Abstract
A 10-year-old male patient with hemoglobin SS suffered a stroke at 7 years of age and was initially transfused at the time of presentation to lower the hemoglobin S concentration to < 30%. You are asked by the family if their child can be treated with oral hydroxyurea rather than monthly transfusions for the secondary prevention of strokes.
Introduction
Patients with sickle cell anemia demonstrate an increased stroke risk throughout their lifetimes. A first (primary) stroke can be clinically devastating and places that patient at a higher risk for a second stroke.1 Standard care for secondary prevention of strokes in countries where blood transfusions are relatively safe is regular blood transfusion therapy initially targeted at keeping the hemoglobin S level at < 30%.2,3 Cessation of regular transfusion therapy places patients at high risk for recurrence.4,5 Therefore, lifelong monthly blood transfusion therapy to keep the HbS level at < 30% or perhaps < 50% has become usual care for secondary stroke prevention.6–8 If a stroke patient has an HLA-matched sibling, a hematopoietic stem cell transplantation should be considered as the first option because the patient can be cured of the disease; there have been no stroke recurrences reported after successful stem cell transplantation.9
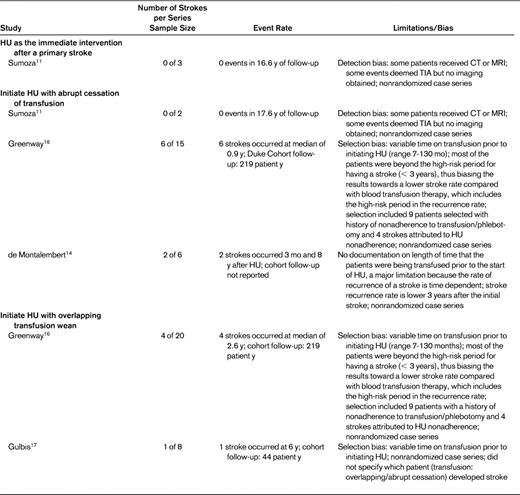
Concerns for the patient burden associated with regular blood transfusion therapy, such as red blood cell alloimmunization, excessive iron stores, infection, and decreased quality of life, have led hematologists to consider hydroxyurea therapy as an alternative to blood transfusion therapy. To examine the current best evidence for the use of hydroxyurea for secondary stroke prevention, a literature search of PubMed using the terms hydroxyurea AND stroke was performed (118 hits), with an additional search using the terms sickle AND stroke (841 hits). Nine case series from 4 centers9–17 and 1 case report10–17 describe the results of using hydroxyurea for secondary stroke prevention (Table 1).
Hydroxyurea instead of blood transfusion therapy as immediate intervention after primary stroke
Even with rigorous blood transfusion therapy, the highest stroke recurrence rate is within the first 3 years after a stroke.4,10 Therefore, the decision to initiate hydroxyurea without blood transfusion should be considered during this high-risk period, especially because the full therapeutic impact of hydroxyurea may take up to 6 months. There are very limited data on the early or immediate use of hydroxyurea after a primary stroke. One case series from Brazil reported 3 patients who were started on hydroxyurea immediately after their primary stroke as the sole therapy for secondary prevention.11 The 3 patients received hydroxyurea therapy at doses of 40, 40, and 30 mg/kg for a mean follow-up of 70.3 months and there was no recurrence of stroke. No larger series or randomized controlled trial has reported on the efficacy of hydroxyurea as the sole therapeutic strategy immediately after a stroke. We recommend against using hydroxyurea as the sole intervention for secondary stroke prevention immediately after an acute stroke. This recommendation is based on two factors: (1) the lack of data supporting this strategy and (2) the risk that if hydroxyurea therapy is assumed to be as effective as blood transfusion therapy, then an excessive number of patients will have stroke recurrences compared with standard care because the prolonged length of time to reach the maximal tolerated dose is during the highest-risk period for stroke recurrence.
Hydroxyurea initiated with abrupt discontinuation of chronic transfusion
Three institutions used a strategy of chronic transfusion followed by initiation of hydroxyurea with an abrupt discontinuation of transfusion therapy. Duke University reported that 6 of 15 patients (40%) initiated on hydroxyurea with an abrupt discontinuation of transfusion developed a recurrent stroke at a median of 2.6 years using a mean hydroxyurea dose of 26 mg/kg.12,13 Two patients with recurrent stroke had poor adherence to hydroxyurea and follow-up visits. The Brazil group reported on 2 patients who were transfused for a mean of 27 months before starting hydroxyurea at 40 mg/kg.11 Neither patient had a stroke while on documented hydroxyurea, but 1 patient was lost to follow-up for 6 months and had a transient ischemic attack. These 2 institutions brought forth an additional concern that adherence to hydroxyurea may bias an evidence-based review of hydroxyurea efficacy. In France, 1 of 6 patients (∼ 15%) developed recurrent stroke after 3 months.14 That study did not report the length of time that patients were on transfusion therapy before initiating hydroxyurea, thus limiting any significant inference about the potential benefit.
One case report describes a 19-year-old patient with history of stroke who developed an intracerebral hemorrhage 1 year after stopping transfusion therapy and initiating hydroxyurea.15 Based on the limited evidence of efficacy and concerns for potential stroke risk during the period needed to optimize hydroxyurea dosing, we suggest against initiating hydroxyurea and abruptly discontinuing chronic blood transfusion for secondary prevention of stroke in sickle cell disease.
Hydroxyurea initiated with overlapping period of chronic transfusion
Given the anticipated time to achieving the maximum tolerated dose of administering hydroxyurea, typically 6 months, an overlapping period between starting hydroxyurea and stopping the transfusion is reasonable. Investigators from Duke University reported on 20 patients initiated on hydroxyurea concurrently with weaning off of transfusion therapy.12,16 Four of the 20 patients (25%) had a second stroke at a median of 2.6 years from initiating hydroxyurea. Three of the 4 recurrent strokes occurred after a minimum of 4 years of hydroxyurea use. In Belgium, 8 patients were started on hydroxyurea (5 with an overlapping period) and 1 patient developed a recurrent stroke after 6 years of hydroxyurea with reported adherence.17 Based on these findings, we suggest that hydroxyurea should not be used as a secondary prevention with an overlapping blood transfusion strategy (grade 2B). If a patient develops contraindications to chronic blood transfusions, the risk of recurrent stroke on hydroxyurea rather than no therapy should be discussed with the patient and with the family.
Disclosures
Conflict-of-interest disclosures: The authors declare no competing financial interests. Off-label drug use: hydroxyurea, a drug not approved by the US Food and Drug Administration for children with sickle cell disease or for secondary stroke prevention.
Correspondence
Jeffrey D. Lebensburger, DO, 1600 7th Ave S, ACC 512, Birmingham, AL 35233; Phone: 205-939-9285; Fax: 205-975-1941; e-mail: JLebensburger@peds.uab.edu.