Abstract
Excellent therapeutic options exist for the treatment of chronic-phase chronic myeloid leukemia (CML) patients. Therefore, managing CML patients has become a more common practice for many physicians. Most chronic-phase CML patients achieve durable cytogenetic and molecular responses on first-line tyrosine kinase inhibitor therapy. However, careful monitoring and assessment of adherence are essential for successful outcomes and to identify patients at risk for failing therapy. The European LeukemiaNet and National Comprehensive Cancer Network provide guidance and strategies for monitoring and managing patients treated with TKIs. These recommendations continue to evolve as approved treatment options expand to include second- and third-generation tyrosine kinase inhibitors. How measurements of response are defined and data supporting recent recommended changes to monitoring are reviewed here. These changes include increasing recognition of the importance of early response. The relevance of achieving deep molecular responses will also be addressed.
Introduction
The increasing prevalence of chronic myeloid leukemia (CML) attests to the success of tyrosine kinase inhibitor (TKI) therapy.1 A recent report estimates that the prevalence of CML in the United States by 2040 may be as high as 200 000 cases,2 a prevalence similar to that for CML, chronic lymphocytic leukemia, acute myeloid leukemia, and acute lymphoblastic leukemia combined as of 2009 and reported in the US Surveillance Epidemiology and End Results (SEER) database (271 880 cases).1 These estimates highlight the excellent survival of CML patients (Figure 1) and the growing importance of both short-term and long-term monitoring. As of 2013, 5 TKIs are available in the United States, 3 as first-line imatinib mesylate (IM), dasatinib (DAS), and nilotinib (NIL), and 5 as second-line (IM, DAS, NIL, bosutinib, and ponatinib). Second-generation TKIs are increasingly used as first-line therapy. The current goals of monitoring chronic phase (CP) CML patients in the short term are reviewed here, with the main focus being the prevention of CML progression and monitoring for intolerance and resistance. In addition, the rationale for long-term monitoring is also reviewed, with the significance and relevance of achieving a persistent deep molecular response addressed.
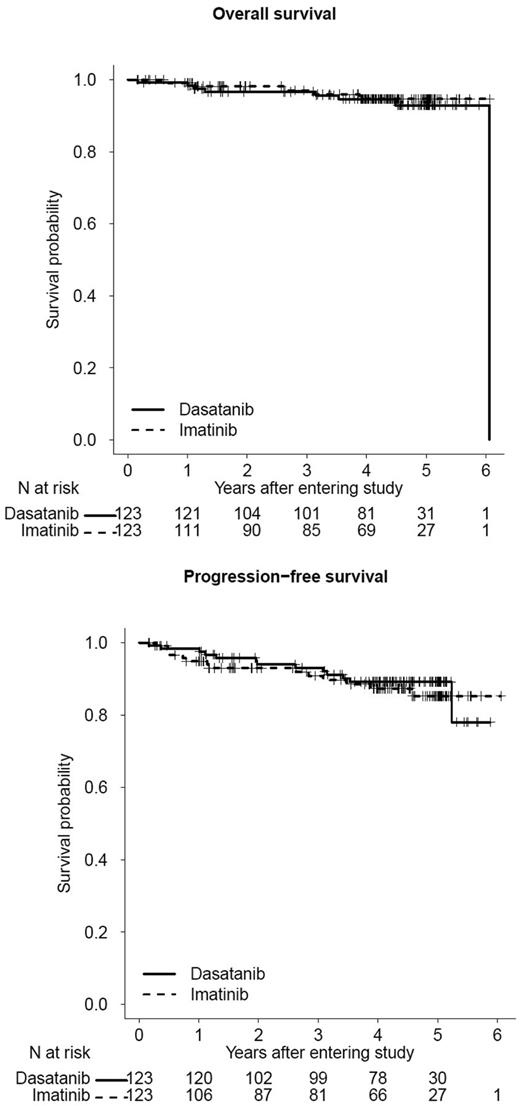
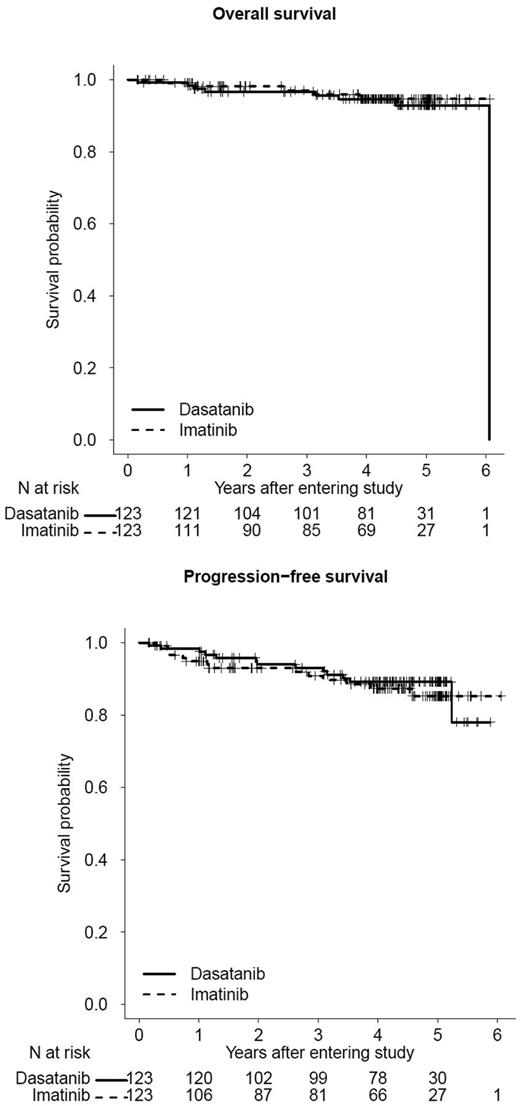
OS and PFS on first-line imatinib or dasatinib therapy. Updated outcomes of Southwest Oncology Group Study S0325 (NCT00070499): “Imatinib Mesylate or Dasatinib in Treating Patients with Chronic Phase Chronic Myelogenous Leukemia” (courtesy of SWOG Statistical Center). The 3-year OS is 97% and 97% for dasatinib and imatinib, respectively; 3-year PFS is 93% and 91% for dasatinib and imatinib, respectively.
OS and PFS on first-line imatinib or dasatinib therapy. Updated outcomes of Southwest Oncology Group Study S0325 (NCT00070499): “Imatinib Mesylate or Dasatinib in Treating Patients with Chronic Phase Chronic Myelogenous Leukemia” (courtesy of SWOG Statistical Center). The 3-year OS is 97% and 97% for dasatinib and imatinib, respectively; 3-year PFS is 93% and 91% for dasatinib and imatinib, respectively.
Approach at diagnosis
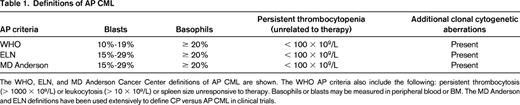
Although CML is easily diagnosed from peripheral blood by FISH or by quantitative or qualitative RT-PCR for BCR-ABL1 transcripts, a bone marrow (BM) examination remains an essential part of the initial workup for CML. Identifying accelerated phase (AP) at diagnosis is important because AP patients have inferior responses on all TKIs compared with CP patients. Definitions of AP CML are shown in Table 1.3,4 Recognizing AP CML affects treatment and monitoring strategies and decisions regarding when to proceed to allogeneic transplantation. Additional clonal cytogenetic aberrations (ACAs) in Philadelphia-chromosome-positive (Ph+) cells are a feature of AP CML. A recent study of 1151 patients found that the more frequent abnormalities in Ph+ cells (trisomy 8, second Ph, isochrome 17q, or trisomy 19), called major-route ACAs, are associated with poorer progression-free survival (PFS) and overall survival (OS) compared with patients without ACAs or with rarer ACAs (called minor route ACAs).5 Conversely, the presence of variant translocations or deletion of the derivative chromosome 9 (der 9q del) does not appear to affect cytogenetic or molecular response or outcomes on IM, and der 9q del does not appear to affect outcomes on NIL or DAS.6-8 Clinical risk as defined by a high risk Sokal or Hasford Risk Score remains prognostic on IM and second-generation TKIs, whereas intermediate risk score patients have similar outcomes to good risk score patients.9,10 A new streamlined risk score, the EUTOS Score, which is derived solely from the baseline basophil percentage in the peripheral blood and spleen size, has been found to predict complete cytogenetic response (CCyR) and 5-year PFS in IM-treated patients.11 Any of these 3 scores may be calculated at presentation to identify high risk score patients. BCR-ABL1 transcript levels from blood or BM at diagnosis do not appear to be prognostic, but this issue is still under study.
Evolving definitions of response
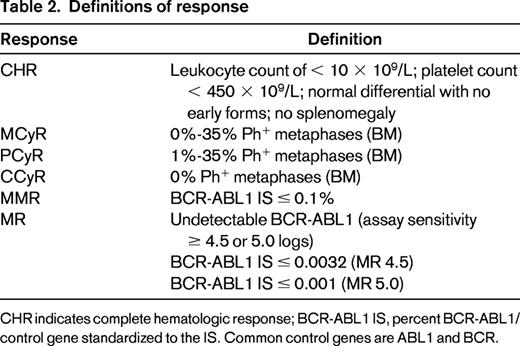
Several organizations, such as the European LeukemiaNet (ELN) and the National Comprehensive Cancer Network (NCCN), provide extensive evidence based recommendations that define response and provide milestones or algorithms to monitor response on TKIs.3,4 Response criteria for hematologic, cytogenetic (based on metaphase cytogenetic preparations), and molecular responses are shown in Table 2. The definitions of molecular response continue to evolve and expand. The Phase 3 International Randomized Interferon vs. STI571 (IRIS) trial identified that patients with a 3-log10 or greater reduction in BCR-ABL1 transcript levels from a specified pooled patient baseline had the best PFS.12 This was the first definition of major molecular response (MMR). Although log reductions remain a valid definition, molecular response is typically defined as a percentage (eg, BCR-ABL1/control gene) and does not require a baseline value at diagnosis. The most common control genes in use are ABL1 and BCR. There are several points that should be understood to ensure reproducible and reliable molecular monitoring in all patients and to guide interpretation of molecular monitoring data. The use of different methodologies, control genes, and reagents has made it difficult to compare results between laboratories. In the absence of universal standards or reagents, the International Scale (IS) was developed to harmonize molecular responses across laboratories.13 IS response is derived by applying a laboratory-specific conversion factor to molecular response data from each individual participating laboratory. This conversion factor is derived from extensive validation and comparison with a reference laboratory.13 In addition, to remain accurate, this process requires monitoring over time for “drift” in IS measurements. All molecular response criteria and recommendations for intervention in the NCCN or ELN guidelines are based upon IS molecular response. Recently, the World Health Organization (WHO) has spearheaded efforts to provide accredited reference reagents directly linked to the BCR-ABL1 IS scale. These secondary reference materials are available from the Institute for Reference Materials and Measurements (Belgium). Supplies of these primary standards are limited, but other methods to provide reference standards are under investigation. The importance of universal standardized testing is reflected in the growing importance of molecular response measurements at early time points after therapy initiation, as well as in measuring MMR and deep molecular responses consistently. Unlike MMR, there are various definitions of deep molecular response (MR), including MR 4.0 (BCR-ABL1 ≤ 0.01%), MR 4.5 (BCR-ABL1 ≤ 0.0032%), and MR 5.0 (BCR-ABL1 ≤ 0.001%). Efforts are under way to standardize definitions and methods to reliably detect and measure such small amounts of BCR-ABL1.13,14 Given the varying and evolving sensitivities of PCR assays, multiple definitions will continue to be used in the near future and these more precise terms are beginning to replace the term “complete molecular response.”13,14
Why does early response matter?
The growing recognition of the importance of early response is reflected by recent changes to the NCCN guidelines. At 3 months, the guidelines suggest that a BCR-ABL1/ABL1 > 10% is a trigger to examine patient adherence and assess for resistance. The recognition of the clinical significance of early response is not a new one, but several studies over the past 3 years have added increasing support to the idea that a very early time point, 3 months after therapy initiation, may be one of the most important treatment milestones. A study of 1440 patients treated on the German CML IV study observed that among the 28% of patients who did not achieve ≤ 10% BCR-ABL1, OS after 5 years was poorer at 87% versus 94% for patients > 1% but ≤ 10% and versus 97% for patients ≤ 1%.15 In another study of 282 patients treated with IM at Hammersmith Hospital in London, BCR-ABL1 > 9.84% was the strongest predictor of poorer event-free survival (EFS), PFS, and OS.16 Molecular response at 6 and 12 months were not as strongly predictive.16 Patients with transcript levels above this cutoff had a significantly poorer OS of 56.9% compared with 93.3% for those below it. These observations suggested that 3-month molecular response may provide more prognostic information than 6-month major cytogenetic response (MCyR) or 12-month CCyR.16 The first study also suggested that molecular response correlates well with cytogenetic response (ie, > 10% with absence of MCyR at 3 months and > 1% with absence of CCyR at 6 months). Consistent with other studies demonstrating that early cytogenetic response correlates with improved EFS,17 the newest iteration of the NCCN guidelines also proposes that absence of a partial cytogenetic response (PCyR; defined as 1%-35% Ph+ cells) at 3 months is also a warning sign. Recent studies have determined that early response at 3 months is associated with response, PFS, and OS in patients treated with first-line and second-line DAS or NIL.18-22 For second-generation TKIs, lower BCR-ABL1 IS transcript thresholds may be more predictive given the more rapid decrease in disease burden seen with these agents.20 One important issue that is unknown at this time is whether therapy should be altered based on response at 3 months. It is possible that poor molecular response at 3 months identifies a subset of patients who respond poorly to all TKIs. The correlation between high-risk EUTOS Risk Score and poor early molecular response supports this idea.15 Nonetheless, given that patients can achieve durable responses on second-line TKI therapy, I do consider, particularly in younger patients, switching early after reviewing therapy adherence and assessing for ABL tyrosine kinase domain (TKD) point mutations. I also watch closely as I do in patients treated with first-line TKIs for AP. I do acknowledge that these data also suggest that most patients who are not switched will have good outcomes, but it is difficult to identify within this subset of patients who will do better versus who will do poorly. In addition, although categorical cutoffs are useful to analyze outcomes, they do require a little common sense before considering a change in treatment strategy. BCR-ABL1 transcript levels of 10.3% versus 9.2% are probably not that different. In addition, a patient whose BCR-ABL1 transcript level decreases from 90% to 10.3% is probably less worrisome than a patient whose BCR-ABL1 transcript levels decreases from 90% to 75%, and may also be less worrisome than a patient whose BCR-ABL1 transcript level decreases from 12% to 9.5%.
Continued importance of a complete cytogenetic response
What is currently in evolution in 2013 is whether molecular monitoring can entirely replace BM examinations with their associated pain and discomfort. This requires reproducible and reliable molecular response surrogates for cytogenetic responses. Although this goal has been achieved in some places, the answer in others is not yet. One important reason driving the recommendation for at least one BM examination during the early monitoring period are the strong data supporting the association between PFS, EFS, and OS and cytogenetic response on IM.17,23 For the IRIS trial at 6 years of follow-up, the EFS rate was 59%, 85%, and 91% for patients with no cytogenetic response, MCyR, or CCyR at 6 months, respectively.23 More recent studies have demonstrated improved OS in patients with CCyR at 6 or 12 months.17 For second-generation TKIs, these milestones occur earlier (∼ 3 months earlier or more) and also result in small improvements in PFS.9,10 Therefore, until standardization of molecular methods is achieved or a centralized resource for molecular testing is available and clear guidelines correlating molecular response and cytogenetic response exist in all laboratories, a BM aspirate should be obtained after initiating TKI to document a CCyR has been achieved within the first year of therapy. A BM examination at 3 months may also be used in lieu of BCR-ABL1 transcripts to assess for poor early responders. In my own practice, I am moving away from performing frequent BMs examinations in patients with ≤ 10% BCR-ABL1 IS at 3 months who have improving molecular responses. I will perform a BM aspirate at ∼ 6 months or earlier when BCR-ABL1 is ≤ 1% to confirm CCyR, because this level typically correlates with CCyR at our center. For standard dose IM-treated patients achieving < 1% BCR-ABL1 IS transcripts may take longer and an argument can be made (especially in laboratories with reliable IS molecular monitoring) to wait up to 12 months if BCR-ABL1 transcripts are decreasing. After a CCyR has been confirmed, the utility of further BMs in responders when reliable molecular testing is available is limited. One caveat is the monitoring of cytogenetic abnormalities in patients with ACA in Ph− cells. Unlike Ph+ ACA, Ph− ACA does not affect outcomes except in the rare cases of myelodysplastic syndrome–associated abnormalities, which are typically accompanied by cytopenias.24 Therefore, these abnormalities may warrant closer follow-up, although no change in treatment strategy is indicated unless evidence of dysplasia is noted. Outcomes data support that CCyR, or perhaps in the future a surrogate for CCyR, remains the gold standard of response and for some patients, such as older patients, may be the only response required.
Why should we aim for deep and persistent molecular responses?
The achievement of MMR or better is of interest for 2 reasons: achieving a stable MMR may provide a safe harbor and achieving a deep molecular response may identify a subset of patient in whom TKIs can be safely stopped. Molecular responses are associated with durability of response, loss of response, EFs, and PFS. Updates of the IRIS trial demonstrated that achievement of MMR affected the durability of CCyR and that patients achieving MMR had the best PFS.12 Although many studies have not yet demonstrated that achieving MMR improves EFS or OS in patients achieving a CCyR,16,25 a recent update from the German CML Study IV suggested that OS is improved in patients who achieve a MMR at 12 months.26 Therefore, MMR at 12 months was the primary end point for many recent clinical trials examining or comparing IM and NIL.
Two studies investigated stopping IM in patients with deep molecular responses: the French STIM (MR 5.0) and the Australasian TWISTER (MR 4.5) studies.27,28 Both studies identified that ∼ 40% of patients maintained deep molecular responses off therapy with a median follow-up of 2 years.27 Although TKIs do not appear to eradicate stem cells in in vitro studies, mathematical modeling studies suggest that in some patients, CML stem cells may be depleted over time, although how this occurs is unknown.29 Several factors affect the achievement and stability of deep molecular responses. A recent study identified 2 factors that predicted achievement of MR4.5: BCR-ABL1 transcript levels at 3 months (in particular, BCR-ABL1 ≤ 0.1% IS) and female sex.30 The STIM study identified that longer duration of MR 5.0 before discontinuation correlated with prolonged MR 5.0 off IM.27 Studies of second-generation TKIs demonstrate not only a higher rate of MMR, but also of deep molecular responses. The cumulative rate of MR 4.5 on the DASISION trial was 22% for DAS-treated patients versus 12% for IM-treated patients at 3 years.10 On the ENESTnd trial, at 3 years, the rate of MR4.5 was 32% for NIL-treated patients versus 15% for IM-treated patients.9 Therefore, achieving deep molecular responses on second-generation TKIs may allow more patients to participate in ongoing TKI cessation trials to study the safety of such a strategy. Based on these observations, I do aim for MMR and, when possible, deep molecular responses, particularly in younger patients. Nonetheless, despite these exciting observations, deep molecular responses are not the goal of therapy and TKIs should not be stopped outside of a clinical trial where careful molecular monitoring is pursued.
Guidelines in brief
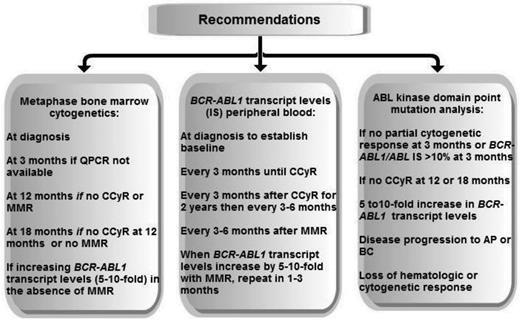
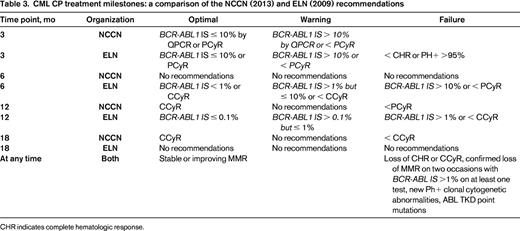
These recent observations have informed changes to the ELN and NCCN recommendations, which are focusing on early achievement of cytogenetic or molecular milestones. These recommendations are also being updated to include second-generation TKIs used as first-line therapy. Recommended testing and a comparison of the guidelines are shown in Figure 2 and Table 3. The NCCN guidelines, updated annually, provide algorithms at 3, 12, and 18 months guiding which tests to perform, when to pursue workup for resistance, and when to consider changing therapy.4 The 3-month evaluation is now based upon molecular response or cytogenetic response, but the remaining evaluations informing workup for resistance and changes in treatment strategy are based upon cytogenetic responses. However, MMR is a secondary goal of therapy endorsed by both the ELN and NCCN and both recommend molecular monitoring at 3-month intervals initially and at 3- to 6-month intervals in patients with CCyR or MMR.3
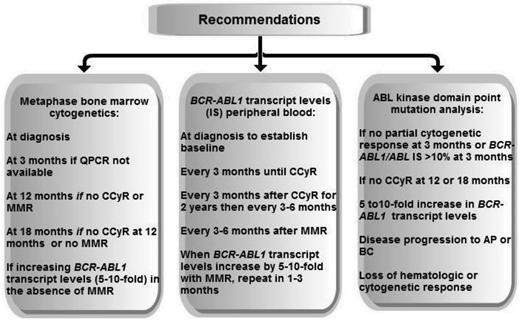
Recommended testing for disease monitoring adapted from NCCN (2013) and ELN (2009 and 2013).
Recommended testing for disease monitoring adapted from NCCN (2013) and ELN (2009 and 2013).
The ELN recommendations categorize response into several categories: optimal, in which no change is indicated; suboptimal (or warning), in which continued benefit may occur from the current treatment strategy but the risk failure is increased; and failure, in which a change of treatment strategy is indicated because these patients have decreased PFS and OS compared with patients with optimal responses.25 The ELN recommendations were updated in 2013 and are similar to the NCCN guidelines but include more extensive recommendations for molecular monitoring.31 An evaluation of earlier ELN recommendations was reported in 2008.25 This validation demonstrated the importance of milestones defined by hematologic and cytogenetic responses within the first year. None of the patients classified as a failure at 3 months (no complete hematological response) or 12 months (no MCyR) reversed their failure status and PFS and OS at 5 years in these patients were significantly different compared with patients never meeting failure criteria.25 Conversely, achieving MMR at 18 months was less important and was not predictive of PFS or OS at 5 years.25 Nonetheless, patients who achieved a CCyR but failed to achieve an MMR at 18 months were statistically significantly more likely to lose their cytogenetic response (24.6% vs 0%).25 Changes to the ELN recommendations for monitoring, like the NCCN guidelines, reflect the importance of early treatment milestones and provide further guidance on molecular monitoring milestones for providers with access to reliable IS molecular monitoring.
Data do not currently support that TKI therapy should be switched or dose altered in patients with stable CCyR due to lack of MMR. Therefore, in CCyR patients without MMR with a molecular response characterized as stable or slowly decreasing or one that “wobbles” around MMR but is not increasing, I do not change therapy but monitor molecular response at 3-month intervals. For some younger patients, after careful discussion, it may be reasonable to switch therapy to achieve MMR or deep molecular response if possible. My reasons for considering this switch include possible enrollment in a stop TKI trial or for young women the consideration of future family planning.
When and how do we look for resistance?
Resistance to TKI therapy is often characterized as primary or secondary (ie, acquired) resistance. The etiology of primary resistance remains largely unknown, but reported mechanisms include altered drug transport and BCR-ABL–independent mechanisms (in which BCR-ABL remains inhibited, but disease is not significantly altered or disease progression occurs). Point mutations in the ABL TKD are rarer in primary resistance, but are the most common cause of acquired TKI resistance. Mutations are currently detected using direct nucleotide sequencing. This is an insensitive technique and the mutated clone must comprise ∼ 15% to 25% of the clone to be detected. More than 80 point mutations have been described after IM exposure, but mutations at 7 sites (G250, Y253, E255, T315, M351, F359, and H396) comprise ∼ 60% of mutations reported in larger surveys.32,33 Subsequent TKI generations have been designed to minimize resistance due to mutations. DAS-resistance-associated mutations include T315I and mutations involving amino acid residues 317 and 299. The Y253H, E255K/V, T315I, and F359C/V mutations are the most frequent mutations associated with NIL resistance and L248V, G250E, V299L, T315I, and F359C are associated with bosutinib resistance.33,34 Ponatinib treats CML with any mutation, including T315I, and is resistant only to certain rare compound mutations (ie, mutations on the same DNA strand).35 An informative review discussing in vitro versus clinical resistance on TKIs was published several years ago, and remains a significant resource.33
ABL TKD mutation sequencing should be pursued when hematologic and cytogenetic milestones are either not achieved or are lost (Figure 2). It is important to pursue ABL TKD mutation studies before changing therapy for treatment failure because these studies will guide second- or third-line treatment selection. For patients who have achieved a CCyR, it has been reported that small changes in BCR-ABL1 may signal the development of point mutations. One study reported that a 2.6-fold rise in BCR-ABL1 mRNA is associated with the emergence of ABL TKD mutations.36 It should be emphasized that these recommendations apply only to patients monitored serially using IS standardized responses, preferably measured in the same laboratory. Furthermore, it is likely that thresholds vary by laboratory. A more conservative approach is based on the observation that PCR values vacillate, particularly when measuring small amounts of BCR-ABL1, and that the meaning of increases in BCR-ABL1 also depend upon the burden of disease. Therefore, one approach for patients with a durable CCyR is to pursue mutation studies in those who lose MMR, never achieve MMR, or who experience a > 5- to 10-fold increase in BCR-ABL1. For patients with smaller increases in BCR-ABL1, I will first repeat the measurement in 1 to 2 months before pursuing sequencing. The application of more sensitive methods to detect ABL TKD point mutations using mass spectrometry has suggested that the presence of multiple low-level mutations is of prognostic significance.37 However, this technology is not currently readily available and its broader clinical significance needs to be defined.
Adherence and treatment failure
Another important contributor to treatment failure is therapy adherence.38-40 Studies have suggested that poor adherence occurs more frequently than either patients or physicians recognize, contributes to poorer outcomes, and may contribute to increased health care costs.38,39,41,42 Studies are difficult to compare head to head given the differences in assessing adherence (eg, chart review vs pill counts vs electronic devices to measure bottle opening vs review of health care databases). Nonetheless, the rates of nonadherence are similar in many of these studies, on average ∼ 25% to 35%, although the range can be quite broad.38-40 Definitions for nonadherence on IM include the use of ≤ 85% or 90% of prescribed drug.38,39 The ADAGIO study examined adherence in 169 patients in Belgium and observed that approximately one-third of patients were nonadherent and only 14.2% of patients were 100% adherent with prescribed IM.38 Nonadherence was associated with poorer cytogenetic response.38 In another study of 87 patients treated at Hammersmith Hospital in London, patients with adherence rates of ≤ 85% had an increased probability of losing CCyR (26.8% vs 1.5%).39 A recent study of 516 patients receiving IM through the Glivec International Patient Assistance Program in India documented the first association between adherence and EFS.41 A statistically significant decrease in 5-year EFS was observed: 76.7% in adherent patients versus 59.8% in nonadherent patients.41
The question then becomes how health care teams can improve adherence. The ADAGIO study identified several factors that adversely affected compliance, including age, living alone, dose of IM, male sex, length of time from diagnosis to treatment, and length of IM treatment.38 Factors that positively influenced adherence included increased knowledge about CML disease and treatment, at least a secondary education, and taking other medications chronically.38 A recent update of the Hammersmith Hospital study of patient adherence further explored issues and behavior contributing to nonadherence.40 The most common reason for unintentional nonadherence was forgetting to take pills and for intentional nonadherence was to minimize side effects.40 Many patients did not think missing doses would contribute significantly to response and patients relied upon their treating physicians to comment on the impact of nonadherence on treatment responses.40 Other studies highlight the importance of quality of life in adherence and the importance of monitoring adverse events. For example, recent studies highlight that issues with chronic fatigue and weight gain on IM are more pervasive than originally thought and can influence patients to skip doses.43,44 Although this observation may seem intuitively obvious, it is important for physicians and their teams to discuss adherence regularly. This can be done through nursing or pharmacist phone calls to assess for adverse effects, dispensing pill boxes to assist taking pills on schedule, recommendations to link TKI use to a regular scheduled daily activity, and taking particular care to discuss these issues with patients who have risk factors for nonadherence. Although improving adherence sufficiently to improve outcomes is difficult, a recent study of 1155 CML patients treated by 405 physicians suggests that a proactive approach may improve adherence.44 Similar to other chronic diseases, long-term adherence once a response has been achieved is difficult and may be the main benefit of continued patient and health care team interactions for patients who have achieved a CCyR.38 Interestingly, a recent study proposed that short BCR-ABL1 transcript doubling time could distinguish nonadherence from resistance and may assist physicians in recognizing nonadherent patients.45 Our institution has recently proposed changes to improve adherence in patients taking oral chemotherapy. These changes include scheduled nursing or pharmacist phone calls or visits to discuss side effects and assess adherence. At this time, these efforts primarily occur early in the treatment course.
Conclusion
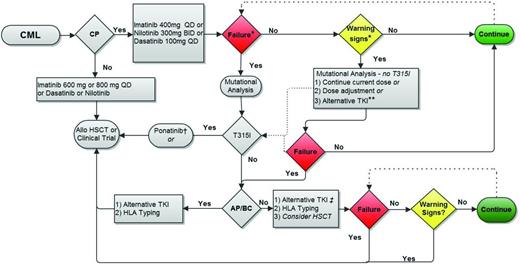
Although most CP CML patients respond to therapy, approximately 20% to 25% of patients become resistant to IM. Adequate monitoring is important to identify these patients early in the treatment course. Recent changes to monitoring recommendations highlight the importance of early responses, which identify both the best and the worst responders. Although it is difficult at times to differentiate poor response due to adherence from poor response due to resistance, these recommendations highlight when closer monitoring should be pursued, adherence should be addressed, and changes in treatment strategies should occur. An overview of my general monitoring approach is shown in Figure 3. However, patient care should be patient driven. Therefore, patient age, medical comorbidities, and the patient's wishes influence how aggressive my approach is and whether the goal should be CCyR, MMR, or even deep molecular responses.
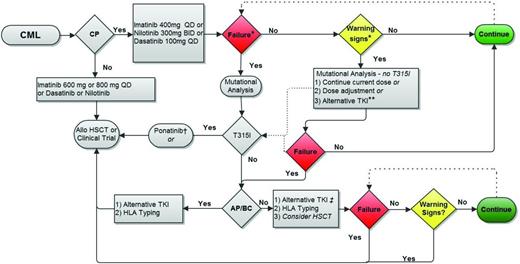
Proposed treatment monitoring strategy. Allo HSCT indicates allogeneic hematopoietic stem cell transplantation. *Failure and warning signs (or suboptimal response) are defined in Table 3. **Choice will be influenced by the type of warning sign. For example, no change may be appropriate for a patient with CCyR and declining BCR-ABL1 transcript level but no MMR at 18 months, whereas the absence of CCyR at 12 months may warrant consideration for switching to an alternative TKI (after assessing for ABL tyrosine kinase domain point mutations). The presence of a resistance-conferring mutation warrants a change in treatment strategy. †For CP patients who develop a T315I mutation on first-line therapy but who have no evidence of additional cytogenetic aberrations or other features of progression, I typically first switch to ponatinib and follow closely to determine the need to proceed to allogeneic transplantation. ‡Monitoring on second-line therapy (nilotinib, dasatinib, bosutinib, or ponatinib) is similar to monitoring on first-line therapy. Figure modified with permission from Coveler and Oehler.47
Proposed treatment monitoring strategy. Allo HSCT indicates allogeneic hematopoietic stem cell transplantation. *Failure and warning signs (or suboptimal response) are defined in Table 3. **Choice will be influenced by the type of warning sign. For example, no change may be appropriate for a patient with CCyR and declining BCR-ABL1 transcript level but no MMR at 18 months, whereas the absence of CCyR at 12 months may warrant consideration for switching to an alternative TKI (after assessing for ABL tyrosine kinase domain point mutations). The presence of a resistance-conferring mutation warrants a change in treatment strategy. †For CP patients who develop a T315I mutation on first-line therapy but who have no evidence of additional cytogenetic aberrations or other features of progression, I typically first switch to ponatinib and follow closely to determine the need to proceed to allogeneic transplantation. ‡Monitoring on second-line therapy (nilotinib, dasatinib, bosutinib, or ponatinib) is similar to monitoring on first-line therapy. Figure modified with permission from Coveler and Oehler.47
Disclosures
Conflict-of-interest disclosure: The author declares no competing financial interests. Off-label drug use: None disclosed.
Correspondence
Vivian G. Oehler, Fred Hutchinson Cancer Research Center, 1100 Fairview Ave N, PO Box 19024, Seattle, WA 98109-1024; Phone: 206-667-1340; Fax: 206-667-2917; e-mail: voehler@u.washington.edu.