Abstract
Marginal-zone lymphoma (MZL) includes three subtypes depending on the site of lymphoma involvement: extranodal marginal zone B-cell lymphoma of mucosa-associated lymphoid tissue (MALT-lymphoma); splenic MZL; and nodal MZL. Beside a common cell-of-origin and similarities concerning a possible chronic antigenic stimulation by microbial pathogens and/or autoantigens, the clinical presentation is very different with symptoms related to lymphoma location. MALT and splenic MZL present with an indolent disease with good performance status, no B symptoms, and no adverse prognostic factors and are associated with long survival. Patients with nodal MZL present with a more aggressive disease and have a shorter failure-free survival. Clinical and biological prognostic factors identified in reported series are heterogeneous. The optimal treatment has yet to be defined for the three subtypes, and current strategies will be described in this review.
Marginal zone B-cell lymphomas (MZL) represent a group of lymphomas whose cells originate from B lymphocytes normally present in a distinct anatomical location, the so-called “marginal zone” (MZ) of the secondary lymphoid follicles.1 These cells are anatomically localized in the lymphoid organs (spleen and lymph nodes) and in the non-lymphoid organs (mucosa-associated lymphoid tissue [MALT] or non-mucosal tissue such as skin, orbit and dura). Depending on the site of involvement, the International Lymphoma Study Group individualized three distinct subtypes of MZL: (1) extranodal MZL of MALT type, (2) splenic MZL (with or without villous lymphocytes), and (3) nodal MZL (with or without monocytoid B cells).2,3 Despite this classification, the relative rarity of these lymphomas and difficulties in distinguishing them from other low-grade lymphoma subtypes are obstacles to conducting epidemiological surveys and to describing clinical features and outcomes. Moreover, no prospective studies on large series have been published to date, making therapeutic decisions difficult. Data regarding clinical and biological prognostic markers are limited, and it is therefore difficult to predict those in whom the disease will be more aggressive. This review will present recent data describing the epidemiology, clinical features, staging, and therapy of these lymphomas.
Epidemiology
MZL account for between 5% and 17% of all non-Hodgkin lymphomas (NHL) in adults depending on the series. MALT lymphoma is the most frequent of the MZL subtypes, representing 50% to 70% of MZL and 7% to 8% of NHL. The splenic and the nodal MZL represent 20% and 10% of MZL, respectively, and account for less than 1% of NHL. Most of the cases occur in adults, with a median age of approximately 60 years, except for splenic MZL with villous lymphocytes (SLVL), occurring in adults at a median age of around 70 years.4–8
Growing evidence indicates that MZL of MALT, splenic and nodal types are associated with chronic antigenic stimulation by autoantigens and/or microbial pathogens, inducing an accumulation of lymphoid tissue in the typical sites of involvement for each lymphoma entity in mucosa or organs that contain no native lymphoid tissue for MALT lymphomas, in spleen for splenic MZL, and in nodes for nodal MZL. In the case of autoimmunity, several diseases have been associated with an increase risk of MALT lymphoma, such as Hashimoto thyroiditis, myoepithelial sialoadenitis (MESA) with or without associated Sjögren syndrome, or lymphoid interstitial pneumopathy. Based on epidemiological studies, molecular investigations, and therapeutic success of lymphoma regression with antibiotics, five distinct microbial pathogens have now been identified to be related to MZL. Helicobacter pylori is the best characterized and is associated with gastric MALT lymphoma.9 Other chronic infections have been described in cases of MZL, although their role in pathogenesis remains to be firmly established. Borrelia burgdorferi associated with Lyme disease has been proposed to play a possible role in cutaneous MALT lymphoma.10Campylobacter jejuni in small intestine has been associated with immunoproliferative small intestinal disease (IPSID),11Chlamydia psittaci infection with ocular adnexal MALT lymphoma,12 and hepatitis C virus (HCV) infection with MALT, splenic and nodal MZL.13,14 Identification of such microbial antigens that may play a pathogenic role in lymphomagenesis through chronic stimulation and indirect transformation of lymphoid cells has important therapeutic implications for these diseases.
MALT Lymphomas
Clinical features of MALT lymphomas
The clinical presentation of MALT lymphomas varies according to the lymphoma location (Table 1; Figure 1; see Color Figures, page 549), but shared characteristics can be described. Most MALT lymphoma patients present at diagnosis with an indolent disease with good performance status (PS), absence of B symptoms, and no adverse biological prognostic factors such as high lactate dehydrogenase (LDH) or β2-microglobulin levels.13,15,16 Disease is localized for the majority of the patients but multifocal lesions are present in 30% to 40% of patients.17 Dissemination of the disease occurs either to other mucosal sites or, more often, by extension from a mucosal site to a non-mucosal site such as spleen, bone marrow, or liver. Bone marrow involvement is detected in 20% of the cases. Risk of dissemination is significantly higher for non-gastrointestinal (GI) tract lymphomas.17
Staging
The staging procedures in MALT lymphomas are not standardized, particularly with regard to the number of the extranodal sites that need evaluation at diagnosis. Early dissemination of the disease occurs in nearly 35% of the patients without changing their outcome.17 Therefore, pre-therapeutic procedures including extensive staging in patients to meticulously assess dissemination is probably not necessary. Guidelines concerning this staging are reported in Table 2 . The second difficulty in staging MALT lymphoma is the application of the traditional staging systems for nodal-type lymphoma to MALT-type lymphoma. The Ann Arbor system is based on the extension from contiguous nodes and can be misleading in MALT-type lymphomas, since the involvement of multiple extranodal sites, particularly within the same organ (e.g., skin, gastric), may not reflect truly disseminated disease. The gastric issue has been particularly discussed in several international meetings and alternative staging systems have been proposed.18
Treatment
Despite abundant literature on pathophysiological features of MALT lymphomas, only few retrospective series of surgery, irradiation or chemotherapy are reported. The International Extranodal Lymphoma Study Group (IELSG) has an ongoing effort to coordinate studies in these diseases.19
The unique pathogenesis of MZL related to possible microbial pathogens that may initiate the disease has an impact on therapy. For localized disease, there is increasing evidence indicating that antibiotics can be effectively employed as the sole initial treatment. In the stomach, eradication of H pylori leads to complete regression of the lymphoma in nearly 80% of the cases.20 In ocular adnexal, skin, and small intestinal MALT lymphomas, an objective clinical response has been observed in some patients after antimicrobial treatment.11,12,21 However it is still unknown whether eradication of microorganisms will definitively cure the lymphoma. In gastric MALT lymphoma, polymerase chain reaction (PCR)-detectable B-cell monoclonality may persist after the disappearance of histological evidence of MALT lymphoma, suggesting that H pylori eradication suppresses but does not eradicate the lymphoma clone.22
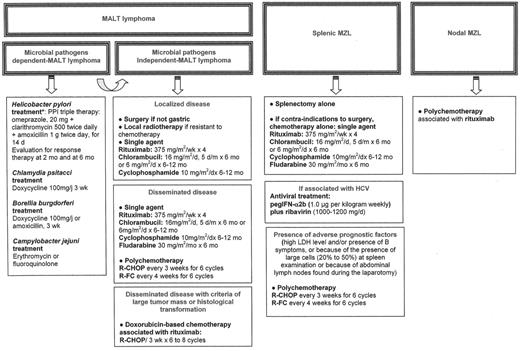
There are no treatment guidelines for the management of patients with MALT lymphoma associated with microbial pathogens who fail antibiotic treatment, or for those with MALT lymphoma not associated with microbial pathogens. For localized disease, local treatment (either radio-therapy or surgery) will usually achieve excellent disease control.23,24 In patients with disseminated disease at presentation, single agents such as alkylating agents (cyclophosphamide or chlorambucil) or fludarabine have been reported to induce a 75% complete remission rate, with projected 5-year event-free and overall survival rates at 50% and 75%, respectively.25 Anthracyline-based chemotherapy has to be reserved for patients with histological transformation or with high tumor mass (i.e., high LDH, mass greater than 7 cm). Recently rituximab has been reported to induce an overall response rate of around 75%, with better results as first-line therapy,19,26 and may have a real place in the management of MALT lymphoma. An ongoing international trial is currently testing whether the combination of rituximab with chlorambucil enhances the activity of chlorambucil (IELSG19). The treatment of these patients, until the conclusion of prospective trials, is summarized in Figure 2F1 .
Outcome and Prognostic Factors
Patients with MALT lymphoma have a favorable outcome with 5-year overall survival reported between 95% and 86% (Figure 3; see Color Figures, page 549), without any significant difference between GI or non-GI lymphoma and between localized and disseminated disease.13,16,17 Median time-to-progression is estimated at around 5 years (Figure 3; see Color Figures, page 549) and is significantly higher for the GI locations compared to the non-GI locations (8.9 versus 4.9 years, respectively; P = .01).17 Recurrences may involve different extra-nodal sites or nodal sites. Histologic transformation to large cell lymphoma is reported to occur in less than 10% of the cases, usually late in the course of the disease and independent of dissemination.13
Prognosis in MALT lymphoma has been reported to be influenced by prognostic factors for lymphoma including poor PS, bulky tumor, and high levels of LDH, β2-microglobulin, and serum albumin.16,27 The presence of a large-cell component at diagnosis is also associated with a poorer outcome.16,27 Influence of systemic dissemination on survival is controversial, being significantly associated with poorer prognosis in some studies.17,28 Interestingly the t(11;18)(q21;q21), a translocation specific of MALT-type lymphoma and detected in 18% to 24% of patients with gastric MALT lymphoma, has been correlated to a resistance to H pylori eradication therapy29 and to alkylating agents30 and but not to rituximab.26
Splenic MZL
Clinical features of splenic MZL
Splenic MZL is rare and overlaps with other indolent lymphomas. The hallmark of the clinical presentation is usually splenomegaly.4,5,31 The splenomegaly becomes symptomatic when massive and/or associated with cytopenias. Early in the disease, however, the splenomegaly may be detectable only on computed tomography (CT) scanning. Small involved splenic hilar lymph nodes are frequently present. Peripheral lymph node involvement is unusual;3 if it is present, the presentation is usually classified as a disseminated nodal and splenic subtype.6
Bone marrow and blood involvement are present in 95% of patients with splenic MZL.31 In 15% of the cases, blood involvement is represented by villous lymphocytes, lymphocytes displaying cytoplasmic protrusions.31 It is controversial whether splenic lymphoma with villous lymphocytes (SLVL) is the leukemic counterpart of all splenic MZL or whether it is a subentity of splenic MZL. It is presently agreed that the SLVL applies to patients with more than 20% villous lymphocytes in the blood. Whereas the serum LDH level is usually normal in splenic MZL, the β2-microglobulin level is increased. A large proportion of patients have a serum monoclonal paraprotein (M-component), mainly of the μ (IgM) isotype.31
In some patients, the first manifestation of the lymphoma is an immune hemolytic anemia or an immune thrombocytopenia. These patients may respond to corticosteroids. Uncommonly, autoantibodies against coagulation factors are present.
Diagnostic procedures and positive diagnosis
Patients with splenic MZL often present with lymphocytosis, cytopenias, or symptomatic splenomegaly. Bone marrow examination shows an involvement in 90% of the cases. The morphology and immunophenotype of these cells usually suggest the diagnosis of splenic MZL. The presence of characteristic cytogenetic abnormalities such as 7q deletion may confirm this. When the blood and bone marrow are not involved, the diagnosis can only be made after splenectomy.
Treatment
Patients with moderate asymptomatic splenomegaly may be followed without any treatment.4,5,31,34 The absence of treatment does not influence the course of the disease and these patients often have stable disease for at least 10 years.5,35
When treatment is indicated (i.e., occurrence of a large symptomatic splenomegaly and/or cytopenia), splenectomy is the treatment of choice.31 Splenectomy is beneficial in terms of improvement of performance status and correction of cytopenias4,31 This benefit, which is due to the disappearance of hypersplenism but also the reduction of marrow infiltration after splenectomy,36 persists for years, with an interval before requiring further treatment of over 5 years, even if the patient develops progressive lymphocytosis.31
Chemotherapy based on alkylating agents (chlorambucil or cyclophosphamide) or purine analogues (fludarabine) has also been reported as an effective treatment in non-randomized studies or in retrospective analyses.31,37 The role of adjuvant chemotherapy in the setting of adverse prognostic factors such as high LDH level and/or presence of B symptoms, or because of the presence of large cells (> 20%) has not been established. Although chemotherapy may increase the number of complete responses, it has no impact on the risk of relapse, histological progression, or survival in comparison to patients who receive no adjuvant chemotherapy.31 Chemotherapy alone may be proposed initially to patients with contraindications to surgery but who required treatment or later for patients with clinical progression after splenectomy. In this setting, good responses have been reported with single-agent fludarabine but not with 2-deoxycoformycin.38 Good responses have been observed with rituximab in patients refractory to standard chemotherapy.
In cases of HCV-associated splenic MZL, antiviral treatment with interferon (IFN)-α or IFN-α plus ribavirin has been reported in a small series to be associated with a marked reduction of lymphocytosis and splenomegaly, whereas antiviral therapy is ineffective in HCV-negative splenic MZL.33 This very interesting observation warrants further follow-up.
Outcome and prognostic factors
Patients with splenic MZL have a long survival in most reported series, with more than 50% survival at 5 years.4,5,31,34 Progression to lymph nodes or extranodal sites may occur. described with a median time to progression is 3.7 years, with no difference between non-SLVL cases and SLVL patients.31
Histological transformation to diffuse large B cell lymphoma (DLBCL) is rare, occurring at time of tumor recurrence in 10% to 20% of patients, and is clinically associated with B symptoms, poor performance status, disseminated disease in nodal and extra-nodal locations, high LDH, and poor outcome (median survival of 26 months31). Transformation may occur at diagnosis (seen on the removed spleen) or at time of progression with a median time to transformation of 12 to 85 months.31,39 Transformation is frequently associated with p53 inactivation or new chromosomal abnormalities.
No specific prognostic factors have been described for splenic MZL, although the International Prognostic Index (IPI) is often used. However, in our experience, none of these criteria (age, PS, stage, number of extranodal sites, and LDH level) influence outcome.31 Leukocyte count > 30 or > 20 x 109/L, lymphocytes < 4 or > 20 x 109/L, β2-microglobulin level, presence of monoclonal component, and initial treatment with chemotherapy have been described as adverse prognostic factors.5,31,34
Nodal MZL With or Without Monocytoid B-Cells
Clinical presentation
Because of the more recent identification of this lymphoma, clinical data restricted to nodal MZL may be found in only three reports in the literature,8,14,40 with limited numbers of patients. The median age for these patients is 50 to 62 years, with a slight female predominance. The vast majority of the patients present with disseminated, peripheral and visceral nodal involvement with bone marrow involvement in less than half of the patients (28%, 43%, and 44%, respectively7,8,14). There is no difference among these groups with respect to B symptoms, elevated LDH, performance status, or IPI score compared to other primary nodal B-cell lymphomas such as follicular lymphomas.7 Elevated β2-microglobulin is found in one-third of the patients, and an M-component is infrequently detected (8%).41 Cytopenias are rare,14 and few cases are reported to be associated with HCV.14
Outcome and prognostic factors
The outcome of patients with nodal MZL is similar to that of patients with splenic MZL but worse than that of patients with MALT lymphoma (Figure 3; see Color Figures, page 549). Estimated 5-year overall survival is reported between 50% and 70%,7,8 without any plateau suggesting that the disease is not currently curable. The estimated median time to progression is between 1 and 2 years.7,41 This may be explained by the fact that 20% of patients present at diagnosis with a component of large cells (> 20%) and a high mitotic count with evidence for transformation into diffuse large B-cell lymphoma.7 Given the small numbers of cases reported, no specific prognostic factors are proposed. At the time of relapse, nodal involvement is usually predominant, although splenic or extranodal involvement may occur.
Treatment
A precise recommendation for therapy is difficult because of the limited available data. The disease is characterized as having a good survival but a short time to progression, and logical treatment options include polychemotherapy, with or without anthracyline, associated with rituximab.42
Conclusion
Although the WHO lymphoma classification has provided significant advances in defining MZL, several issues regarding the clinical course of these lymphomas remain to be defined. Collaboration of clinicians and pathologists in defining stringent diagnostic criteria, will help in designing prospective clinical trials to define the optimal therapeutic approach to these diseases.
Suggestions for treatment of patients with marginal zone B-cell lymphoma (MZL).
Abbreviations: HCV, hepatitis C virus; IFN, interferon; LDH, lactate dehydrogenase; R-CHOP, rituximab, vincristine, doxorubicin, cyclophosphamide, predisolone; R-FC, rituximab, fludarabine, cyclophosphamide
Suggestions for treatment of patients with marginal zone B-cell lymphoma (MZL).
Abbreviations: HCV, hepatitis C virus; IFN, interferon; LDH, lactate dehydrogenase; R-CHOP, rituximab, vincristine, doxorubicin, cyclophosphamide, predisolone; R-FC, rituximab, fludarabine, cyclophosphamide
Acknowledgments: The author thanks Bertrand Coiffier, Pascale Felman, Françoise Berger, Evelyne Callet-Bauchu, Alexandra Traverse-Glehen, Lucile Baseggio, Sophie Gazzo, and Martine Ffrench for their input and assistance.