Abstract
High-level production of β-globin, γ-globin, or therapeutic mutant globins in the RBC lineage by hematopoietic stem cell gene therapy ameliorates or cures the hemoglobinopathies sickle cell disease and beta thalassemia, which are major causes of morbidity and mortality worldwide. Considerable efforts have been made in the last 2 decades in devising suitable gene-transfer vectors and protocols to achieve this goal. Five years ago, the first βE/β0-thalassemia major (transfusion-dependent) patient was treated by globin lentiviral gene therapy without injection of backup cells. This patient has become completely transfusion independent for the past 4 years and has global amelioration of the thalassemic phenotype. Partial clonal dominance for an intragenic site (HMGA2) of chromosomal integration of the vector was observed in this patient without a loss of hematopoietic homeostasis. Other patients are now receiving transplantations while researchers are carefully weighing the benefit/risk ratio and continuing the development of further modified vectors and protocols to improve outcomes further with respect to safety and efficacy.
Introduction
Approximately 3 of 1000 new births worldwide are affected with a severe form of β-hemoglobinopathy, and approximately 350 000 children are born with these diseases each year. The degree of severity is related to the number of defective genes inherited, the type of mutation that changes β-globin conformation or impairs β-globin gene expression, and possible mitigating effects conferred by inherited polymorphisms in modifier loci that include the γ-globin genes.
In β-thalassemia major, absent β-globin chains (β0/β0-Thal, also referred to as Cooley anemia) or a profound decrease in the synthesis of β-globin (eg, severe cases of βE/β0-Thal or β+-Thal) trigger the precipitation of unpaired α-globin chains in erythroid cell precursors, leading to BM cell death, dyserythropoiesis, and decreased production of RBCs. Peripheral RBC lifespan is also shortened. The resulting anemia induces erythropoietin production and increased expansion of erythroid cell precursors with secondary hepatosplenomegaly, osteoporosis, and growth retardation. Repeated RBC transfusion causes iron overload and, ultimately, hemochromatosis with life-threatening complications that include cardiomyopathy, liver disease, and endocrine dysfunction.
Sickle cell disease (SCD) presents as a hemolytic anemia accompanied with several other acute and chronic clinical manifestations. One amino-acid substitution (a valine for a glutamic acid) leads to the polymerization of hemoglobin S and intravascular sickling of RBCs, which is compounded by their decreased deformability and increased adhesion, thereby triggering the obstruction of small blood vessels with impaired oxygen delivery to tissues and, ultimately, irreversible organ damage. Patients suffer from acute painful crises known as vasoocclusive events, increased vulnerability to severe infection, increased risk of stroke and silent cerebral infarcts, and impaired neurocognitive function, retinopathy, nephropathy, and pulmonary disease. This constellation of pathologic features results in high morbidity with frequent hospitalization, massive health care costs, and an increased incidence of premature deaths.
Most patients with a severe β-hemoglobinopathy in developing countries die before the age of 5 years, whereas most of affected children born in high-income countries survive for several decades, although they live with a chronic and often debilitating disorder with palliative treatments that are of high cost to society. Even under modern and specific care, severe complications are frequent, and according to a recent survey from the United Kingdom and Italy, only 50%-65% of β-thalassemic major patients live beyond 35 years of age.1–2 The mortality rate in infancy can be higher than 90% for patients with SCD in sub-Saharan Africa, although the survival of children could be substantially improved with their accelerated introduction into immunization programs and the prevention of bacterial infections with the early use of antibiotics. Once systematic neonatal screening and early care are the rule in Africa, the number of young people diagnosed and living with SCD is likely to increase to a scale never imagined before.
HbF induction
β-Thalassemia and SCD patients with inherited persistently high-level production of fetal hemoglobin (HbF) have a milder clinical course. The S-phase cell-cycle inhibitor hydroxyurea (HU) triggers a proportionate increase in erythroid precursors that contain HbF, which decreases blood leukocyte counts and has been shown to reduce pain severity, frequency of hospitalization, and the occurrence of acute chest syndrome in both adults and severely affected children.3 Although HU provides significant benefit to many patients, at least 25% of patients with SCD are nonresponders. HU can also reduce RBC transfusion requirements in patients with β-thalassemia intermedia and may be beneficial for a selected subset of patients with β-thalassemia major.4 Although HU may decrease morbidity and mortality rates in patients with SCD, concerns have been raised about abnormal spermatogenesis and teratogenesis in the fetal offspring. Furthermore, HU does not appear to reverse established end-organ damage and its putative long-term leukemogenicity remains a concern.
5-Azacytidine, short-chain fatty acid derivatives, histone deacetylase inhibitors, and recombinant erythropoietin, alone or in combination, have also been shown to be beneficial for a few β-thalassemic patients.5 To identify new targets for reactivation of HbF and to develop novel pharmaceutical approaches, efforts have been made to understand the molecular mechanisms of fetal to adult hemoglobin switching. Genetic linkage analysis and genome-wide association approaches have identified susceptibility loci for the persistence of HbF in adulthood, including the β-globin cluster, HBS1L/MYB (6q locus), and BCL11A (2p locus), the latter playing the most important role in patients with the β-hemoglobinopathies. Recent evidence suggests that BCL11A and SOX6 cooperate in adult erythroid cells to repress γ-globin gene expression. Because it was shown that repression of BCL11A in a transgenic SCD mouse model reverses certain disease-associated defects, molecular screening studies to find specific inhibitors that target BCL11A are ongoing.6
Allo-HSCT
At present, only allogeneic hematopoietic stem cell transplantation (allo-HSCT) by BM cells or mobilized CD34+ cells after myeloablative conditioning (MAC) can result in a definitive cure in selected patients with severe β-hemoglobinopathies. MAC-HSCT with HLA-matched sibling donor (MSD) cells is the treatment of choice, providing an excellent outcome in most recipients, and this therapeutic approach may be applied to many pediatric patients with a compatible intrafamilial donor. Benefit, risks, and limitations are well established. With more stringent inclusion and exclusion criteria and in the absence of an MSD, HLA-matched unrelated donor (MUD) or minimally mismatched cord blood products and haploidentical donor cells have been used, although these approaches present a lower benefit/risk ratio and thus remain experimental. Many fewer patients with SCD than β-thalassemia major have proceeded to allo-HSCT thus far because of the variable course of SCD, making predictions of long-term severity difficult, and because of limitations in patient eligibility.
Allo-HSCT with MSD cells in β-thalassemia patients
Allo-HSCT for β-thalassemia patients was initiated in the early 1980s. Patients between the ages of 1 and 15 were divided into 3 classes by the Pesaro group according to the absence (class 1) or the presence of 1 or 2 (class 2) or 3 (class 3) risk factors (hepatomegaly of more than 2 cm, portal fibrosis, and irregular chelation history). Overall survival after MAC (busulfan and cyclophosphamide) and allo-HSCT with MSD was reported to be higher in class 1 (94%) and class 2 (80%) than for class 3 (61%).
Transplantation of MSD cells in pediatric patients with β-thalassemia gives excellent results for patients with class 1 and 2 disease. In recent years, only few modifications have been brought to this protocol for class 1 and 2 patients, except for the use of thiotepa in those below 4 years of age and the addition of antithymocyte globulin (ATG) to reduce rejection rates. Disease-free survival (DFS) rates for class 1 and 2 patients oscillate between 85% and 95%.7–8
In young class 3 patients, a slight reduction in the dose of cyclophosphamide, the addition of preconditioning chemotherapy and fludarabine to ablate the expanded thalassemic BM and to increase immunosuppression, and hypertransfusion to maintain Hb levels above 14 g/dL have increased the rate of DFS to 85%.9 The inclusion of ATG in the preconditioning regimen has also improved outcomes.7
Most adult patients, who have more advanced disease due to longer exposure to iron overload, belong to class 3. The MAC-HSCT protocol that is used for pediatric or class 1 and 2 patients (busulfan and cyclophosphamide at myeloablative and immunosuppressive doses) is highly toxic and most adult class 3 patients die from organ failure or acute GVHD. Reducing the dose of cyclophosphamide in adult class 3 patients decreases the mortality to 37% and increases the DFS rate to 62%.10 However, preablation of thalassemic BM and hypertransfusion, as for pediatric patients, does not further improve outcome. Other modifications of the myeloablative preconditioning regimen, such as inclusion of thiotepa, treosulfan, fludarabine, and ATG, are being assessed.
Allo-HSCT with MSD cells in SCD
The transplantation of MSD cells in pediatric patients with SCD provides excellent outcomes with high survival rates, few transplantation-related complications, and stabilization or restoration of organ function. Despite continuous improvement in survival due to advances in noncurative supportive care (> 90% of patients live to become adults), this curative therapy could be proposed to most children suffering from this debilitating disease when a compatible intrafamilial donor is available. Results reported in the last 15 years for approximately 300 young patients worldwide (mostly younger than 16 years of age) indicate an overall survival rate of 90%-100%, disease free-survival of 85%-90%, rejection rates of < 10% after MAC (busulfan 14-16 mg/kg, cyclophosphamide 200 mg/kg ± ATG 15-90 mg/kg or total lymphoid irradiation), and pharmacologic prophylaxis of GVHD.11
Only a few adult patients with SCD have received transplantations because of the accepted toxicity of the intensive conditioning regimen (ie, a myeloablative dose of busulfan and high-dose cyclophosphamide) in older patients with accumulated disease burden. However, despite continuous progress in the post-HU era, the median age of survival in adult patients remains lower than normal (approximately 40 years), with a large proportion of premature deaths caused by stroke, cardiopulmonary events, and liver, kidney, or multiorgan failure. This has provided the motivation to propose allo-HSCT to adult SCD patients, although with reduced-intensity pretransplantation conditioning. Indeed, mixed donor chimerism has often been observed in young patients and is sufficient to reverse the SCD phenotype. Nonmyeloablative conditioning (NMAC) approaches may represent an effective strategy for sicker patients overall, because this conditioning strategy is less likely to result in GVHD. Intermediate-intensity regimens have been reported to allow engraftment of donor cells, but severe acute and chronic GVHD occurred and was fatal in some. It is difficult to find the correct conditioning regimen in which the level of T-cell engraftment and the allogeneic effect on stem cells are sufficient to establish stable donor chimerism but not too high to trigger acute GVHD. Trials based on protocols for older patients with hematologic malignancies (30 mg/m2/d for 5 days of fludarabine, 200 cGy of total body irradiation ± ATG, and postgrafting immunosuppression) were disappointing, and full autologous hematopoietic reconstitution occurred after tapering down posttransplantation immunosuppression, suggesting that immune tolerance was not reached.12 Combining NMAC with an anti-CD52 Ab (alemtuzumab) and mycophenolate mofetil or rapamycin to induce tolerance seemed well tolerated and more efficient, resulting in stable mixed chimerism and reversion of the SCD phenotype.13
Allo-HSCT with non-MSD cells
One of the main barriers to allo-HSCT is the scarcity (fewer than 20%) of HLA-matched sibling donors. Cord blood (CB) transplantation is a viable alternative, but the probability of finding an HLA-matched donor CB unit is no better than that of finding an MSD. Furthermore, low cell numbers are an issue with CB, especially in the NMAC setting. Unrelated, HLA-matched CB transplantation has been reported for young patients with SCD and β-thalassemia with both MAC and NMAC strategies. In a study from Taiwan in which patients were infused with CB after MAC, most patients were disease free but there was a high rate of GVHD.14 In a trial including SCD patients, an NMAC regimen was associated with graft failure and MAC with GVHD.15 In a study combining β-thalassemic and SCD patients and MAC and NMAC regimens, less than 50% of patients engrafted with CB.16 Multivariate analysis indicated that engraftment and DFS rates were higher with the highest cell dose used. DFS was 50% for SCD patients and 20% for patients with β-thalassemia, which is in contrast to other results.14 There was no correlation with conditioning regimens. Lastly, in the SCD unrelated transplantation (SCURT) trial initiated in April 2008, 5 of 8 patients failed to engraft after NMAC with a highly immunosuppressive conditioning regimen, and the CB arm of the trial was closed.17
Results using MUD BM or mobilized peripheral CD34+ cells in SCD patients are not yet available. Transplantation of MUD BM cells coupled with MAC has shown promising results for β-thalassemia major patients,18 with 80% DFS for class 1 and 2 patients. This is comparable to the probability of DFS in young patients receiving MSD transplantations and is therefore an acceptable therapeutic approach in thalassemia when a sibling donor is not available. Continuing investigation will hopefully offer allo-HSCT to an increasing number of patients. Haploidentical allo-HSCT is under investigation with CD34+ selected products, and early successes have been reported in SCD19 and in thalassemia patients.20
First phase 1/2 study with anticipated benefit for hemoglobin disorders using gene therapy: conversion of a patient with β-thalassemia major to transfusion independence
For subjects lacking a suitable donor, and even for those who do have a compatible donor but nevertheless face the risk of GVHD after allo-HSCT, much of the drawbacks may be avoided by gene therapy of HSCs. Direct homologous recombination/repair of the defective β-globin gene would be ideal, but is not yet feasible in HSCs. Gene addition by vector-based transfer and chromosomal integration of a therapeutic globin gene remains the approach of choice. However, efficient modification of HSCs and high expression of globin genes in erythroid cells have presented major technical challenges that require resolution before human trials can be initiated.
The genetic elements required for high and erythroid-specific expression while decreasing the occurrence of position effect variegation include the βA-globin gene with its introns, promoter, and β-locus control region (β-LCR), the discovery of which was essential to achieving position-independent expression in transgenic mice. Beta-LCR elements have been subsequently reduced to smaller sizes suitable for inclusion in gene-transfer vectors. Unlike γ-retroviral vectors derived from oncoviruses such as the Moloney murine leukemia virus, which have posed major issues of genetic instability and low titers, lentiviral vectors have proven capable of transferring these elaborate structures with fidelity and high titers.21–22 Because one of the advantages of lentiviral vectors over γ-retroviral vectors is to package full-length unspliced RNA due to the presence of a strong RNA export element (rev-responsive element or RRE) that binds the viral REV protein, longer LCR elements could be included in lentiviral vectors and were shown to improve the likelihood of β-globin expression, resulting in much higher expression levels in vivo. Another important advantage of replication-defective HIV-derived vectors over mouse oncoretroviral-derived vectors is their capability of transducing cells arrested at the G1-S boundary of the cell cycle and, to a lesser extent, at the G0 stage. This is highly significant for the transduction of HSCs because most are quiescent at any given time.23 Lentiviral vectors have thus emerged as an important advance for gene therapy in the β-hemoglobinopathies.
The first and only clinical trial to date for gene therapy in the β-hemoglobinopathies was initiated in France.24 The first patient in this trial engrafted without injection of back-up cells was 18 years of age at the time and received the transplantation on June 7, 2007 with his own cells after ex vivo gene transfer. This patient had βE/β0-thalassemia major, was dependent on monthly transfusions, and was first transfused at age 3 because of poorly tolerated anemia (6.7 g/dL despite residual HbF) and major hepatosplenomegaly. As he grew older, transfusion requirements rapidly increased to once a month (2-3 RBC packs each time; 157 mL of RBCs/kg the year before transplantation). The patient was splenectomized at age 6. Despite this, his Hb levels decreased several times to as low as 4 g/dL, and HU therapy was ineffective. Iron chelation was initiated at age 8 by parenteral deferoxamine overnight 5 times a week. The patient did not have a sibling HLA-matched donor and ex vivo therapeutic gene transfer in HSCs could therefore be attempted.
The viral vector used to transfer the β-globin gene in human HSCs (LentiGlobin; Bluebird Bio) in this trial is very similar to the vector we described in 2 preclinical studies, in which we showed correction of SCD and β-thalassemia in mice.22,25 For safety reasons, the vector was rendered self-inactivating and only contains nonviral, physiologic promoter/enhancer elements, thereby limiting the risk of genotoxicity by insertional mutagenesis and transcriptional activation of adjacent oncogenes. Furthermore, a tandem core element of the chicken chromatin insulator HS4, which was shown to reduce the likelihood of promoter/enhancer interactions in lentiviral vectors,26 was included (Figure 1), although 1 of these core elements showed instability in a fraction of transduced cells.24,27 The therapeutic gene encodes a mutated β-globin (βA-T87Q). This specific globin chain inhibits polymerization of HbS in SCD22 and can be easily distinguished from βA contained in transfused RBCs for quantification by HPLC.
LentiGlobin vector and experimental design. (A) Diagram of the human β-globin (βA-T87Q) lentiviral vector (LentiGlobin; LG). The 3′ β-globin enhancer, the 372-bp IVS2 deletion, the βA-T87Q mutation (ACA[Thr] to CAG[Gln]) and DNase I Hypersensitive Sites (HS) 2, HS3, and HS4 of the human β-globin Locus Control Region (LCR) are indicated. Safety modifications including the 2 stop codons in the ψ+ signal, the 400-bp deletion in the U3 of the right HIVLTR, the rabbit β-globin plyA signal, and the 2 × 250-bp cHS4 chromatin insulators are indicated. HIVLTR indicates human immunodeficiency type-1 virus long terminal repeat; ψ+, packaging signal; cPPT Map, central polypurine tract; RRE, Rev-responsive element; βp, human β-globin promoter; and ppt, polypurine tract.
LentiGlobin vector and experimental design. (A) Diagram of the human β-globin (βA-T87Q) lentiviral vector (LentiGlobin; LG). The 3′ β-globin enhancer, the 372-bp IVS2 deletion, the βA-T87Q mutation (ACA[Thr] to CAG[Gln]) and DNase I Hypersensitive Sites (HS) 2, HS3, and HS4 of the human β-globin Locus Control Region (LCR) are indicated. Safety modifications including the 2 stop codons in the ψ+ signal, the 400-bp deletion in the U3 of the right HIVLTR, the rabbit β-globin plyA signal, and the 2 × 250-bp cHS4 chromatin insulators are indicated. HIVLTR indicates human immunodeficiency type-1 virus long terminal repeat; ψ+, packaging signal; cPPT Map, central polypurine tract; RRE, Rev-responsive element; βp, human β-globin promoter; and ppt, polypurine tract.
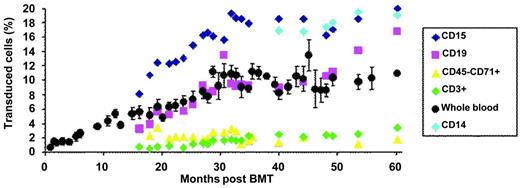
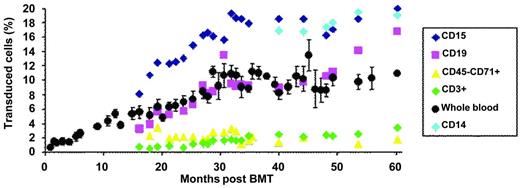
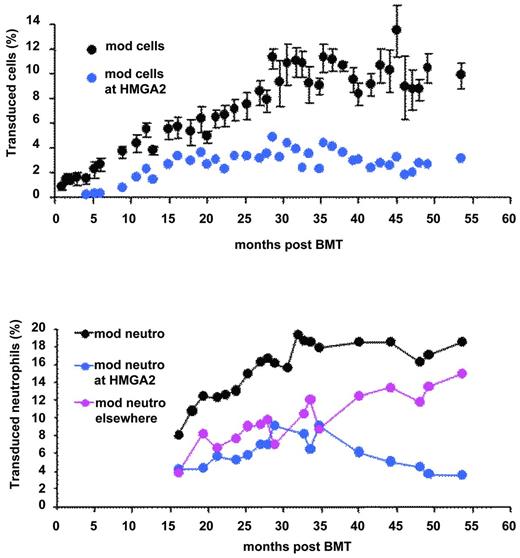
The transduction efficiency of bulk CD34+ cells was approximately 30%. The patient was conditioned by IV busulfan (Busulfex; Otsuka) at 3.2 mg/kg/d for 4 days without the addition of cyclophosphamide, before the injection of transduced CD34+ cell (approximately 3.9×106 CD34+ cells/kg). Neutropenia (neutrophil granulocytes < 500/μL) and thrombocytopenia (platelets < 20 000/μL) lasted 26 and 41 days, respectively. Hematopoietic reconstitution was subsequently complete and without adverse events. There was a progressive increase in the number of genetically modified nucleated blood cells that stabilized at approximately 10%, 30 months after BM transplantation (BMT; Figure 2). There was a concurrent increase in vector-bearing myeloid and lymphoid B cells. The reason for the progressive increase in vector copy numbers in the peripheral blood rather than an initially high vector copy number followed by stabilization at a lower level, as was observed in the lentiviral vector trial for adrenoleukodystrophy,28 has not yet been elucidated. Additive effects of the antiproliferative activities of busulfan and cyclophosphamide on hematopoietic cells in the adrenoleukodystrophy gene therapy trial28 may have created more opportunities for homing and proliferation of short-term stem/progenitor donor cells, whereas this globin trial made use of busulfan alone. The slow and intermediate rates of appearance of modified lymphoid T and B cells were anticipated because of the absence of destruction of the immune system by cyclophosphamide, which was not used, and, as a consequence, the persistence of long-lived memory lymphocytes. Similar observations were previously reported in another retroviral trial.29 Another finding is the contrast between the very low level of vector copy numbers in erythroblasts from the peripheral blood (approximately 0.02 copy per cell 36 months after BMT) versus BM erythroblasts (approximately 0.33 copy per cell, 36 months after BMT). In normal BM, cell-cell and cell-matrix interactions, hallmarks of erythroid niches, influence and regulate cell differentiation, enucleation, and cell adherence. In β-thalassemia, the accumulation of excess unmatched α-globin chains leads to RBC membrane changes and abnormal protein composition, especially integrins that bind fibronectin. The low level of gene marking of blood erythroblasts compared with that of BM-derived erythroblasts is likely to result from the fact that a great number of thalassemic erythroblasts expressing the therapeutic globin protein are capable of differentiating into mature RBCs in the BM, whereas uncorrected erythroblasts fail to do so and thus are released into the circulating blood.
Proportion of transduced cells in peripheral blood. The percentage is deduced from the vector copy number measured in purified cell population, assuming that most cells did not contain more than 1 copy of the vector per cell.
Proportion of transduced cells in peripheral blood. The percentage is deduced from the vector copy number measured in purified cell population, assuming that most cells did not contain more than 1 copy of the vector per cell.
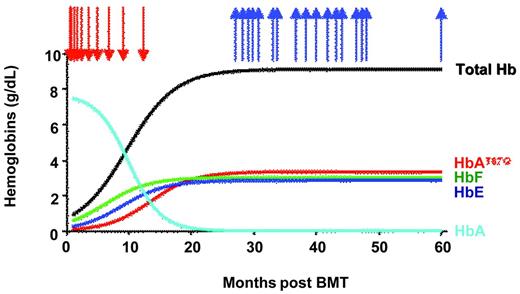
In agreement with the progressive increase in the proportion of vector-bearing cells in the peripheral blood, vector-encoded βA-T87Q-globin was first detected by HPLC 6 months after BMT and gradually increased before stabilizing approximately 2 years after BMT at approximately 3.5 g/dL. We observed a concurrent increase in the levels of HbβA-T87Q, HbE, and HbF. Expression of βA-T87Q-globin in an increasing number of thalassemic erythroid cells and the associated increase in β-like chains (γ and βE) per RBC progressively selected for the cells with the highest level of β-like production, including βE and γ-chains. This phenomenon is likely caused by the stimulatory effect of the absence of RBC transfusions on the expression of the endogenous globin genes.30
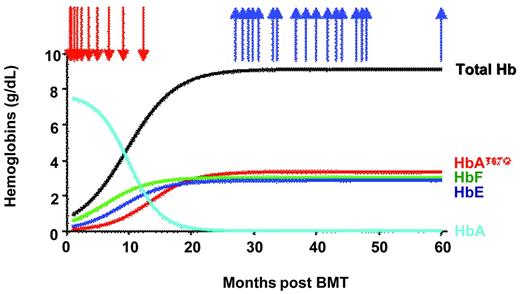
The total Hb level reached 8.5-9 g/dL and abrogated the need for RBC transfusion (Figure 3). The number of peripheral blood erythroblasts decreased by approximately 3-fold and RBC survival improved. Mean corpuscular hemoglobin content was fully corrected at 28.4 pg (the average for βE/β0-Thal patients is 19 pg). The calculated expression of βAT87Q was greater than 70% of the normal endogenous βA-globin level on a per-gene basis. All of these phenotypic changes are indicative of partial disease correction. Phlebotomies to withdraw blood (200 mL each) were initiated to accelerate reduction of the iron overload, and ferritin plasma levels decreased by > 2-fold. Despite these repeated phlebotomies, the patient has not required nor received any blood transfusion for more than 4 years (5 years after transplantation and gene therapy).
Concentration of hemoglobins in blood. Arrows indicate RBC transfusion (red) and phlebotomies (blue).
Concentration of hemoglobins in blood. Arrows indicate RBC transfusion (red) and phlebotomies (blue).
Insertional mutagenesis by integrative vectors
Activation of protooncogenes upon chromosomal integration of γ-retrovirus–based vectors in their vicinity has been involved in lymphoproliferative and myelodysplastic syndromes observed in patients undergoing gene therapy for severe combined immune deficiency,31 Wiskott-Aldrich syndrome,32–33 and chronic granulomatous disease.29,34 As demonstrated in patients and animal models, oncogene activation was mostly caused by the viral enhancers contained in the integrated vectors. The risk of oncogenicity related to insertional mutagenesis is believed to be lower for lentiviral than oncoretroviral vectors because, unlike oncoretroviral vectors, lentiviral vectors do not have a preference for integration near promoters.35 Removal of viral promoter/enhancer and production of self-inactivating vectors is now the rule and has improved the safety of integrative vectors. However, as for other lentiviral vectors, the LentiGlobin vector displayed a strong bias toward integration within genes, particularly in introns,36 and may therefore disrupt genes by compromising gene splicing37 and/or causing transcriptional termination at premature sites. Furthermore, even if cellular promoters are theoretically safer than viral promoters, the LCR elements contained in the vector may disturb the expression of genes up to 600 kb away from the integration sites.38 To limit this effect, we have introduced a tandem repeat of the cHS4 chromatin core insulator in the β-globin vector, although 1 of these core elements showed instability in a fraction of transduced cells.24,27
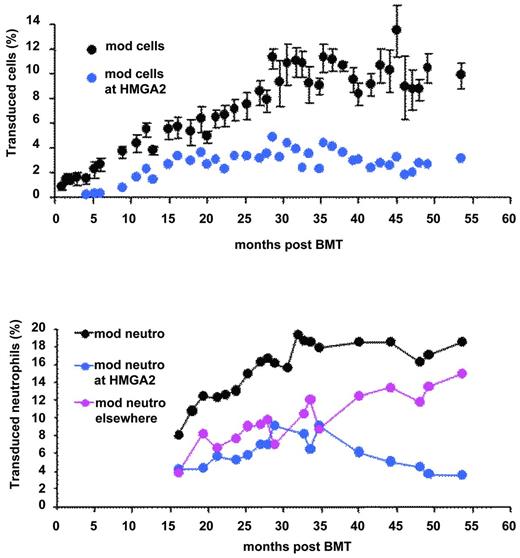
We performed a thorough pan-genomic analysis of LentiGlobin chromosomal integration sites (IS) in the peripheral blood and in purified hematopoietic cell populations of the βE/β0-Thal patient treated by LentiGlobin gene therapy. Of approximately 300 different IS identified, 24 of them were found in both lymphoid and myeloid lineages. Two of the most abundant and stable multilineage IS (lymphoid and myeloid) lay within the RFX3 and ZZEF1 genes. Their contribution, as monitored by quantitative PCR, remained below 0.5% and 1% for RFX3 and ZZEF1, respectively. There was a relative dominance over time of an IS at the high-mobility group AT-hook 2 (HMGA2)24 locus in both granulocytes and erythroid cells (Figure 2). However, HMGA2 IS was undetectable by quantitative PCR in fresh or expanded B or T lymphocytes. The cell clone was first detected 4 months after BMT, has stabilized since month 15, and now represents approximately 2%-3% of the circulating nucleated cells (Figure 4). Because translocation events in the HMGA2 gene and overexpression of a truncated form of HMGA2 have been involved in neoplasia39 (mostly benign), there has been careful and regular follow-up of this patient and so far, no breach of hematopoietic homeostasis has been observed. The proportion of neutrophils bearing the vector at this site is stable and has even decreased slightly (Figure 4). Careful analysis of vector insertion and expression studies has revealed the following features: (1) the vector is inserted within the third intron of HMGA2; (2) HMGA2 is overexpressed in erythroid cells but is not detectable in neutrophils; and (3) the HMGA2 transcript is truncated: the third exon of the gene is fused to the vector due to splicing of the donor sequence with a cryptic splice acceptor site localized in the LentiGlobin vector and premature termination. Overexpression of the truncated transcript in erythroid cells occurs both through deletion of let7 miRNA-binding sites (located in exon 5 of HMGA2) and by LCR-mediated activation of the HMGA2 promoter.24 Because the HMGA2 insertion site was not detected in lymphoid cells in vivo, we hypothesize that the triggering event has occurred in myeloid-biased stem cells. Alternatively, abnormal expression of HMGA2 may have modified the fate and the self-renewal properties of a cell with otherwise limited proliferative capacity.
Transduced peripheral blood cells. All cells (top) and neutrophils (bottom) at all sites (black) at the HMGA2 site (blue) or elsewhere (pink).
Transduced peripheral blood cells. All cells (top) and neutrophils (bottom) at all sites (black) at the HMGA2 site (blue) or elsewhere (pink).
Although a recent study in transgenic mice reported that ubiquitous deregulation of HMGA2 caused a proliferative advantage reminiscent of that seen in patients with myeloproliferative neoplasms,40 observations made in patients with paroxysmal nocturnal hemoglobinuria, who have abnormally high expression of HMGA2,41 and transplantation of mouse cells expressing a truncated form of HMGA241 both suggest that only benign expansion of hematopoietic cell clones occurred. The detection of IS near HMGA2 in other clinical studies,33,42–43 also in the absence of myeloproliferative syndrome, suggests that a possible explanation for the detection of this target on multiple occasions may not reflect in vivo selection, but rather an intrinsic bias of vector to integrate at specific loci at the time of CD34+ cell transduction. The observation that a similar proportion of cells bear the integrated LentiGlobin vector within the HMGA2 gene in all myeloid compartments, including long-term culture initiating cells, whereas HMGA2 expression at the RNA and protein levels is only detected in the erythroid lineage, led us to conclude that myeloid-biased hematopoiesis may have been triggered by insertional mutagenesis or may simply be physiologic and revealed by vector marking.44
Conclusion
Long-term clinical benefit (ie, complete transfusion independence for more than 4 years) has been achieved in the first treated patient (without injection of backup cells) with severe, transfusion-dependent βE/β0-thalassemia. Another patient was treated in November 2012 and is being analyzed. Data will be presented as soon as their release is permitted according to the trial protocol schedule. Other patients are in the process of being treated. This study has increased our knowledge of the occurrence of endogenous gene activation upon lentiviral vector integration and the dynamics of human hematopoiesis. Larger series of patients will ultimately shed light on benefit/risk ratios for the gene therapy of the β-hemoglobinopathies and for hematopoietic gene therapy as a whole. Alternative approaches continue to be evaluated in cell lines and animal models. Such approaches include the potential use of induced pluripotent stem cells derived from patients,45–46 although many hurdles to acceptable safety and efficacy remain to be surmounted.
Disclosures
Conflict-of-interest disclosure: P.L. is on the board of directors or an advisory committee for, has received research funding from, has consulted for, has equity ownership in, and holds patents with or receives royalties from Bluebird Bio. E.P. has consulted for Bluebird Bio. Off-label drug use: None disclosed.
Correspondence
Philippe Leboulch, MD, Department of Medicine, Division of Genetics, Brigham and Women's Hospital, Nrb, 75 St Francis St, Boston, MA 02115; Phone: 617-525-4742; Fax: 617-525-4705; e-mail: leboulch@rics.bwh.harvard.edu.

![Figure 1. LentiGlobin vector and experimental design. (A) Diagram of the human β-globin (βA-T87Q) lentiviral vector (LentiGlobin; LG). The 3′ β-globin enhancer, the 372-bp IVS2 deletion, the βA-T87Q mutation (ACA[Thr] to CAG[Gln]) and DNase I Hypersensitive Sites (HS) 2, HS3, and HS4 of the human β-globin Locus Control Region (LCR) are indicated. Safety modifications including the 2 stop codons in the ψ+ signal, the 400-bp deletion in the U3 of the right HIVLTR, the rabbit β-globin plyA signal, and the 2 × 250-bp cHS4 chromatin insulators are indicated. HIVLTR indicates human immunodeficiency type-1 virus long terminal repeat; ψ+, packaging signal; cPPT Map, central polypurine tract; RRE, Rev-responsive element; βp, human β-globin promoter; and ppt, polypurine tract.](https://ash.silverchair-cdn.com/ash/content_public/journal/hematology/2012/1/10.1182_asheducation.v2012.1.276.3807841/5/m_bep0011202640001.jpeg?Expires=1763837865&Signature=YfM~ftt9zde3FaYj1auUmS8i6fThkDQu~Mu7fzHQ00MLQ9-yjnaxfXaOWkGxfkM34pYZF5hZ9grh4L8c6U2Tfe~i9UcA1jiKb7N4Js3SAHRJpe3qV3azPQhIrSLQyJ8zcvZ5Y4dNv5mB8VVxuy4JJaVlp2Eq-HXU~0dmvjCpwvZpLV-61pdQSi4hoSN0Z6L92QXae5nFr~afCuX72ZJN8qT58XYWpuaUDgvfkgCHfKmjie78htLmF-MIbuYAp5qPbug7mkutakjLnc5bkTU~rnF-n2ja48CcvRBQXhrvC6rkJyUw9orp--SSdiLvwHoqZseye-cWS9pE2suO7pNO9w__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 1. LentiGlobin vector and experimental design. (A) Diagram of the human β-globin (βA-T87Q) lentiviral vector (LentiGlobin; LG). The 3′ β-globin enhancer, the 372-bp IVS2 deletion, the βA-T87Q mutation (ACA[Thr] to CAG[Gln]) and DNase I Hypersensitive Sites (HS) 2, HS3, and HS4 of the human β-globin Locus Control Region (LCR) are indicated. Safety modifications including the 2 stop codons in the ψ+ signal, the 400-bp deletion in the U3 of the right HIVLTR, the rabbit β-globin plyA signal, and the 2 × 250-bp cHS4 chromatin insulators are indicated. HIVLTR indicates human immunodeficiency type-1 virus long terminal repeat; ψ+, packaging signal; cPPT Map, central polypurine tract; RRE, Rev-responsive element; βp, human β-globin promoter; and ppt, polypurine tract.](https://ash.silverchair-cdn.com/ash/content_public/journal/hematology/2012/1/10.1182_asheducation.v2012.1.276.3807841/5/m_bep0011202640001.jpeg?Expires=1763837866&Signature=TB7DJe1517gqle1CW3t8U-AqeD-l1tjM-EcuBLYH8ty15FkUbneIGyhPgLg7xSrrnoTxWYF0tG6bNXvMbVL1r0EqnUOs2eIK3V0rg5~cH3eSXwEGfx0ahBu4d~Hta1FNXOOyzMisULxY6Gq6qGdDnBG4tMHs1rhkJ5jZXXtPcep2OS~deVeGKmh8APigPVbuSRcYqPmyEfYC4r-QlWcEXHgmGnsAIkgC4maq0zX5x8BLZcOLa6qFh1cQ-VFY0qfwjL3aL2Cz-joD4w14-SUNDpmfJsQsu2cqXFUxA2llcNTh5slziZRfXuaSwjZ3wduQhU7B5XPDLabz0OVexcNwXg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)