Abstract
Significant advances in the treatment of Philadelephia chromosome (Ph)– or BCR-ABL–positive acute lymphocytic leukemia (ALL) have been made since the discovery of the selective ABL tyrosine kinase inhibitors (TKIs). Whereas the outcome with standard chemotherapy was previously dismal, incorporation of imatinib mesylate into frontline therapy has improved relapse-free and overall survival. The benefit of imatinib extends particularly to instances where allogeneic stem cell transplantation in first complete remission is prohibited by older age, comorbidities, or lack of a suitable donor. However, the emergence of resistance to imatinib presents new therapeutic challenges. The development of novel TKIs with enhanced inhibitory potency against ABL and other kinases may further improve on the results observed with imatinib. Optimal use of these novel agents in the treatment schema of Ph+ ALL will be paramount in ensuring continued success in the eradication of this disease. Herein, the new approaches to the management of Ph+ ALL are reviewed.
Introduction
The Philadelphia (Ph) chromosome is the most frequent karyotypic aberration in adults with acute lymphocytic leukemia (ALL).1 It occurs in 20% to 30% of adult patieints with ALL overall, with the incidence rising to more than 50% in patients aged 50 years or older.2 The Ph chromosome results from the reciprocal translocation that fuses the BCR (breakpoint cluster region) gene from chromosome 22 to the ABL (Abelson tyrosine kinase) gene from chromosome 9.3–6 This translocation [t(9;22)(q34;q11)] ultimately results in a constitutively active tyrosine kinase protein. The location of the breakpoint within the BCR gene results in either the p190bcr-abl protein exclusively observed in Ph+ ALL, or the p210bcr-abl protein common to 20% to 40% patients with Ph+ ALL and nearly all patients with Ph+ chronic myelogenous leukemia (CML).7 Overexpression of the BCR-ABL fusion gene activates a number of downstream signaling pathways involving Ras/ Raf/mitogen activated protein kinase and Jak-STAT (Janus kinase signal transducer and transcription activator of transcription).8–11 Development of growth factor–independent malignant clones ensues, contributing further to the pathogenesis of the disease.
Chemotherapy for Ph+ ALL in the Pre-Imatinib Era
Historically, prior to the advent of tyrosine kinase inhibitors (TKIs), outcome after chemotherapy for Ph+ ALL was dismal. Although the complete remission (CR) rates with conventional and intensive ALL regimens ranged from 60% to 90%, long-term disease-free survival (DFS) rates were less than 20% in the absence of allogeneic stem cell transplantation (SCT).12–14 Median survival ranged from 8 to 16 months owing to relapse-related mortality. Improved CR rates with the more intensive regimens did not translate into an increase in durability of response. The quality of the molecular response as measured by log reduction in the level of BCR-ABL transcripts after frontline chemotherapy correlated with outcome, even prior to the availability of TKIs. In a study using high-dose anthracycline chemotherapy, chemosensitive patients who achieved at least a 3-log reduction in BCR-ABL transcripts by quantitative real-time polymerase chain reaction (RT-PCR) after consolidation chemotherapy had 2-year DFS and overall survival rates of 27% and 48%, respectively, not dissimilar from the outcomes observed after allogeneic SCT in first CR.15 None of the patients who had less than a 3-log reduction in BCR-ABL transcripts were alive at 2 years. This further emphasized the importance of achieving an optimal molecular response in order to improve outcome, providing the impetence for development of selective small-molecule ABL inhibitors.
Allogeneic Stem Cell Transplantation for Ph+ ALL in the Pre-Imatinib Era
Given the dismal outcome with chemotherapy alone, allogeneic SCT was established as the only potential curative modality. However, allogeneic SCT was often feasible only in younger patients without significant comorbidities and for whom a suitable donor was identified prior to disease recurrence, limiting the applicability of this approach. Although the long-term survival rates improved to 27% to 65% after allogeneic SCT in first CR, relapse remained the primary cause of failure, with persistent detection of BCR-ABL by RT-PCR heralding eventual recurrence.13,16–18 In a retrospective review of 197 patients with Ph+ ALL who underwent allogeneic SCT, the 5-year survival rates were 34% for patients in first CR, 21% for those in second or subsequent CR, and 9% for those with active disease (P < .0001).19 Multivariate analysis identified that younger age, CR at the time of SCT, conditioning with total body irradiation, and an HLA-identical sibling donor were factors associated with improved survival. Newer modalities such as nonmyeloablative conditioning regimens or donor cells derived from umbilical cord blood have increased the applicability of allogeneic SCT, but a significant proportion of patients remain ineligible for this potentially curative approach and are in need of novel therapy.20
Imatinib Mesylate in Previously Treated Ph+ ALL
Imatinib mesylate (Gleevec/Glivec; Novartis, Basel, Switzerland) is an oral selective inhibitor of the ABL, c-kit and platelet-derived growth factor receptor (PDGFR) tyrosine kinases.21–23 Imatinib binds to the inactive moiety of Bcr-Abl while partially blocking the ATP binding site, preventing a conformational switch to the activated form of the oncoprotein. The activity of single-agent imatinib was initially investigated in patients with relapsed or refractory Ph+ ALL. A phase 1 clinical trial of imatinib at doses of 300 to 1000 mg daily led to a 70% hematologic response rate with a 20% CR rate.24 A phase 2 trial of intermediate-dose imatinib yielded a CR rate of 29%.24,25 Disease recurrence was usually observed within a median of 2 months, and responses were durable only in a minority of patients. Relapse in the central nervous system (CNS) was not uncommon, as imatinib concentrations in the cerebrospinal fluid only reach 1% to 2% of detectable serum levels, emphasizing the need for concurrent CNS prophylaxis.26,27 Toxicity profile was similar to that observed in the trials of single-agent imatinib for Ph+ CML, and included transient myelosuppression, fluid retention syndrome, nausea, muscle cramps, rash, and transient elevations in hepatic transaminases.
Imatinib-Based Chemotherapy for de novo Ph+ ALL
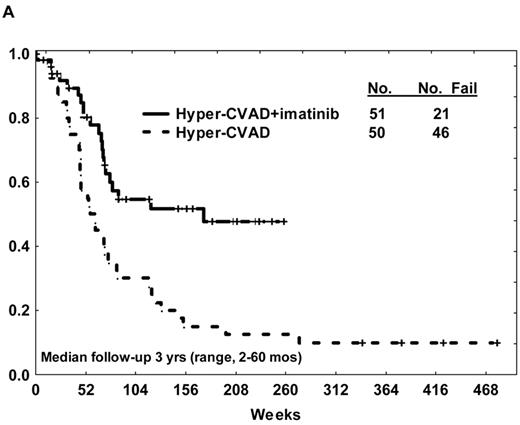
Although monotherapy with imatinib demonstrated modest activity in the setting of recurrent or refractory disease, durability of responses was suboptimal. Imatinib was thus incorporated into combination chemotherapy regimens typically used for de novo Ph+ ALL, either concurrently (simultaneous imatinib and chemotherapy) or sequentially (alternating imatinib with chemotherapy) (Table 1 ). The first report of a clinical trial of this nature included 20 patients with de novo or minimally treated Ph+ ALL (no age restrictions).28 Imatinib was given concurrently with the hyper-CVAD regimen (fractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone alternating with cycles of high-dose methotrexate and cytarabine); CR rate was 96%, with a 2-year DFS rate of 85%. The molecular remission rate or negativity for BCR-ABL transcripts by RT-PCR and nested PCR approached 60%. The addition of imatinib to the chemotherapy improved outcome (Figure 1A ).29 Lee et al30 also reported favorable outcomes after incorporating imatinib into a conventional L-asparaginase–based ALL regimen for newly diagnosed Ph+ ALL (up to age 67 years). Not surprisingly, the use of concurrent imatinib and L-asparaginase often resulted in hyperbilirubinemia, requiring dose interruptions or modifications of therapy. Yanada et al31 observed a CR rate of 96% and molecular remission rate of 71% in de novo patients with Ph+ ALL aged less than 65 years after concurrent imatinib and induction chemotherapy followed by alternating blocks of imatinib and consolidation chemotherapy. Long-term DFS and overall survival rates were significantly superior to the historical experience in these studies.
In a subsequent report of outcome with imatinib-based frontline chemotherapy, two sequential cohorts of patients with de novo Ph+ ALL were treated according to German Multi-Centre Acute Lymphoblastic Leukemia (GMALL) protocols.32 First, a treatment regimen consisting of alternating blocks of chemotherapy and single-agent imatinib was designed because of concerns of potential toxicity. Once the feasibility and tolerance of concurrent imatinib and chemotherapy was demonstrated by other investigators, a concurrent regimen was implemented. The superiority of the latter approach was evidenced by a higher rate of molecular remission (52% vs 19%; P =.01), although the greater antileukemia efficacy did not translate into significant improvements in DFS or overall survival compared with the alternating regimen. Allogeneic SCT was successfully used in more than 70% of the patients regardless of the imatinib regimen, significantly higher than the historical experience of 50% for the LALA and GMALL multicenter group cooperative trials.
Interim therapy with single-agent imatinib has also been used as a bridge to allogeneic SCT after completion of frontline imatinib plus chemotherapy.33 This approach also reduced the relapse rate prior to allogeneic SCT with-out an apparent worsening in the acute transplantation-related morbidity or mortality in comparison with historical controls.
Allogeneic Stem Cell Transplant in the Imatinib Era
Because of the universally dismal prognosis of de novo Ph+ ALL in the pre-imatinib era, all patients who achieved CR were recommended to undergo allogeneic SCT as feasible, inclusive of all stem cell sources such as matched unrelated marrow and umbilical cord blood. The benefits of SCT in first CR were attributed to the intense myeloablative therapy and graft-versus-leukemia effect; the high risk of transplantation-related mortality was accepted given the alternative of poor outcomes with chemotherapy alone. Two large multicenter trials confirmed the benefit of allogeneic SCT in the pre-imatinib era. In the Ph+ subset of patients with ALL (n=167) enrolled in the UKALL XII/ ECOG E2993 trial, the 5-year relapse risk was decreased from 81% with either chemotherapy alone or autologous SCT to 32% with allogeneic SCT.34 Five-year event-free survival (EFS) and overall survival rates improved from 17% to 36% and 19% to 42%, respectively. Furthermore, the prospective multicenter French, Belgian, Swiss and Australian LALA-94 trial of 154 patients with Ph+ ALL showed that achievement of negativity for BCR-ABL by RT-PCR and undergoing allogeneic SCT predicted for improved DFS and overall survival.14
The role of allogeneic SCT for de novo Ph+ ALL in the imatinib era continues to be refined, with feasibility of this approach still limited by the availability of an appropriate donor, absence of significant comorbidities, and ability to sustain a complete remission. Advances in SCT such as the application of nonmyeloablative reduced intensity conditioning regimens to older patients or those with co-morbidities prohibiting traditional myeloablative regimens, in addition to increased availability of umbilical cord blood as a source of stem cells, have allowed this modality to be applied in a more systematic fashion.20
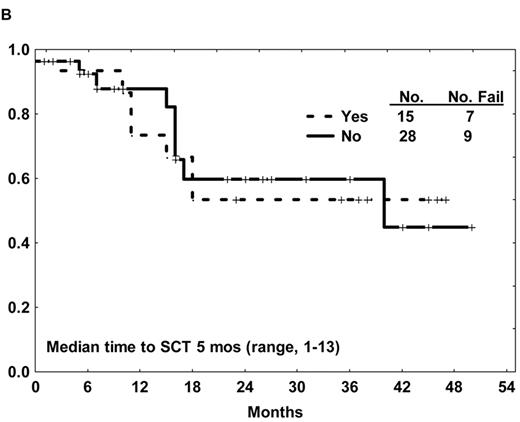
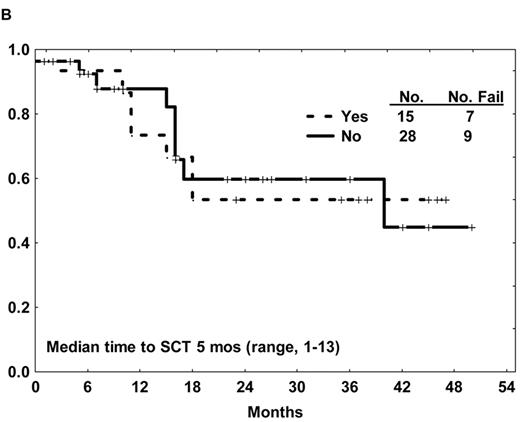
Several studies have reported an improvement in the rate of allogeneic SCT in first CR after imatinib-based therapy compared with the prior experience.28,31,32,35 This success is in part related to (1) an increase in the proportion of sustained remissions, offering additional time for identification of a suitable donor, and to (2) an improvement in the quality of the remissions (e.g., lower levels of BCR-ABL transcripts after imatinib-based therapy), resulting in a lower pretransplantation tumor burden. Two of the early non-randomized studies of imatinib-based chemotherapy for de novo Ph+ ALL applied allogeneic SCT in first CR as standard of care when feasible. Similar survival outcomes were observed with or without allogeneic SCT, despite the selection biases favoring SCT (Figure 1B ).28,29,31,35 Additional experience and longer follow-up is needed to clarify whether allogeneic SCT can be deferred in a select group of patients with Ph+ ALL otherwise eligible for this modality.
Postallogeneic SCT maintenance strategies are also being explored, particularly as the detection of minimal residual disease (MRD) following SCT predicts imminent relapse in the absence of intervention.13 Wassmann et al36 investigated the use of single-agent imatinib in the post transplantation setting after detection of MRD by quantitative RT-PCR for BCR-ABL. Standard-dose (400 mg) imatinib resulted in eradication of molecular disease in 52% of the 27 patients treated. Notably, failure to achieve molecular remission within the first 6 weeks of therapy heralded overt leukemia relapse despite other additional manipulations (e.g., donor lymphocyte infusions). Using imatinib in the post-transplantation setting in a prophylac-tic manner, immediately after engraftment and prior to the detection of MRD, may further improve outcome by preventing resurgence of the leukemia clone. Two small series have shown that this approach is feasible, with transient elevations in hepatic transaminases usually responding to dose interruptions or modifications.37,38 Additional experience will be required to determine whether imatinib monotherapy after transplantation would eventually lead to development of resistance.
How I treat:De novoPh+ALL
Patient: 55-year-old male presenting with untreated Ph+ ALL. The patient commences frontline TKI-based chemotherapy and achieves a complete remission. The patient has no siblings, and an unrelated donor and cord blood search fails to identify a suitable match.
My frontline therapy options would include the hyper-CVAD and dasatinib regimen in the context of a clinical trial, or alternatively the hyper-CVAD and imatinib regimen given its established efficacy (using imatinib 600 mg days 1-14 of induction chemotherapy followed by continuous imatinib concurrently with the remainder of the intensive chemotherapy cycles, further dose-escalated to 800 mg as tolerated with maintenance therapy, with extension of the maintenance phase for 24 months followed by imatinib indefinitely).
In either case, I would monitor levels of quantitative PCR for BCR-ABL and minimal residual disease by multipa-rameter flow cytometry with bone marrow aspirations on days 14 and 21 of the induction phase, then every 3 months from the start of therapy. Once the concordance of peripheral blood and marrow assessments was established, I would confine the quantitative PCR analyses to peripheral blood with bone marrow samples held in reserve if resistance was suspected.
As the presence of ABL KD mutations at diagnosis do not currently appear to affect response outcome, this would not influence my frontline therapy choices. If a rising level of quantitative RT-PCR for BCR-ABL was noted, I would screen for ABL KD mutations at that time. If this patient achieved a complete molecular remission, I would consider an autolo-gous stem cell harvest; however, this generally requires cessation of the TKI for approximately 3 weeks prior to and 2 weeks after the stem cell collection, a less than ideal situation. Although molecular remissions can now be achieved with TKI-based chemotherapy, the role of autologous SCT in Ph+ ALL has yet not been revisited in a systematic fashion. Data from the recent MRC UKALL XII/ECOG E2993 study suggests that is unlikely this modality would offer benefit over continuing TKI-based chemotherapy alone.
Treatment of Elderly Patients with de novo Ph+ ALL
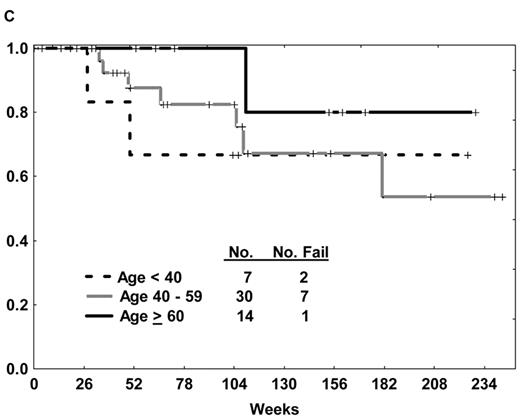
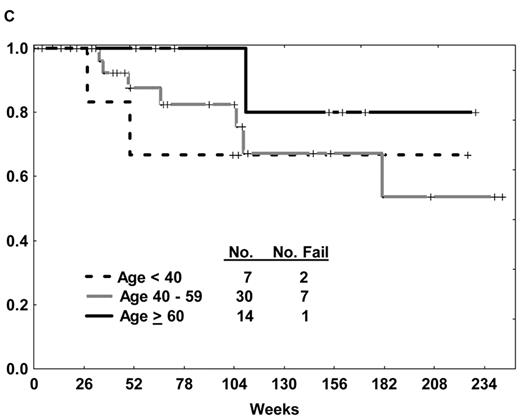
The prognosis of ALL in elderly patients has traditionally been poor, irrespective of the presence of the Ph chromosome. Overall CR rates with frontline ALL therapy yield CR rates of 46% to 79%, with 3-year survival rates less than 20%.2 An optimal treatment approach to the elderly patient with Ph+ ALL is paramount, particularly given that the incidence of the Ph chromosome increases with advancing age. Intolerance of chemotherapy, inability to undergo allogeneic SCT due to comorbidities, and heretofore unrecognized differences in biology of the disease partly account for the inferior outcomes in the elderly subgroup compared with their younger counterparts. Several approaches using imatinib-based therapy for elderly patients with de novo Ph+ ALL have thus been explored (Table 1 ). The experience with the hyper-CVAD and imatinib regimen was extended to the elderly group; DFS was similar regardless of age (Figure 1C ).29 Delannoy et al39 treated patients age 55 years and older with induction chemotherapy alone, followed by intermediate-dose (600 mg) imatinib alternating with consolidation chemotherapy. An improvement in the 1-year DFS and overall survival rates was observed compared with historical controls (58% vs 11% and 66% vs 43%, respectively); however, the relapse rate approached 60%. Notably, there was relatively minimal use of imatinib in the consolidation and maintenance phase (3 blocks of 60 days over a 730-day period) compared with other regimens. No data regarding the incidence of ABL kinase domain (KD) mutations at the time of recurrence was provided. Vignetti and colleagues40 explored the efficacy of induction therapy with high-dose imatinib (800 mg) and intermediate-dose oral prednisone for patients aged 60 years or more. Nearly all patients responded, continuing single-agent imatinib until disease recurrence, with 1-year DFS and overall survival rates similar to those reported by Delannoy et al despite the absence of chemotherapy.39
Ottmann et al41 conducted a randomized study of front-line induction therapy for patients with de novo Ph+ ALL more than 55 years old comparing single-agent imatinib with standard induction chemotherapy followed by consolidation with concurrent imatinib and chemotherapy. As expected, response rates with single-agent intermediate-dose imatinib exceeded 96%, compared with a CR rate of 50% for chemotherapy alone (due to induction mortality and disease resistance). Patients refractory to the induction chemotherapy were often successfully salvaged with imatinib and chemotherapy. However, there were no significant differences in long-term outcome between the two frontline approaches (Table 1 ). Achievement of a molecular remission with either approach was associated with a longer median DFS (18.3 vs 7.2 months; P =.002). Disease recurrence appeared to be associated with a high rate of ABL KD mutations.42
Recurrence after Imatinib-Based Chemotherapy
In the GRAAPH 2003 study, prephase corticosteroid insensitivity appeared to predict a higher probability of disease recurrence despite earlier incorporation of imatinib into the chemotherapy.35 Relapse rates after imatinib-based frontline chemotherapy with or without allogeneic SCT ranged from 19% to 32% in the studies confined to younger patients.29,30,43 Relapse rates after imatinib-based regimens tailored for the elderly group appear higher, ranging from 41% to 60%, likely related to the inherent differences in the biological features of Ph+ ALL with older age. Dose attenuated chemotherapy and/or frequency of ABL KD mutations likely are in part responsible for these observed differences.39–41
Mechanisms of Resistance to Imatinib
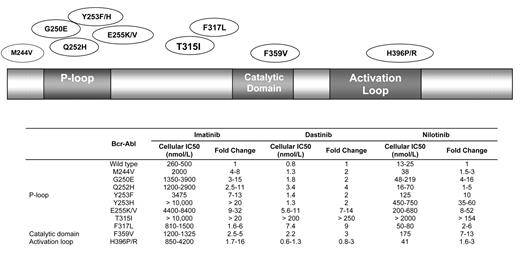
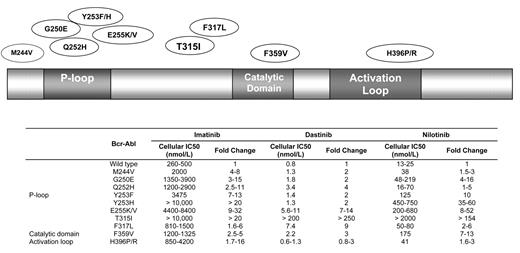
Imatinib continues to have a favorable toxicity profile with ongoing monitoring.44 However, the development of clinical resistance to imatinib has now surfaced in several arenas. Acquisition of point mutations in the ABL tyrosine kinase domain that interfere with the binding of imatinib appear to be the most influential (Figure 2 ). ABL KD mutations generally are comprised of two categories. The first includes mutations that directly impede contact between imatinib and Bcr-Abl, such as the gatekeeper mutations T3151 or F317L.45 The second involves mutations that alter the spatial conformation of the Bcr-Abl protein by affecting one of the two flexible loops: (1) the P-loop containing the ATP binding pocket, or (2) the activating loop.46–48 To date, more than 50 ABL KD mutations have been identified. Although the prognostic significance of many of these remains unclear, the T315I mutation has been associated with a particularly adverse outcome since it disrupts a hydrogen bond critical for binding of the TKI to the ATP-binding site. It has been identified in up to 20% of patients with imatinib-resistant Ph+ ALL, and also confers resistance to the second-generation TKIs nilotinib and dasatinib.49
Data on the frequency and incidence of ABL KD mutations in Ph+ ALL has been relatively sparse until recently. In the GMALL study for elderly patients with Ph+ ALL,41 the incidence of ABL mutations by direct cDNA sequencing at the time of disease recurrence was 84%. In the patients with ABL KD mutations, P-loop mutations predominated at a frequency of 57%, followed by the T315I mutation at 19%.49 The mutated clone comprised more than 50% of the ABL clones in all patients. Pfeifer et al49 also demonstrated that these ABL KD mutations were present in nearly 40% of patients with de novo imatinib-naïve Ph+ ALL, with a distribution of P-loop mutations in 80% and the T315I mutation in 17%. However, the mutated ABL clone always comprised less than 2% of the sample, in contrast to the predominance of the mutated clone when associated with disease recurrence. These low-level ABL KD mutations in imatinib-naïve samples required more sensitive methods for detection (e.g., high-performance liquid chromatography). The presence of ABL KD mutations prior to imatinib did not correlate with known prognostic factors. There was no difference in the probability of achieving CR or molecular response based on the presence or absence of ABL KD mutations prior to imatinib therapy. No difference in remission duration was observed other than for those with the T315I mutation, which adversely affected outcome. In nearly all patients with an ABL KD mutation identified pretreatment, the same mutation was noted at the time of disease recurrence. Conversely, approximately 67% of patients without an ABL KD mutation detected prior to imatinib had developed one at the time of disease recurrence.
How I Treat: Relapsed Ph+ALL
Patient: 40-year-old female with de novo Ph+ ALL who achieved CR after induction chemotherapy with the hyper-CVAD and imatinib regimen. The patient received 2 cycles of consolidation chemotherapy concurrently with imatinib, then proceeded to HLA-identical allogeneic SCT in first CR. No therapy with imatinib was administered after transplantation. Transplantation course was uncomplicated, without graft-versus-host disease. Overt recurrence of disease was subsequently noted 12 months later.
In this case, the presence or absence of ABL KD mutations will influence therapy choices. Given that the patient has had a first remission duration of more than 1 year, reinduction with TKI-based chemotherapy is more likely to be successful than not. If no ABL KD mutations (or those not associated with resistance to dasatinib) were identified, I would recommend reinduction chemotherapy with the hyper-CVAD and dasatinib regimen in the setting of a clinical trial. Donor lymphocyte infusion(s) or alternatively second allogeneic SCT followed by maintenance TKI therapy could then be considered in the setting of second CR, although data on these approaches in this setting are limited. If the T315I ABL KD mutation was identified, I would refer the patient for a specific clinical trial using one of the novel agents with specific inhibitory activity of this aberrancy, such as MK-0457.
The discovery of novel acquired ABL KD mutations has also been reported in Ph+ ALL after sequential therapy with imatinib followed by the second-generation TKI dasatinib. Soverini et al50 reported the development of the T315A and F317I (as opposed to the T315I or F317L) mutations that have inherent resistance to dasatinib. These ABL KD mutations could be suppressed by either imatinib or nilotinib given the lower IC50 with these compounds, although retreatment with imatinib after a prior failure would likely be ineffective due to the potential role of other coexisting mechanisms of resistance. Resistance screening with nilotinib, the other second-generation TKI, yielded only a limited spectrum of point mutations.51 This suggests a lower rate of ABL KD mutations after nilotinib therapy; however, additional analyses of ongoing clinical trials is needed to support this contention.
Other mechanisms of resistance to imatinib and other TKIs include increased drug efflux,52 amplification of the BCR-ABL gene,46 and signaling independence of BCR-ABL after secondary transforming events (e.g., Src kinase pathway53). Theoretically, dose escalation of imatinib or the use of more potent ABL inhibitors could circumvent the first two events, whereas use of novel Src inhibitors or multitargeted inhibitors would be required to restore sensitivity in the latter case.
Newer Tyrosine Kinase Inhibitors for Imatinib-Resistant Ph+ ALL
Second-generation TKIs such as dasatinib (BMS-354825; SPRYCEL; Bristol-Myers Squibb, New York, NY) and nilotinib (AMN107, Novartis) are increasingly potent inhibitors of ABL. Several of the more recently developed multitargeted agents in ongoing clinical trials can also inhibit c-kit, PDGFR, FLT3, and other kinases with varying potency (Table 2).
Dasatinib is a dual Src/Abl inhibitor with 325-fold more in vitro, and 30- to 50-fold more in vivo potency than imatinib against wild-type Bcr-Abl; it also inhibits the c-kit, PDGFR, and ephrin A receptor kinases.54 Unlike imatinib, it binds to both the inactive and active forms of the Bcr-Abl protein. Dasatinib has demonstrated in vitro efficacy against all imatinib-resistant KD mutations tested, with the exception of T315I and F317L.54 In a phase 1 trial of dasatinib, a hematologic response rate of 80% was observed in 10 patients with imatinib-resistant Ph+ ALL.55,56 In a phase 2 program with START (Src/Abl Tyrosine Kinase Inhibition Activity: Research Trials of Dasatinib) using single-agent dasatinib 70 mg twice daily in 36 patients with imatinib-resistant Ph+ ALL, a CR rate of 33% was achieved.57 Responses were observed even with the presence of ABL KD mutations other than T3151. Based on the efficacy demonstrated in these and other trials, dasatinib was granted approval by the U.S. Food and Drug Administration for the treatment of all phases of CML and Ph+ ALL resistant or intolerant to imatinib. Adverse events associated with dasatinib were often amenable to dose modifications, and included myelosuppression, diarrhea and peripheral edema. The unique toxicity of pleural effusions occurred in 5% to 20% of the patients, and has been attributed to dasatinib’s potent inhibition of PDGFR. Although these effusions are amenable to dose interruptions and cor-ticosteroids, in some patients the effusions recur with rechallenge despite dose reductions.
Nilotinib is an aminopyrimidine derivative of imatinib which inhibits c-kit and PDGFR like its parent compound.51 However, it is 20- to 50-fold more potent than imatinib as an ABL kinase inhibitor, and retains much of this potency against imatinib-resistant cell lines.58,59 Phase 1 and 2 clinical trials of nilotinib in imatinib-resistant Ph+ ALL demonstrate hematologic responses in 30% to 35% of the patients.60 Dose-dependent adverse events included myelosuppression, transient indirect hyperbilirubinemia, pruritis, and rash.
Development of third- or fourth-generation novel TKIs which target specific ABL KD mutations is now the focus of developmental therapeutics. Several of these agents have dual activity against the Src/ABL kinases, and are currently being investigated in clinical trials (e.g., bosutinib or SKI-60661,62 and INNO-40663). Differential selectivity for the other tyrosine kinases besides ABL, or lack thereof, as in the case of bosutinib (which does not inhibit c-kit or PDGFR) may improve clinical outcome simply by altering the safety profile. The multitargeted agent MK-0457 (previously VX-680), an inhibitor of the Aurora, FLT3, JAK2 and ABL kinases, is a promising agent with preliminary clinical activity against the T315I mutation, as it does not require interaction with threonine 315 for efficient binding.64
Several other agents are in preclinical stages of development: (1) dual specific Abl and Src kinase inhibitors such as AZD0502 and AP23464; (2) the ABL, Src, and PDGFR inhibitor ON012380 with activity in T315I mutated cell lines; and (3) pyrimidine Src/Abl inhibitors such as PD166326.65 Combination TKI therapy may prove of interest for further study as in vitro data suggest that nilotinib and/or dual Src/ Abl inhibitors such as dasatinib or AP23464 further enhance the ability of imatinib to prevent autophosphorylation of wild-type Bcr-Abl.59,66 Carefully designed clinical trials of such combinations will require frequent monitoring for MRD and ABL KD mutations given the potential for selection of TKI-specific mutations.
Conclusions and future directions
Emerging data has established that the standard of care for de novo Ph+ ALL should be imatinib-based chemotherapy. The concurrent approach appears superior from an efficacy perspective and feasible from a safety perspective. New challenges have emerged with respect to induction of resistance to imatinib via ABL KD mutations. The development of novel TKIs with increased potency for ABL inhibition and their ongoing incorporation into frontline therapy should further improve outcome, although resurgence or emergence of novel ABL KD mutations will likely remain a therapeutic challenge. Strategies designed to optimize the achievement of a complete molecular response to TKI-based chemotherapy while circumventing development of resistance will be paramount to ensuring eradication of the disease. These mutations, in turn, will be selected or de-selected depending on the spectrum of sensitivity and inherent resistance to the specific TKI used. Rationally designed clinical trials of combination TKI therapy with or without chemotherapy should be explored based on the preclinical models suggesting additive or synergistic effects. The role of allogeneic SCT in first CR will continuously need to be redefined as further improvements in outcome are noted, likely a question best answered in the setting of a prospective randomized clinical trial. An optimal approach to the treatment of de novo Ph+ ALL now includes frontline TKI-based chemotherapy followed by al-logeneic SCT in first CR (as feasible or as indicated) with adoption of a posttransplantation “maintenance” regimen of TKI monotherapy on an investigational clinical trial.
(A) Overall survival of de novo or minimally treated Ph+ ALL with the hyper-CVAD and imatinib regimen compared with historic experience with hyper-CVAD alone. (B) Survival by allogeneic stem cell transplantation in first CR after therapy with hyper-CVAD and imatinib. (C) Disease-free survival by age after therapy with hyper-CVAD and imatinib.
(A) Overall survival of de novo or minimally treated Ph+ ALL with the hyper-CVAD and imatinib regimen compared with historic experience with hyper-CVAD alone. (B) Survival by allogeneic stem cell transplantation in first CR after therapy with hyper-CVAD and imatinib. (C) Disease-free survival by age after therapy with hyper-CVAD and imatinib.
Over 50 ABL kinase domain mutations have been identified with differential potency of the tyrosine kinase inhibitors imatinib, dasatinib and nilotinib.58,59 Selected mutations are depicted.
Abbreviations: IC, inhibitory concentration.
Department of Leukemia, University of Texas M. D. Anderson Cancer Center, Houston, Texas