac disease. The disease was sometimes called “bronzed diabetes.” But in the 1970s the definition of the disease gradually changed. Instead of being applied only to patients who had severe clinical manifestations of iron storage, patients who had elevated serum transferrin saturation and ferritin levels were also designated as having hemochromatosis, especially if they had the HLA type A4 B14 that was commonly associated with the disease. Even larger numbers of patients were considered to have hemochromatosis after the HLA-linked gene that was associated with the disease, HFE, was discovered.
But who, then, should be considered to have hemochromatosis? Only those with disease? Or those who merely have the genotype? No consensus exists concerning what definition is to be used.
Classification
Hemochromatosis may be divided into primary (or genetic) hemochromatosis and secondary hemochromatosis.
Primary (hereditary) hemochromatosis
As shown in Table 1 , the hereditary form can be divided into several subgroups. A numerical classification has been proposed (http://www.ncbi.nlm.nih.gov/htbin-post/Omim/dispmim?235200), but such a system does not add to our understanding, since most of the forms of hemochromatosis can simply be designated by the mutation that is their cause, and some forms of iron storage disease (e.g., African iron overload) are not included.
Secondary hemochromatosis
Secondary hemochromatosis can arise in many disorders, inborn or acquired. These disorders have in common the fact that the patient is anemic. Transfusions of erythrocytes add, nearly stoichiometrically, to the body iron burden: each milliliter of red cells contains one milligram of iron. When anemia is accompanied by ineffective erythropoiesis, inappropriate absorption of iron from the gastrointestinal tract seems to be activated. Patients with anemias in which ineffective erythropoiesis does not play a role seem much less prone to hyperabsorb iron. Thus, it is patients with ineffective erythropoiesis who develop the largest iron burdens. Among the hereditary forms, the most common are the thalassemias; among the acquired forms, the acquired sideroblastic anemias predominate. Table 2 lists some of the diseases with which hemochromatosis has been associated.
Penetrance
The penetrance of a mutation may be defined as the extent to which a phenotypic effect is exerted in individuals carrying the mutation. But which phenotypic effect? Clearly, the penetrance is a function of which endpoint is selected.
Penetrance of the homozygous HFE mutation
The C282Y mutation of the HFE gene is a very common one. About 15% of the northern European population is heterozygous; accordingly, one would expect over 5 per 1000 in the population to be homozygous, and this is, indeed, the case.
Biochemical penetrance: Relatively few studies have been conducted in which an unbiased population was screened for the C282Y mutation and the transferrin saturation and ferritin levels of the homozygotes were determined. Deugnier et al16 screened over 9000 individuals (3367 men and 6029 women) in France and found 10 homozygous men and 44 homozygous women. Although the population was relatively young, 80% of the men had transferrin saturations over 55%, and 44% of the women had transferrin saturations over 50%. In our study of patients in the health appraisal clinic of Kaiser Permanente in San Diego we found that among 152 homozygotes, 75% of men and 40% of women had a transferrin saturation higher than 50%.17–,19 Serum ferritin levels were increased in 76% of the men and 54% of the women. In another small study all 5 homozygotes detected have transferrin saturations greater than 55%.20 Thus, there is agreement that homozygotes for the HFE C282Y mutation usually have increased serum transferrin saturation levels and increased serum ferritin levels. Clearly, there is a subset of homozygotes who do not show these biochemical stigmata. A few of these prove to be frequent blood donors, but most of them are not. It is simply that even on a biochemical level the homozygous state is not always expressed.
Clinical penetrance: Clinicians do not encounter many cases of full-blown hemochromatosis. Our attempts to obtain samples from patients with iron overload who manifested diabetes, cirrhosis, cardiomyopathy, and darkening of the skin from our own clinic and from major centers in which many patients with hemochromatosis are treated have met with very little success. Most of the many patients that have been diagnosed as having hemochromatosis have been diagnosed on the basis of biochemical changes and non-specific symptoms, such as fatigue and arthropathy. Neither common clinical experience21,22 nor autopsy series21,23–,25 suggest that hemochromatosis is a common cause of death. However, it has been a common belief that milder symptoms are, in contrast, very common in patients homozygous for the C282Y mutation, and it has been suggested that most of the homozygous males will develop symptoms by the time they are 40 years of age.26 This impression that a mild phenotype exists (and the accompanying assumption that this leads to the more severe phenotype if not treated) has been based largely on uncontrolled observations in which the patients being assessed and the physician performing the assessment knew the diagnosis and could well have been influenced by it.
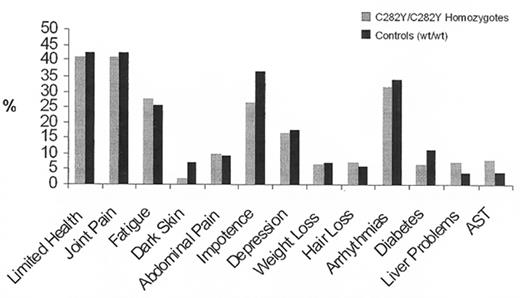
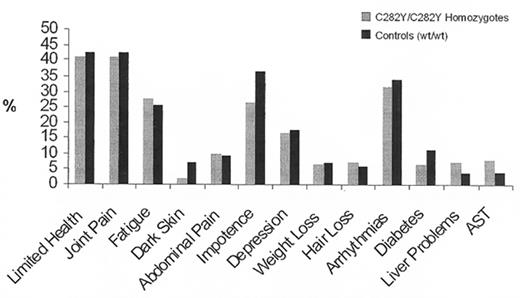
Between 1998 and 2001 we had the opportunity for the first time to study a large population, genotyping all participants for the HFE mutations and comparing symptoms, laboratory findings, and survival in homozygotes for the HFE C282Y mutation, C282Y/H63D compound heterozygotes, and homozygous wildtype individuals. When we first presented our results at the ASH meeting in 2000, having analyzed data from 26,000 genotyped subjects,27 they were surprising. Although many homozygotes manifested the non-specific symptoms that are associated with hemochromatosis—fatigue, arrhythmias, impotence, and arthralgias—the prevalence of such symptoms proved to be no higher than those in homozygous wildtype controls (Figure 1 ). There was no demonstrable effect on lifespan. The only significant difference found between homozygotes for the C282Y mutation and controls was a higher prevalence of abnormal liver function tests. Upon completion of the study,17– 19 the preliminary findings were confirmed. Only one of 152 homozygotes had the typical clinical syndrome of hemochromatosis and we estimated the clinical penetrance of the homozygous state to be of the order of 1% (Figure 1 ).
No one had expected the penetrance to be so low, and predictably, the results were greeted with considerable skepticism. In attempting to reconcile our data with the concept that the homozygous state had a much higher penetrance, it was suggested that the data were “flawed” in a number of respects. It was proposed that we were dealing with an unusually healthy population28 or a population with an extraordinarily healthy life style. Alternatively, it was proposed that our population was unusually “sickly” and that the manifestations of hemochromatosis had been obscured by the poor health of the controls.29 Obviously it is impossible to reconcile these two objections: the population cannot be too well and too sickly at the same time. But, in fact, neither criticism applies. The most cogent objection was that our study was biased by selecting a healthy population. If, indeed, patients with symptoms had been excluded because they did not attend a health appraisal clinic, having died or being taken care of in a more intense medical setting, we might have erroneously concluded that the penetrance of the homozygous state is very low. But there is a straightforward way to address this problem. If homozygotes were systematically excluded then the number found in the population should fall short of the number predicted by the Hardy-Weinberg equilibrium based on the gene frequency in the population. But in fact, the number of homozygotes actually exceeds the predicted number.17–,19,30–,32 Another way to examine the possible lethal effect of the hemochromatosis mutation is to examine the age distribution of homozygotes. Since hemochromatosis is a late-onset disease, one would expect underrepresentation of the homozygous genotype in the elderly if the disease caused an appreciable number of early deaths. No significant shift in age distribution has been observed. In fact, extensive meta-analysis of 161 publications giving gene frequency data and the number of homozygotes in each population confirms these results (J Waalen et al, unpublished). In the past two years numerous studies from different parts of the world have all confirmed these findings: The homozygous state is only rarely associated with illness17,22,33– 36 (Figure 2 ).
Why, then, is there a controversy about the penetrance of hemochromatosis? One issue that seems to have muddied the waters is the interpretation of non-specific symptoms such as fatigue, joint pains or impotence. Suggestions that symptoms are common come from uncontrolled studies in which the subjects knew their diagnosis.37,38 But to be meaningful the history must be elicited before the patient has been informed of the diagnosis and must be compared with age, sex and ethnically matched wildtype controls. Studies that have been carried out in this manner show that no symptoms are statistically significantly more common in homozygotes than controls in any study.17,34 The only possible exception is a small French study in which 7 of 10 male homozygotes complained a fatigue, a number that was statistically significant, but had not been corrected for multiple comparisons.16 The other issue is the significance of the abnormality in liver function tests and biopsy interpretations in homozygotes. The reading of liver biopsies is subject to observer bias and, unfortunately, there are never control biopsies with which to compare the patient cohort. Nonetheless, there is considerable consistency in the data. Olynyk38 reported that of 16 homozygotes (of whom two refused biopsy) 3 had fibrosis, and one alcoholic subject had cirrhosis (25%). Bulaj37 found 16 patients with cirrhosis and 17 with fibrosis out of 210 homozyotes (15%), and Åsberg et al39 found 12 of an estimated 400 homozygotes had fibrosis or cirrhosis (3%). For ethical reasons we could not perform liver biopsies on our patients, but found that 8.2% had elevated SGOT levels compared to 3.2% of controls. Serum collagen IV levels, considered a surrogate for hepatic fibrosis, were elevated in 25.8% of the homozygotes in our study compared with only 11.1% of matched controls. Notably, the elevated liver function tests were not age-related. Thus, all of the data, including our own (except for a small French study that found 3/54 [5.5%] homozygotes had elevated ALT levels, compared to 5% in controls16), indicate that there is a subset of patients, considerably larger than 1% who have abnormal liver function tests. Those who hold that the penetrance of hemochromatosis is higher than the approximately 1% estimate can point to the presence of hepatic fibrosis as an indication that iron overload is clinically important. We, however, take the point of view that since the fibrosis did not produce any clinical symptoms in the vast majority of subjects, and that it does not appear progressive, it is not important for the person to whom it should matter the most, the patient. Thus, to some degree the disagreement about penetrance comes down to the single issue of whether hepatic fibrosis seen on liver biopsy by pathologists or abnormal liver function is important if it is not associated with measurable morbidity or mortality.
Penetrance of the compound heterozygous C282Y/H63D HFE mutation
On the average, compound heterozygotes manifest significantly higher transferrin saturations and serum ferritin levels than do individuals with the wildtype genotype. Because the H63D mutation is very prevalent in the population, this compound heterozygous genotype is very common in the population. Among patients who had been classified as having “hemochromatosis” on the basis of increased biochemical parameters there is an increased number of compound heterozygotes, and it has been calculated that the biochemical penetrance of this genotype is only about 1% of that of the homozygous genotype.40 Accordingly, patients with this genotype who develop severe cirrhosis and other clinical manifestations of hemochromatosis are very rare.
Penetrance of the simple heterozygous genotype
It is clear from large studies that simple heterozygotes for the C282Y or H63D mutations have, on the average, very slightly higher transferrin saturations and ferritin levels than do homozygotes for the wildtype. Numerous claims have been made that these minor changes translate into increased prevalence of a variety disorders including diabetes,41,42 heart disease,43,44 and cancer.45,46 None of these claims has been widely substantiated,18,36,47,48 and it seems unlikely that the heterozygote for these common mutations suffers ill health because of them with one notable, rather uncommon exception. Carrying either the C282Y or H63D does appear to be a risk factor for porphyria cutanea tarda.49,50 In general, however, it is much more likely that mutations that have gained a high prevalence in the genome have a beneficial effect, i.e., that they constitute a balanced polymorphism. Their beneficial effect is probably that of preventing iron deficiency in women.51,52
Diagnosis
It is a tragedy when a patient dies of a treatable disease such as hemochromatosis without receiving the benefits of therapy. However, the enthusiasm for general population screening for hereditary hemochromatosis has abated with the realization that the penetrance is very low.53 The burden for making the diagnosis of hemochromatosis therefore falls squarely upon the physician. The diagnosis of hemochromatosis should be considered in patients with cirrhosis of the liver, particularly those with diabetes. Alcoholism is no bar to consideration of hemochromatosis in a cirrhotic patient; indeed, a high proportion of patients with clinical hemochromatosis ingest excessive amounts of alcohol54,55 and cirrhosis is much more common in patients with the hemochromatosis genotype who have heavy alcohol intake.56
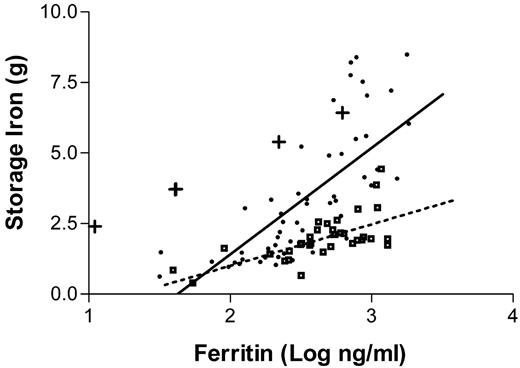
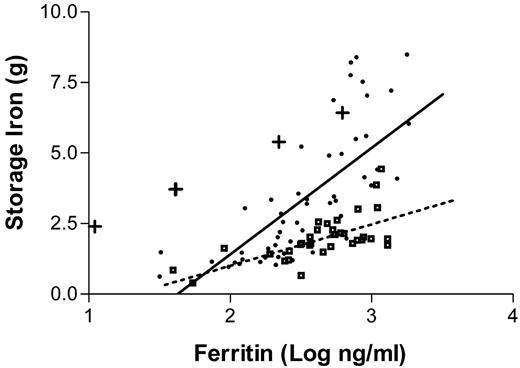
The most efficient approach to diagnosis of patients who are suspected to have the disease is to measure the serum transferrin saturation and ferritin levels. Increased transferrin saturation is an important hallmark of the disease. If the transferrin saturation is < 40% it is very unlikely that the patient has hemochromatosis. About 90% of patients with genotypic hemochromatosis will have a transferrin saturation at least this high, and the few who do not are unlikely to have clinical manifestations of the disorder. While transferrin saturation is considered to be an indicator of the underlying genetic defect, serum ferritin concentrations more closely mirror the total body iron content. It is sometimes assumed that there is a linear relationship between body iron burden and serum ferritin levels. In reality, the ferritin values only provide a rough approximation of the body iron burden.57Figure 3 presents the results of a recent study in which ferritin levels are compared with the actual amount of iron in the body as measured by phlebotomy. Serum ferritin levels are of considerable value in predicting the likelihood that a given patient has cirrhosis: patients with serum ferritin levels of under 1,000 ng/mL are extremely unlikely to have cirrhosis.58,59
Genotyping patients for the C282Y and H63D mutations is useful to confirm the diagnosis and for family studies, but it must be borne in mind that in the US some 20% of patients who have been diagnosed as having hereditary hemochromatosis do not have mutations of HFE.60 In southern Europe, the proportion of hemochromatosis patients who do not carry this mutation is even larger.61 The presence of excessive iron stores in a patient with putative hemochromatosis can be confirmed by carrying out serial phlebotomies (see “Treatment”). Some physicians consider liver biopsy as an essential part of the workup of a patient with possible hemochromatosis. Liver biopsy will show whether the iron is deposited chiefly in liver parenchymal cells, which is characteristic of the disease, it will allow quantitation of liver iron, and it will establish whether the patient has cirrhosis. However, serial phlebotomy will quantitate the iron. Therefore, the major benefit to the patient of undergoing liver biopsy is to establish whether cirrhosis is present. Hepatic cirrhosis affects prognosis, but not treatment, and many or most patients may not be eager to know what their estimated lifespan will be. Cirrhosis also increases greatly the risk of hepatocellular carcinoma, an important complication in patients with hemochromatosis and cirrhosis. Patients with cirrhosis may therefore be better candidates for periodic screening for this disease. As treatment for hepatocellular carcinoma becomes more effective, establishing whether or not cirrhosis is present may be important for optimal management and may therefore be a reason for performing a biopsy.
Treatment
The aim of treatment of iron storage disease is to remove from the body the excess iron that has accumulated. In the case of patients without primary disorders of hematopoiesis (i.e., patients with one of the forms of primary hemochromatosis), this is best achieved by phlebotomy, since regeneration of erythrocytes by the marrow utilizes iron, which is therefore withdrawn from various body pools. Phlebotomy is only occasionally feasible in patients who have augmented iron stores because of ineffective erythropoiesis and is generally precluded in those in whom the iron overload is the result of multiple transfusions. Such patients require treatment with an iron chelating agent, as discussed in Section II.
One ml of erythrocytes contains 1 mg of iron. Hence, removing one unit (~450 mL) of blood with a hematocrit of 45% removes approximately 200 mg of iron from the body. Iron absorption is increased in patients with hemochromatosis who are undergoing phlebotomy, and may be more than 5 mg per day.62 But with even this level of iron absorption, it is relatively simple to achieve a negative iron balance by instituting a phlebotomy program. Initially, some patients feel enervated by phlebotomies, and I find that compliance is improved by initially phlebotomizing every two weeks. But after this has been done for a month or two, weekly phlebotomies are almost always well tolerated. The aim of phlebotomy is to deplete the body iron stores, and the most easily appreciated endpoint is mild iron deficiency anemia. One of the easiest guides to incipient iron depletion is a falling MCV.63 The other useful parameter is the serum ferritin level, which should be brought to under 10 ng per ml. Some clinicians prefer a higher cutoff, but there is usually appreciable residual, difficult-to-mobilize tissue iron even when the erythron is iron depleted, and the mobilization of this iron and enhanced gastrointestinal iron absorption that is present in iron-depleted patients with hemochromatosis will quickly correct any deficiency that may have been induced. Maintenance phlebotomy is initiated when the ferritin level rises to 80 or 100 ng/ml, and the rate of maintenance phlebotomy required varies widely between patients. Some physicians prescribe an iron-poor diet, but this seems to me to represent more interference with the patient’s lifestyle than is justified by the slight decrease in intervals between phlebotomies that may be achieved by restricting iron intake.
One of the manifestations of hereditary hemochromatosis is increased susceptibility to infection. Death due to overwhelming sepsis is not uncommon in severely affected patients. Because of the risk of infection with organisms such as Yersinia enterocolitica or Vibrio vulnificus, patients are usually advised to avoid the ingestion of raw shellfish. Because of the fulminating nature of some infections in patients with hemochromatosis, vigorous early treatment of febrile diseases is recommended.
The prevalence of hepatomas is increased in patients with hemochromatosis, particularly those with cirrhosis; a relative risk of 1.8 (CI 1.1–2.9) has been reported.64 Some cases have been documented in non-cirrhotic patients,65,66 but these are only case reports, and it is unclear whether the relative risk is increased. It is probably prudent to monitor the liver by ultrasound every 6 to 12 months, although the value of this procedure is in some doubt since the treatment results for hepatoma are so unsatisfactory.
It is universally stated that phlebotomy improves the health and increases the lifespan of patients with hemochromatosis. This assumption is based upon a study in which it was shown that phlebotomized patients with “hemochromatosis” had a normal lifespan.67 We now recognize that unphlebotomized patients with hemochromatosis also have a normal lifespan when the selection criterion is based on either genotype or chemical phenotype. The fact is that there are no data that allow us to show definitively that phlebotomy improves survival. No such data can be obtained because of ethical issues. Nonetheless, the concept that removing iron from a patient whose disease is due to an excess of iron will be helpful is compelling, and therefore treating patients with hemochromatosis who have clinical disease is mandatory. The recognition that most patients with the genetic and biochemical stigmata of hemochromatosis will have a normal lifespan without intervention raises the question of whether all patients with hemochromatosis should be phlebotomized. Fortunately, the treatment is almost entirely risk free and potentially beneficial to society. Moreover, our current state of knowledge is such that we cannot predict which of the patients with hemochromatosis will be the rare ones who develop serious clinical consequences. Thus, it seems prudent to phlebotomize all patients with hemochromatosis who have elevated body iron stores as estimated by serum ferritin levels. It is useful in this regard to consider the fact that cirrhosis is very largely limited to patients with the serum ferritin levels higher than 1000 ng/mL.59,68 There is no harm, however, in phlebotomizing patients with less elevated ferritin levels. Family studies are also potentially useful, since homozygous relatives of non-expressing homozygotes are potentially more seriously affected.
The management of patients with the juvenile hemochromatosis is similar to that of patients with HFE hemochromatosis, but knowing that this is an aggressive form of disease, a vigorous phlebotomy program should be initiated early.
Summary
There are many forms of iron storage disease, primary (or hereditary) and secondary to various hematologic disorders. The most common of the primary forms are associated with mutations of an HLA-linked gene designated HFE. Five per thousand northern Europeans are homozygous for the common C282Y mutation of HFE. Very few of these develop any clinical symptoms, but uncommonly cirrhosis, diabetes, arthropathies, and bronzing of the skin may result, and approximately 25% will have elevated serum collagen IV levels, a surrogate marker for hepatic fibrosis, compared with 11% of controls. Hereditary hemochromatosis is characterized by an increased serum transferrin saturation. It is treated by serial phlebotomy to remove the accumulated iron. Secondary hemochromatosis has clinical manifestations similar to those of primary hemochromatosis, but by necessity treatment usually consists of the administration of iron chelating agents.
II. Iron Chelation Therapy
A. Victor Hoffbrand, DM, FRCP*
Royal Free Hospital, Pond Street, NW3 2QG London, UK
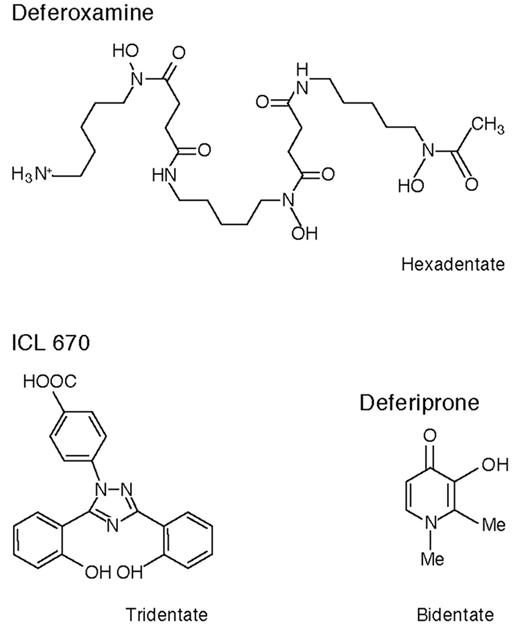
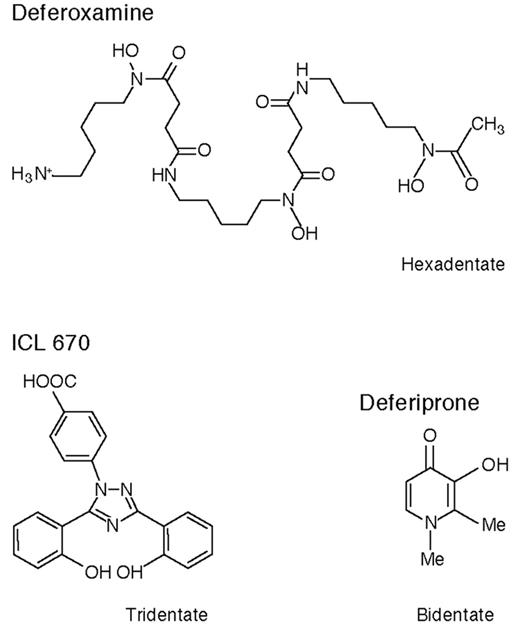
These are exciting times for patients with thalassemia major (TM) and other transfusion-dependent patients with refractory anemias who need chelation therapy. Although at present deferoxamine (DFO) remains the standard of care recent data suggest that deferiprone, orally active and in clinical trials for 16 years, may for many patients be a safe and effective alternative to the more cumbersome drug DFO. There is the new possibility of combination chelation therapy with DFO and deferiprone, and a second orally active iron chelator, ICL 670, is now in early clinical trials. Figure 4 shows the chemical structures of these three compounds.
Approximately 72,000 patients with TM or thalassemia/hemoglobin E disease receive regular blood transfusions worldwide. Because DFO is expensive and cumbersome to administer, about 42,000 receive no chelation therapy; about 25,000 are prescribed DFO and 5000 (mainly in India) receive deferiprone (C.B. Modell). Some 2000–4000 thalassemia patients die each year from iron overload. The lack of an inexpensive orally active iron chelator has been a major reason why iron chelation therapy is not considered for these patients in poor countries. In many of these countries, regular blood transfusions, which in the absence of iron chelation will inevitably lead to death from iron overload, are not even contemplated. Indeed, only about 3500 of the 27,000 transfusion-dependent children born each year are transfused at all.
There are two other large groups of patients requiring iron chelation therapy: (1) patients with non-transfusion-dependent but nevertheless severe genetic diseases of hemoglobin synthesis (thalassemia intermedia) who become iron overloaded because of increased iron absorption but are too anemic to undergo phlebotomy to reduce iron overload; and (2) regularly transfused patients with, for instance, sickle cell anemia, myelodysplasia, myelofibrosis, red cell aplasia, aplastic anemia, congenital dyserythropoietic anemia, and congenital sideroblastic anemia.
Requirements of Iron Chelation Therapy
The chelator must result in excretion of sufficient iron to prevent damage to the endocrine organs, liver, and most importantly heart. In TM, about 100–200 mL of pure red cells/kg/y are transfused, equivalent to 0.32–0.64 mg/kg/d of iron.1 In thalassemia intermedia, iron absorption is about 5–10 times the normal amount, around 0.1 mg/kg/d. Excretion levels of these rates must be achieved to maintain a “safe” level of body iron. Monitoring of iron chelation therapy requires: (1) estimation of the iron content of different organs, and (2) assessment of the function of the heart, liver, and endocrine glands, the organs particularly damaged by iron overload. These aspects have recently been extensively reviewed and are only briefly discussed here.2,3
Estimation of Tissue Iron
Serum ferritin
This is a useful technique for assessing changes in body iron, although the absolute level is an imprecise measure of body iron. This is partly because inflammation—for example, hepatitis C—raises the level, while vitamin C deficiency lowers it, both frequent complications of TM. Most studies have found a wide range in liver iron at any given serum ferritin level. The Thalassemia International Federation guidelines1 recommend maintaining serum ferritin levels around 1000 μg/L; nevertheless, levels below this may in some individuals be associated with cardiac complications. One study in TM patients receiving DFO found that those with at least two-thirds of serial serum ferritin estimations less than 2500 μg/L had significantly less cardiac disease than those with higher levels.4 More recently, a level consistently below 1500 μg/L was found to be associated with few complications in 32 patients with TM followed for approximately 15 years.5 When effective chelation therapy is initiated, the serum ferritin falls more rapidly than body iron. This may happen partly because of improvement in liver function and partly because serum ferritin may reflect predominantly reticular endothelial iron rather than parenchymal iron in the liver and other organs.6
Liver iron
Liver iron has been described as the “gold standard” for determining body iron and has been recently shown to correlate with total body iron stores.7 It can be measured chemically after liver biopsy (which can be inaccurate because of fibrosis, cirrhosis, or uneven distribution of iron) or noninvasively by the superconducting quantum interface device (SQUID) (available in only a few centers) or by magnetic resonance imaging (MRI). Brittenham et al8 studied 59 TM patients who were more than 7 years old. All patients who died had liver iron concentrations > 15 mg/g dry weight, and this level has been subsequently regarded as an index of high risk of death from cardiac disease. More recently, Angelucci et al7 have shown that this level is also associated with liver fibrosis and cirrhosis. The level of 7 mg/g is the upper limit found in carriers of genetic hemochromatosis. For levels between 7 and 15 mg/g, Angelucci et al found no evidence of liver damage except in patients who had hepatitis C and were messenger RNA positive; the combination of iron overload and hepatitis C infection is particularly damaging to the liver.
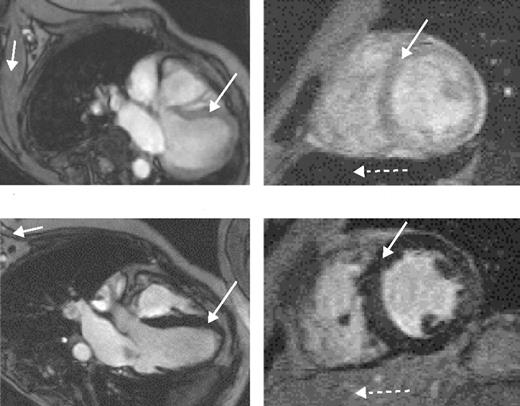
The value of liver iron, whether > 15 mg/g or in the range of 7 to 15 mg/g, as a predictor of cardiac iron has recently been questioned. MRI data using the T2* technique (Figure 5 )9 and spin-echo10 have shown no correlation between cardiac and liver iron, although other MRI techniques, possibly less sensitive and accurate, have shown such a correlation. Possible explanations for these discrepant observations have been discussed.3,9
Cardiac iron
Direct measurement of cardiac iron by endomyocardial biopsy of the right atrium is inappropriate since iron locates mainly to the myocardium of the ventricles. The recent development of a reproducible, sensitive, and accurate indirect measure of cardiac iron using the MRI T2* technique11 has provided substantial important new data. A T2* value less than 20 ms has been found to correlate with the presence of cardiac dysfunction, detected by echocardiography, 24-hour monitoring, or the need for cardiac therapy. It is also valuable for monitoring changes in cardiac iron during intensive chelation therapy.12
Non-transferrin-bound iron
In severely iron-loaded patients, non-transferrin-bound iron (NTBI) is present in plasma. It occurs in 80% of patients with TM and represents a highly toxic species causing tissue iron loading. NTBI is also found in patients receiving chemotherapy or undergoing heart bypass operations and those having other conditions in which large amounts of iron from hemoglobin breakdown are released into the circulation.13 NTBI is removed by administration of DFO or DFP but reappears rapidly (i.e., usually within 1 hour of discontinuing intravenous DFO therapy) unless body iron burden is substantially reduced.
Urine iron excretion
Iron excreted in response to a single dose of DFO or deferiprone has been taken as an index of body iron burden. It will vary, however, with the dose of chelator used and, for DFO, whether vitamin C is also given and with the hemoglobin level. There is also considerable day-to-day variation, even with apparently the same conditions.2 Nevertheless, in one recent study it has been found to correlate closely with cardiac iron measured by MRI.10
Estimation of Iron-Induced Tissue Damage
In addition to measuring iron status, it is important to assess the function of the heart, liver, and endocrine glands, the organs particularly damaged by iron overload. Early detection of cardiac dysfunction is especially important so that increased chelation therapy can be instituted before cardiac damage is irreversible. Once the patient has started chelation therapy, it will also be necessary to monitor for potential side effects of the iron chelator being used (Table 3 ).
Chelation Therapy
Deferoxamine
The management of iron overload using subcutaneous DFO has been extensively reviewed.2 DFO is hexadentate, 1 molecule binding 1 atom of iron (Table 3 ). Standard therapy is with 40 mg/kg infused subcutaneously over a period of 8–12 hours on 5–7 nights each week using a battery-operated infusion pump. Therapy is usually begun in children after 10–20 transfusions have been given or when serum ferritin levels reach 1000 μg/L. Vitamin C, 200 mg, given orally when the infusion is started, enhances urine iron excretion. Alternative routes of administration that have been tried include twice-daily bolus subcutaneous injections,14 continuous infusions over 24 or 48 hours using disposable prefilled balloons,15 and continuous intravenous infusion using an indwelling central line or Portacath.16
First introduced in 1976 as subcutaneous treatment for TM, DFO has substantially improved the life expectancy in the disease.4,8,17 Deaths continue to occur from cardiac failure due to iron overload, but these are mainly caused by lack of compliance.17,18 Defining compliance as more than 250 infusions a year, Gabutti and Piga17 found that 95% of compliant patients are alive at 30 years of age, compared with only 12% of noncompliant patients. Modell et al18 reported that only 50% of TM patients in the UK reach 35 years, the poor result again being attributable to cardiac failure due to poor compliance.
DFO can reverse iron-induced cardiomyopathy in some but not all patients.16,19 Continuous intravenous DFO results in comparatively rapid improvement in ventricular function compared with the slow clearance of cardiac iron, which can remain high even after 1 year.12 Recent studies show that liver iron clears more rapidly and, despite severe iron overload initially, may be normal at 6 months.12
Although cost and lack of compliance are the main obstacles to DFO therapy, complications may also exclude some patients. High-frequency hearing loss, deafness, and retinal damage with impaired vision (e.g., night blindness) can occur when large doses of the drug are given to less severely iron-loaded patients, especially children, in whom growth retardation and skeletal damage have also been reported. Generalized hypersensitivity is rare, but painful local reactions at the injection site are common and often lead to lack of compliance. Infection with Yersinia is increased, and on rare occasions other infections (e.g. Klebsiella) are precipitated.
Deferiprone
The orally active bidentate iron chelator deferiprone (1,2 dimethyl-3-hydroxy-pyrid-4-one, also known as L1, CP20, Ferriprox, or Kelfer) was designed in R.C. Hider’s laboratories (Figure 4 ).20 First tested clinically in 1987, this drug is now licensed in 25 countries for patients with TM unable to be effectively treated with DFO. Reviews of its chemistry, pharmacology, and clinical results have recently been or are being published.20– 22
Pharmacokinetics
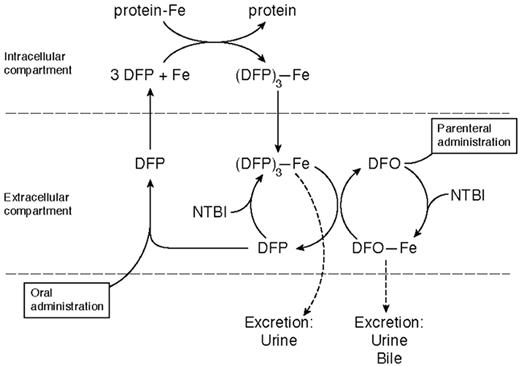
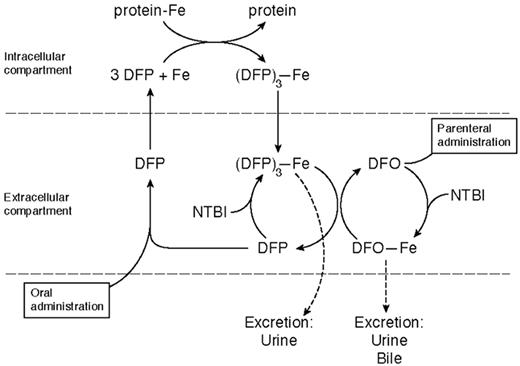
Deferiprone is rapidly absorbed, appearing in plasma within 15 minutes of ingestion, with a peak plasma level within 45–60 minutes (Table 3 ). It forms a 3:1 chelator:iron complex that is excreted with the free drug in urine. Only 4% of a single oral dose of the drug is excreted bound to iron, even in heavily iron-loaded patients. Its iron chelation site is inactivated by glucuronidation, the speed of which varies from patient to patient. This explains much of the individual variation in response, the area under the curve of the concentration of free drug in plasma being related to the amount of iron excreted.23 Deferiprone mobilizes iron from parenchymal and reticuloendothelial pools and from transferrin, ferritin, and hemosiderin. Unlike DFO, it is also capable of chelating iron from intact red cells in vitro and in vivo, shown in patients with sickle cell anemia24 and thalassemia intermedia.25 The enhanced ability of DFP to cross cell membranes may underlie what is emerging as its superior ability, compared with DFO, to protect the heart from iron and also the “shuttle effect” for iron when the 2 drugs are given simultaneously (Figure 6 ).
Clinical studies
Short-term studies showed that iron excretion occurs in the urine with negligible amounts in feces, although some subsequent studies have suggested excretion up to 33%. Iron excretion increases with the dose of the drug and the transfusional iron load of the patients. Although initial studies showed that 100 mg/kg/d was more effective,26,27 the dose used in most trials has been 75 mg/kg/day fractionated into 3 doses. This dose was reported in early studies to be as effective as standard-dose DFO at increasing urine iron excretion.28,29 In most patients urine iron excretion around 0.5 mg/kg/day was achieved with no indication of a diminishing response with time. There were early concerns that doses higher than 75 mg/kg might produce more side effects, e.g., arthropathy.27 Balance studies suggest that total iron excretion with 75 mg/kg deferiprone is somewhat less than that with 40 mg/kg DFO given over 8 hours subcutaneously, but only small numbers of patients have been studied and there is wide patient-to-patient variability.30
Final mean serum ferritin concentrations in 9 published trials in patients (mainly DFO ‘failures’ with TM) treated from 1 to 56 months with deferiprone have ranged from 1779 to 3273 μg/L.22 In 7 of the trials, there was a significant fall in serum ferritin, and in 2 there was no significant change. Serum ferritin levels fell mainly in the patients starting with the highest levels. In 6 studies in which serial liver iron determinations were made, hepatic iron fell significantly in 1, rose significantly in 1, and did not change significantly in the other 4.22 When SQUID technique was used in 54 thalassemia patients (aged 7–22 years), median liver iron concentration rose from 1456 mg/g liver to 2029 mg at 2 years and 2449 mg at 3.2 years.6 These patients were well chelated before starting deferiprone, but their iron intake from transfusions rose substantially during the study. Recent MRI data retrospectively comparing 15 patients treated long term with deferiprone with 30 matched patients treated with DFO, in which liver MRI T2* was converted to estimated dry-weight liver iron, showed significantly higher liver iron: 5.1 versus 3.5 mg/g in the deferiprone compared with the DFO group.9 In a short-term study, raising the dose of deferiprone to around 100 mg/kg led to reduction of serum ferritin levels in patients inadequately chelated at 75 mg/kg.31 Prospective trials are needed to assess whether doses of deferiprone (around 100 mg/kg daily) can safely be given long term and will result in more effective iron chelation in patients inadequately chelated on 75 mg/kg daily. There is also a need for further (> 3 years) long-term studies to determine liver iron levels in large numbers of patients receiving deferiprone to determine more accurately the proportion of patients in whom liver iron is adequately controlled.
The most important aspect of iron chelation therapy is protection of the heart. Two recent studies, albeit retrospective, show significant benefit for the patients receiving deferiprone compared with DFO. In a London study, 15 TM patients who had received deferiprone 75 mg/kg/d for 3 years showed a lower incidence of cardiac disease (assessed by echocardiography and need for cardiac drug therapy) and lower cardiac iron estimated indirectly by MRI T2* than 30 age- and sex-matched patients who had received DFO 40 mg/kg/d subcutaneously on 5–7 days each week (median myocardial T2* 34.0 vs 11.4 ms, P = .02).9 Excess myocardial iron (T2* < 20 ms) was significantly less common in the deferiprone group (27%) than in the DFO group (67%), P = .025.
In a Turin study, 54 patients who had received deferiprone were compared retrospectively with 75 patients who had received DFO; the drugs were given at standard doses over an average of 6 years.32 No patient in the deferiprone group compared with 3 in the DFO group died of cardiac failure. Deterioration of preexisting cardiac dysfunction or new cardiac disease occurred in 2 (4%) of the deferiprone-treated patients compared with 15 (20%) of the DFO group (P = .007).32 Formal prospective studies are needed to confirm the apparent greater cardioprotective effect against iron toxicity of deferiprone compared with subcutaneous DFO suggested by these two retrospective studies. In a short-term (12 months) prospective randomized study, Maggio et al33 found no difference in any of the parameters used to detect cardiac abnormalities between patients receiving DFO and patients receiving deferiprone.
Complications
The incidence of the now well-established complications of deferiprone therapy—agranulocytosis, neutropenia, arthralgia, gastrointestinal symptoms, transient changes in liver enzymes, and zinc deficiency—has been established in recent prospective trials34–,36 and reviewed.22 Hepatic fibrosis has also been suggested in one small retrospective study to be a consequence of deferiprone therapy.37 However, recent evidence based on 56 repeat biopsies in patients treated for a mean of 3.1 years shows no evidence for this.38 No other study has reported significant increase in hepatic fibrosis ascribed to deferiprone. Transient changes in alanine aminotransferase (ALT) levels, especially in the first few months of therapy and in hepatitis C antibody–positive patients, have been observed. The mean ALT levels did not increase among 151 patients treated for 3 years.36 Occasional patients have, however, been withdrawn from therapy in some trials because of concerns about raised ALT levels.34
Agranulocytosis, the most serious complication of deferiprone, occurs in about 1% of patients and appears to be idiosyncratic; it is probably more frequent in females. Patients with agranulocytosis should be permanently withdrawn from therapy, although a proportion of patients with less severe degrees of neutropenia have successfully been re-exposed to the drug. Most patients with the other side effects can usually continue with the drug, often after a period of withdrawal and retreatment initially at a lower dose.
Combination therapy
Combination therapy with DFO and deferiprone commenced in 1998, when it was reported that DFO and deferiprone could be safely given simultaneously and that the urine iron excretion achieved is at least equivalent to the iron excretion resulting when the 2 drugs are given on separate days.31 Six clinical studies of combination therapy have now been reported (Table 4 ). All show decreasing serum ferritin levels and, where measured, decreasing liver iron. Mourad et al,40 for instance, report that deferiprone 75 mg/kg 7 days a week and DFO 40 mg/kg subcutaneously over 8–12 hours 2 days a week gives approximately equivalent iron chelation, based on serum ferritin levels, to 5 days a week of DFO. Compliance is likely to be improved longer term for a patient needing 2 rather than 5 days of subcutaneous infusions.
The basis for this additive or synergistic effect is given by the studies of Grady et al30 and Breuer et al.45 These suggest that deferiprone enters cells and chelates iron, which it brings into plasma. The iron is then transferred to DFO for excretion in urine and feces (Figure 6 ). If combination therapy in longer-term studies does not show any unexpected toxicity, it is an exciting therapeutic advance for improving compliance and avoiding large, potentially toxic doses of either drug.
Alternating (sequential) therapy with DFO and deferiprone has also been studied in 7 children noncompliant to DFO.46 Compliance was improved when deferiprone was given for 4 days and then DFO for 2 days each week. Over 6 months, liver iron fell significantly and there was a nonsignificant fall in mean serum ferritin from 5536 to 3778 μg/L. More prolonged studies are needed to determine the place of this approach.
Thalassemia intermedia
Oral iron chelation therapy is a potentially attractive option for patients with iron overload who are too anemic for phlebotomy. Olivieri et al47 first reported a patient with thalassemia intermedia in whom deferiprone was effective in reducing both liver iron and serum ferritin to normal within 12 months of therapy. Pootrakul et al25 have recently extended these observations. They found in 8 thalassemia intermedia patients in Thailand (mainly suffering from thalassemia/hemoglobin E) that deferiprone at the low dose of 50 mg/kg/d not only significantly reduced serum ferritin and red cell membrane liver iron over 12 months but also resulted in an increase in hemoglobin and serum erythropoietin levels and improvement in weight and appetite. No side effects requiring drug withdrawal were encountered.
Other transfusion-dependent anemias
Similar results to those in TM have been obtained with deferiprone in patients with myelodysplasia, myelofibrosis, and other acquired marrow diseases. Although there has been theoretical concern that agranulocytosis may be more frequent in these acquired bone marrow disorders than in TM, there are no data to suggest this.
ICL 670
ICL 670 (4-[3,5-bis(2-hydroxyphenyl)-1,2,4-triazol-1-y1] benzoic acid) (Figure 4 ) was developed by Novartis Pharma AG after many hundreds of potential orally active iron chelators were screened. Preclinical studies show that it forms a 2:1 chelator:iron complex and produces an increase predominantly in fecal iron excretion after a single oral dose, only 6% of iron excretion accruing in the urine (Table 3 ). It is highly selective for iron, is rapidly absorbed, and circulates for several hours. In the non-iron-loaded marmoset and rat, its main toxic effect was on the renal tubular epithelial cells, but this effect was abrogated in iron-loaded marmosets and substantially reduced in iron-loaded rats.48
Short-term clinical trials have recently been reported.49 Single daily doses of 10, 20, and 40 mg/kg body weight were studied. Peak plasma concentration after a single oral dose occurred at about 2 hours, and the drug was still detectable in plasma in almost all patients at 24 hours; the mean elimination half-life was between 11–16 hours after multiple dose administration. Net iron excretion after 6 days of exposure was linearly related to the dose of the drug. Iron excretion at 12 days was related to the area under the curve of the concentration of free drug in plasma. Five of 6 patients receiving 20 mg/kg were calculated to excrete iron equivalent to the amount received in blood transfusions. The main side effect was skin rashes that required withdrawal of 4 patients given the highest dose of 40 mg/kg over 8–10 days. Sporadic transaminase rises occurred in 1 of these patients and in 4 other patients. Mild nausea, diarrhea, and abdominal pain—none requiring discontinuation of the drug—occurred in other patients. Longer-term studies of the drug at the dose of 20 mg/kg have been carried out.50 These showed that total body iron excretion ranged from 7.7–28.5 mg iron/d. These did not show any additional toxic effects and showed that liver iron decreased in 12 (57.1%), was unchanged in 8 (38.1%), and rose in 1 (4.8%) of 21 patients studied using the SQUID technique.
Conclusions
The prospect for transfusion-dependent patients to receive effective iron chelation therapy has substantially improved in the last few years. Subcutaneous DFO 40 mg/kg over 8–12 hours on at least 5 days a week protects most compliant patients against cardiac disease and other serious complications and remains the first choice. Its cost, frequent lack of compliance, and complications means that alternative approaches are needed. After many years of short-term clinical trials of the orally active agent deferiprone and much controversy about its efficacy and toxicity, recent published data have been favorable on both aspects. These suggest that the drug at a dose of 75 mg/kg/d may be at least as effective as DFO in protecting patients from iron-induced cardiomyopathy. Hepatic fibrosis does not appear to be a problem, and the established side effects do not lead to the need for discontinuation of the drug in the majority of patients.
Combination therapy with DFO and deferiprone is an exciting new possibility for those patients inadequately chelated on either drug alone. ICL 670, a new oral iron chelator in early clinical trial, promises to expand further the range of possibilities for effective and safe iron chelation therapy for patients with TM and other iron-loaded transfusion-dependent or -independent patients with severe refractory anemias. Whichever chelation regimen is chosen, patients must be closely monitored both for effectiveness of therapy, with particular attention to cardiac iron and function, and for toxic side effects of the chelating drug.
III. Newer Aspects of the Diagnosis and Treatment of Iron Deficiency
James D. Cook, MD*
University of Kansas Medical Center, 3901 Rainbow Boulevard, 1417 KU Hospital, Kansas City KS 66160-7233
Iron deficiency is by far the most common hematological disorder encountered in general practice. The basic approach to its diagnosis and management is well established and outlined in most medicine and hematology texts. My emphasis in this selective review is on soluble transferrin receptor (sTfR) measurements, iron deficiency induced by recombinant erythropoietin (rHuEPO) therapy, and parenteral iron therapy with sodium ferric gluconate.
Diagnosis of Iron Deficiency
There are two main categories of laboratory methods for identifying iron deficiency: screening measurements that detect iron deficient erythropoiesis (IDE) and definitive measurements that evaluate tissue iron status (Table 5). Newer tests include the percentage of hypochromic erythrocytes, reticulocyte hemoglobin content (CHr), and sTfR. It should be added that a therapeutic trial iron has been proposed as a convenient method to diagnose iron deficiency. This is a reasonable approach in otherwise healthy populations, such as teenage girls and pregnant women, at high risk of deficiency. However, in most clinical settings, it is preferable to make a definitive diagnosis at the outset.
Screening measurements
The initial screening method for iron deficiency in clinical practice is invariably the hemoglobin or hematocrit but additional laboratory evidence is needed, except perhaps in patients with obvious blood loss. Screening measurements to detect IDE narrow the diagnostic possibilities but are not specific. The traditional measurement is the transferrin saturation, which has the advantages of low cost and wide availability. However, marked diurnal variation and the numerous clinical disorders that affect the transferrin saturation limit its clinical utility. A normal or elevated value is as useful for excluding iron deficiency anemia as a low value is for diagnosing it.
Other screening methods are based on signs of restricted iron supply in circulating red cells. Examination of the peripheral smear is reliable in experienced hands but not cost-effective in routine clinical practice. The traditional measurement is the mean corpuscular volume (MCV) but it is one of the last parameters to change with the onset of IDE. The latter can be detected earlier by an increased percentage of hypochromic erythrocytes measured by selected hematology analyzers, but it is also a relatively late indicator of IDE.1 On the other hand, the reticulocyte hemoglobin content (CHr) declines within a few days of the onset of IDE because of the short 1–2 day lifespan of circulating reticulocytes.1–,3 Falsely normal values of the CHr can be observed with an elevated MCV or thalassemia4 but the main limitation to its wider use is that it can be measured by only one model of analyzer (Bayer Advia). The low specificity of the percent hypochromic erythrocytes and the CHr is reflected in a recent study reporting that 62% of predominantly anemic patients had abnormal values on admission to hospital.1
The measurement of erythrocyte zinc protoporphyrin (ZPP), a product of abnormal heme synthesis, is a simple and precise measure of IDE but still not widely used for clinical purposes.5 One important advantage is the ability to measure the ratio of ZPP/heme directly on a drop of whole blood using a dedicated portable instrument called a hematofluorimeter. The ZPP is ideally suited for field surveys of iron status or pediatric and obstetrical clinics where uncomplicated iron deficiency is relatively common. The ZPP is redundant in laboratories equipped for percentage hypochromic red cell measurements because the two tests provide similar information. ZPP increases with lead toxicity and falsely elevated values and can occur in patients with elevated serum bilirubin or on regular hemodialysis, although the interference can be eliminated with prior washing of the red cells.6
Storage iron and serum ferritin
The serum ferritin is a universally available and well-standardized measurement that has been the single most important laboratory measure of iron status during the past quarter century. Phlebotomy studies in normal subjects have demonstrated that 1 μg/L serum ferritin corresponds to 8–10 mg or 120 μg storage iron/kg body weight,7 although a log transformation gives a more accurate estimate.8 Numerous studies have demonstrated its superiority over other iron-related measurements in identifying iron deficiency anemia. In 55 studies culled from 1179 relevant citations, receiver-operator characteristic curves in 2579 subjects gave a mean area for the serum ferritin of 0.95 ± 0.1 (95% confidence limits) as compared with 0.77 for the ZPP, 0.76 for the MCV, and 0.74 for the transferrin saturation.9 The well-known limitation of the serum ferritin is the elevation in values independent of iron status that occur with acute or chronic inflammation, malignancy, liver disease, and alcoholism.
Many hematologists still rely on the assessment of stainable iron on aspirated marrow smears or biopsy for the definitive diagnosis of iron deficiency anemia. Although still widely regarded as the gold standard for the diagnosis of iron deficiency, the reliability of the marrow iron stain is often suboptimal when used for routine clinical purposes. In a recent study, 108 consecutive bone marrow specimens from unselected hematology patients reported to have absent iron were reviewed.10 One-third of the reports were incorrect due either to an inadequate specimen or detectable iron stores, and less than half of the patients with absent marrow iron had clinical evidence that supported the diagnosis of iron deficiency anemia. In another recent review of iron stains, high intra-observer variability in pathological diagnosis led the authors to conclude that the bone marrow is not a perfect gold standard.4 It should also be noted that the bone marrow is no longer reliable in diagnosing iron deficiency anemia after parenteral iron therapy. While marrow iron staining continues to play a critical role in validating newer laboratory measurements of iron status when performed and reviewed under standardized conditions in prospective studies by experienced investigators, bone marrow examinations should seldom be performed solely to diagnose iron deficiency because of the expense, discomfort, and technical pitfalls with this approach.
Tissue iron and serum transferrin receptor
Transferrin receptors are membrane glycoproteins that serve as the gateway for circulating transferrin iron to the interior of all body cells. In addition to erythroid precursors that contain 80% of the total body receptor mass, rapidly dividing cells and the placenta contain a high density of receptors. The synthesis of ferritin and transferrin receptors is precisely regulated by a common mechanism involving the iron response protein and a nucleotide sequence termed the iron response element.
The sTfR is a soluble form of the cellular receptor lacking the first 100 amino acids and composed of the extracellular domain. Countless articles have been published on the clinical utility of the sTfR since it was first discovered in 1986 by Kohgo and coworkers.11 Ferrokinetic studies have demonstrated that the sTfR is directly correlated with the total mass of erythroid precursors over the complete spectrum of hematological disorders ranging from marrow aplasia to thalassemia major.12 Its use for assessing erythropoiesis has been reviewed recently.13 The only determinant of the sTfR other than the erythroid precursor mass is tissue iron deficiency, which increases the sTfR in proportion to the severity of iron deficit.14 Several commercial assays are now available, but wider application of sTfR measurements is limited by the divergent values reported with different assays,15 differences that could probably be eliminated by the development of an international standard.
Isolated iron deficiency
Isolated or uncomplicated iron deficiency in the absence of other diseases that influence measurements of iron status is seen most often with rapid growth or during gestation, and in patients with excessive uterine or gastrointestinal blood loss. The key laboratory measurement for its identification is the serum ferritin. A low hemoglobin concentration in a patient with a serum ferritin < 30 μg/L is diagnostic of iron deficiency anemia.
One drawback of relying solely on a low hemoglobin and serum ferritin is that milder iron deficiency without anemia goes undetected. Individuals with baseline hemoglobin values in the upper normal range must lose 20%–30% of their body iron before iron deficiency can be detected by anemia. A method for measuring body iron quantitatively using the log(sTfR/serum ferritin) ratio that permits detection of mild tissue iron deficiency has been described recently.16 The method estimates in mg/kg the surplus of storage iron in replete individuals or the tissue deficit in those with iron deficiency. Serial measurements in an individual remain constant over several months allowing those at risk of recurrent iron deficiency to be monitored prospectively. The relevance of iron deficiency without anemia that can be assessed with this approach has been unclear largely because of the difficulty in identifying it. Greater improvement in exercise performance was observed in iron-depleted nonanemic females given iron supplements as compared with unsupplemented controls despite no effect on hemoglobin values.17 Mild iron deficiency without anemia could explain chronic fatigue in some individuals.18,19 It is also preferable to detect declining tissue iron levels in individuals with recurrent iron deficiency before overt anemia develops.
Iron deficiency and chronic disease
The diagnosis of iron deficiency would be simple if it were not for the many clinical disorders that influence the internal iron cycle. The anemia of chronic disease is a common hematological disorder that is easier to recognize than it is to define. Because it alters screening tests for iron status in the same manner as true iron deficiency, the distinction between the anemia of chronic disease and iron deficiency anemia requires tissue-related iron measurements. To avoid the need for bone marrow examinations in patients in whom iron deficiency anemia is suspected, reliance is often placed on the serum ferritin concentration despite the well-known elevation with acute or chronic inflammation. The optimal cut-off values of the serum ferritin to distinguish iron deficiency anemia from the anemia of chronic disease was examined in a landmark study in 259 anemic patients over 65 years of age.20 In 36% of patients with iron deficiency anemia based on bone marrow examination, the serum ferritin was the only test of several that added useful diagnostic information. Only 2 of 49 patients with serum ferritin < 18 μg/L did not have iron deficiency anemia and only 8 of 116 with a serum ferritin > 100 μg/L had iron deficiency anemia. Between 18 and 100 μg/L, 40% had iron deficiency anemia although only 1 such patient had a serum ferritin > 45 μg/L. The authors later proposed serum ferritin values < 40 and < 70 μg/L to diagnose iron deficiency anemia in anemic patients without and with inflammation respectively.9 A serum ferritin < 50 μg/L has been proposed as the best cutoff to identify iron deficiency anemia in patients with liver disease.21
Because the sTfR concentration remains normal in patients with the anemia of chronic disease,22 it is an invaluable addition to the serum ferritin measurement. The sTfR cannot only distinguish iron deficiency anemia from the anemia of chronic disease but it can also identify iron deficiency anemia when it occurs in patients with the anemia of chronic disease. In 129 consecutive anemic patients receiving a bone marrow for stainable iron, iron deficiency anemia was identified in 48, the anemia of chronic disease in 64 and both disorders in 17.23 The sTfR was normal in all patients with the anemia of chronic disease, elevated in 41 of 48 patients with iron deficiency anemia and in 13 of 17 patients with both the anemia of chronic disease and iron deficiency. The separation between the 3 groups was further improved using the sTfR/log(serum ferritin) ratio; no patients with iron deficiency anemia overlapped those with anemia of chronic disease and all but 1 patient with both the anemia of chronic disease and iron deficiency anemia had higher values than patients with the anemia of chronic disease only. A recent study has indicated that even better discrimination can be obtained with the log(sTfR/serum ferritin)24 as described above for quantifying body iron. Use of the receptor/ferritin ratio can eliminate the need for bone marrow examination to detect iron deficiency in patients with chronic inflammatory joint or bowel disease who are usually reluctant to undergo this unpleasant procedure.
Iron deficiency and rHuEPO therapy
Iron status in patients with chronic renal failure has varied widely over the past half century. Transfusional iron overload was invariable until the introduction of hemodialysis when iron deficiency emerged due to dialyzer blood loss. Vigorous parenteral iron therapy led to reports of significant iron overload but with the introduction of rHuEPO therapy, iron deficiency again became widespread. Vigorous parenteral iron therapy has again raised concern about iatrogenic iron overload.25
The term functional iron deficiency has arisen mainly in the nephrology literature in reference to the IDE induced by rHuEPO therapy in dialysis patients with residual iron stores.26 The diagnosis is usually based on one or more screening measurements (Table 5 ). Aggressive parenteral iron therapy is commonly advised for its treatment on the assumption that functional iron deficiency is the major cause of resistance to rHuEPO therapy. While this is undoubtedly true in many patients, the laboratory features are also typical of the anemia of chronic disease and there is sparse information about the role of inflammation in so-called functional iron deficiency. The sTfR is not an optimal guide to the need for additional parenteral iron because of the enhancing effect of rHuEPO on erythropoiesis and consequently on the sTfR. More data is needed on the extent to which inflammation contributes to functional iron deficiency and whether a rise in sTfR concentration in patients on stable doses of rHuEPO can be used as a guide to parenteral iron therapy.
In a dialysis patient without laboratory evidence of inflammation, the amount of iron that should be given during the first 2–3 weeks of initiating rHuEPO therapy can be calculated from the anticipated increase in circulating hemoglobin (roughly 4 mg iron/kg body weight for each 10 g/L hemoglobin rise) minus iron stores based on 8 mg available iron per μg/L serum ferritin or 3–4 mg/μg/L serum ferritin if the CRP is elevated. After achieving a hematological response, parenteral iron should be continued to maintain a serum ferritin > 100 μg/L if the CRP is normal and > 200–300 μg/L if elevated. There is evidence that serum ferritin values above 500–800 μg/L increase the risk of infections in hemodialysis patients26 and this risk could be even greater in pancytopenic patients given rHuEPO therapy who are already at increased risk of infection.
Treatment of Iron Deficiency
Oral iron therapy
It is preferable to treat iron deficiency with oral rather than parenteral iron. One iron tablet taken daily without food is as effective as 3 tablets with meals, and the disparity is even greater in patients with atrophic gastritis, chronic suppression of gastric acid secretion, and gastric stapling or bypass surgery. The major obstacle to successful oral therapy is the nausea and epigastric discomfort that occurs 30–60 minutes after taking iron, symptoms that are dose-related but often subside after 2–3 days with continued treatment. Reducing the dose or taking a tablet at bedtime is usually helpful in reducing side effects. Diarrhea or constipation are not dose-related and should be managed symptomatically. Commercial iron preparations promoted on the basis of fewer side effects are invariably less well absorbed. A brief follow-up clinic visit 2–3 weeks after initiating oral therapy can be helpful in tailoring an oral iron regimen in patients having troublesome side effects. Parenteral iron should not be used simply as a convenience for the patient or physician.
Parenteral iron therapy
The main indications for parenteral iron are uncontrolled blood loss, intolerance to oral iron, intestinal malabsorption, and poor adherence to an oral regimen. Iron malabsorption can be detected by observing an increase in serum iron of less than 100 μg% over baseline in a fasting patient 1 or 2 hours after taking 60 mg iron as ferrous sulfate.
Until recently, iron dextran has been the only parenteral iron preparation available in the United States. It is a low molecular weight dextran complexed with ferric oxide and supplied as a dark brown solution containing 50 mg iron per mL. Although intramuscular administration is still recommended by current manufacturers, intravenous (IV) administration is preferred by most physicians because of the ability to administer larger doses. In hemodialysis patients in whom multiple treatments can be given conveniently, 6–10 injections of 100 mg iron can be administered on consecutive dialysis days.27 In other patients, a major advantage of iron dextran is the ability to administer relatively large doses of 500–2000 mg iron on a single occasion.28,29 After diluting the dose in 500 mL normal saline, and premedicating with diphenylhydramine with or without steroids, the first 20–30 mL is given slowly over 5 minutes as a test dose. If no allergic reaction occurs within the first 15–30 minutes, the remainder of the dose is given over the next 3–4 hours.
The major drawback of iron dextran is a severe anaphylactic reaction that occurs within a few minutes of initiating the infusion and is sometimes fatal. At least 30 deaths have been attributed to iron dextran use in the US since 197630 and because anaphylaxis can occur in those who have not reacted to iron dextran in the past, a test dose is always required. Another drawback of iron dextran is a less serious delayed reaction occurring 24–48 hours after the infusion. Characterized by myalgia, arthralgia, headache, and malaise, delayed reactions occured in over 10% of patients given total dose infusions.31 These symptoms usually respond promptly to nonsteroidal anti-inflammatory drugs but the reaction can be severe and prolonged in patients with inflammatory joint disease.
Sodium ferric gluconate (SFG)
This form of parenteral iron was approved in the US in 1999 under the Food and Drug Administration’s priority drug review, although it has been used in Europe for many years. SFG in sucrose is a stable macromolecular complex supplied as a deep red solution in 5 mL ampoules containing 62.5 mg elemental iron. The current product label advises administration of 5–10 mL as a direct IV push at a rate of 12.5 mg per min for 10 minutes for a recommended dose of 125 mg iron. No test dose is required. Unlike iron dextran, which can require several weeks for complete processing by macrophages, roughly 80% of SFG is delivered to transferrin within 24 hours. One report suggested that saturation of circulating transferrin can be exceeded following the infusion of SFG32 but this was later explained by the release iron from the ferric gluconate during the serum iron determination.33
The appeal of SFG administration is avoidance of the risk of the serious anaphylactic reactions associated with iron dextran. In a review of data from the World Health Organization and pharmaceutical manufacturers, no deaths were reported with 25 million infusions of SFG as compared with 31 deaths from about half the number of administratons of iron dextran.30 Adverse reactions with SFG in a multicenter, randomized, crossover, double-blind, placebo-controlled prospective study in 2534 hemodialysis patients was recently reported.34 Adverse events were similar between SFG and placebo groups, 12.3% versus 9.8%. Life-threatening events (immediate reactions requiring resuscitation measures) and drug intolerance rates (any event that precluded further SFG infusions) were compared to historical adverse events in 3768 patient exposures to iron dextran. A life-threatening event was observed in a single patient given SFG as compared with 23 patients given iron dextran while drug intolerance occurred in 11 patients receiving SFG as compared with 64 patients given iron dextran (Table 6 ). These results are a compelling reason for using SFG rather iron dextran in hemodialysis patients and in most patients requiring parenteral iron. The feasibility of large single doses with iron dextran provides a useful alternative in noncompliant patients or those who are seriously inconvenienced by multiple iron infusions.
Iron sucrose
Iron sucrose (saccharate) was approved for use in the US in late 2000, providing a third option for intravenous iron therapy. Iron sucrose has also been used for several decades outside of the US although recently published experience is more limited than with SFG. Iron sucrose is supplied as an aqueous brown solution in 5 mL vials containing 100 mg of elemental iron. No test dose is required. It is recommended that 5 ml iron sucrose (100 mg iron) either be infused directly or first diluted in 100 mL normal saline and infused over 15 min to reduce the risk of hypotensive episodes. The safety profiles of iron sucrose and SFG are similar although most studies have evaluated efficacy rather than adverse reactions.
Summary and Conclusions
In otherwise healthy individuals at higher risk of iron deficiency due to rapid growth, pregnancy, or excessive blood loss, a low hemoglobin and serum ferritin < 30 μg/L is diagnostic of iron deficiency anemia. In hospitalized or elderly patients who have a higher prevalence of chronic disease, a CRP and sTfR should be added to the initial laboratory evaluation. If the CRP is normal, anemia and a serum ferritin < 30 μg/L establish iron deficiency anemia. If anemia is present and the CRP is elevated, iron deficiency anemia can be diagnosed in patients with anemia of chronic disease by an elevated sTfR. Prospective trials are needed to determine the optimal laboratory approach for monitoring initial and maintenance parenteral iron therapy in patients receiving rHuEPO therapy. When parenteral iron is indicated because of iron malabsorption, heavy iron losses or intolerance to oral iron, sodium ferric gluconate or iron sucrose is preferable to iron dextran because of reduced acute and delayed drug reactions.
Symptoms and laboratory findings in white homozygotes for the C282Y mutation (light bars) and in wildtype controls (heavy bars).
Adapted from the data of the Kaiser/Scripps study19
Symptoms and laboratory findings in white homozygotes for the C282Y mutation (light bars) and in wildtype controls (heavy bars).
Adapted from the data of the Kaiser/Scripps study19
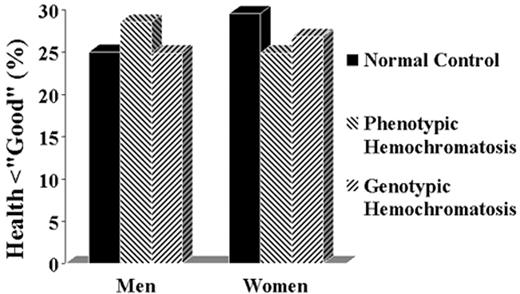
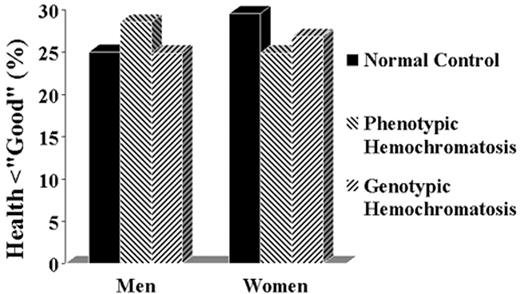
The percentage of homozygotes for the C282Y mutation (genotypic homozygotes) and of subjects with persistently elevated transferrin saturation and ferritin (phenotypic homozygotes) who consider themselves to be in less than good or excellent health.
Based on the data from Åsberg et al34
Figure reprinted with permission from
The percentage of homozygotes for the C282Y mutation (genotypic homozygotes) and of subjects with persistently elevated transferrin saturation and ferritin (phenotypic homozygotes) who consider themselves to be in less than good or excellent health.
Based on the data from Åsberg et al34
Figure reprinted with permission from
The relationship between serum ferritin levels and storage iron as determined by serial phlebotomy.
The dots represent patients homozygous for the C282Y mutation. Squares represent patients with a diagnosis of hemochromatosis who are not homozygous for the C282Y mutation. The plus signs represent those whose phlebotomy program had not yet been completed.
Figure reprinted with permission from
The relationship between serum ferritin levels and storage iron as determined by serial phlebotomy.
The dots represent patients homozygous for the C282Y mutation. Squares represent patients with a diagnosis of hemochromatosis who are not homozygous for the C282Y mutation. The plus signs represent those whose phlebotomy program had not yet been completed.
Figure reprinted with permission from
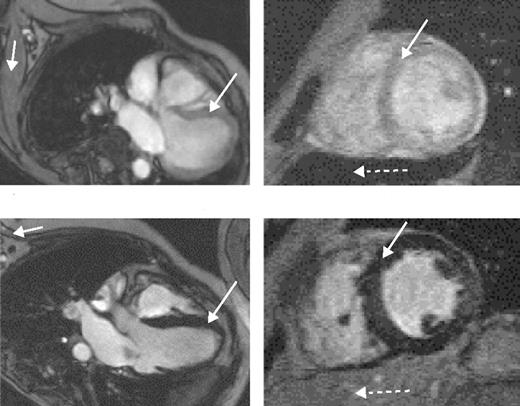
Magnetic resonance scans in patients with thalassemia major.Left scans are horizontal long axis, the right ones mid-short axis. (A) Low myocardial iron deposition. The left ventricular volumes are normal, and myocardial signal intensity (long arrow) is similar to that arising from skeletal muscle (short arrow). Left ventricular ejection fraction was 70%. In this case, the liver is very dark (dotted arrow), indicating heavy hepatic iron deposition despite the normal myocardial appearances. (B) Severe myocardial iron overload. The myocardial signal intensity is dark (long arrow) compared with skeletal muscle (short arrow). The ventricle is dilated and thickened. Cine imaging showed greatly reduced systolic function (left ventricular ejection fraction 39%) with a restrictive filling pattern. Liver signal in this case is well preserved (dotted arrow).
Reproduced with permission from
Magnetic resonance scans in patients with thalassemia major.Left scans are horizontal long axis, the right ones mid-short axis. (A) Low myocardial iron deposition. The left ventricular volumes are normal, and myocardial signal intensity (long arrow) is similar to that arising from skeletal muscle (short arrow). Left ventricular ejection fraction was 70%. In this case, the liver is very dark (dotted arrow), indicating heavy hepatic iron deposition despite the normal myocardial appearances. (B) Severe myocardial iron overload. The myocardial signal intensity is dark (long arrow) compared with skeletal muscle (short arrow). The ventricle is dilated and thickened. Cine imaging showed greatly reduced systolic function (left ventricular ejection fraction 39%) with a restrictive filling pattern. Liver signal in this case is well preserved (dotted arrow).
Reproduced with permission from
The concept of combination therapy.
Abbreviations: DFO, deferoxamine; DFP, deferiprone; NTBI, non-transferrin-bound iron.
Modified from Liu et al.20
The concept of combination therapy.
Abbreviations: DFO, deferoxamine; DFP, deferiprone; NTBI, non-transferrin-bound iron.
Modified from Liu et al.20