Abstract
Diffuse large B-cell lymphoma (DLBCL) is the most common subtype of non Hodgkin lymphoma in the Western world, and is potentially curable with standard R-CHOP chemoimmunotherapy. Historically, clinical risk assessments provided prognostic information, but did not define treatment approach. We are now in an era where the heterogeneity of DLBCL is defined genetically and molecularly, and rational subset-specific therapeutic targets are guiding clinical trials. Primary mediastinal DLBCL is a unique clinicopathologic entity, and alternatives to R-CHOP may confer superior outcome. Rearrangement of the myc oncogene occurs in ∼10% of patients with DLBCL, and confers a very poor prognosis with standard R-CHOP, particularly when there is concomitant rearrangement of bcl-2, a condition referred to as “double-hit” DLBCL. A larger subset of DLBCL demonstrates overexpression of both myc and bcl-2 by immunohistochemistry. Cell of origin, determined by gene expression analysis, immunohistochemistry algorithms, or a novel Lymph2Cx platform, provides prognostic information, and guides therapeutic decisions in both relapsed and de novo disease. This article will define specific subsets of DLBCL and provide subtype-specific treatment options, including novel approaches under investigation. Understanding these key features of the pathology report, and limitations of these assays defining subsets of DLBCL, allows for an evolving precision medicine approach to this disease.
Learning Objectives
Define double-hit and double-protein DLBCL, and list optimal treatment strategies for these populations of patients
Explain how cell-of-origin genotype affects DLBCL treatment options in the de novo and relapsed settings
Provide features in the pathology report that suggest primary mediastinal DLBCL, and explain how that information is translated to therapeutic decisions
Diffuse large B-cell lymphoma (DLBCL) is the most commonly occurring lymphoma in the United States, and significant improvements in overall survival for a substantial subset of patients have resulted from the incorporation of rituximab into the CHOP chemotherapy regimen.1 Clinical prognostic scoring systems may be used to determine prognosis of patients newly diagnosed with DLBCL, and these remain robust in the rituximab era. For example, the NCCN-IPI discriminates between low- and high-risk subgroups (5-year overall survival: 96% vs 33%) in two large retrospective datasets.2 These clinical indices, although prognostic, have not impacted care of patients, as R-CHOP has remained the backbone of initial therapy for all risk groups, with consideration of consolidative high dose therapy and autologous stem cell transplantation for only the highest risk, young patients.3
Clinical prognostic features are at least in part surrogates for differential biology within DLBCL. Gene expression studies have indicated that DLBCL is a heterogeneous disease entity, as cell-of-origin studies reveal at least three distinct subtypes: primary mediastinal, activated B cell, and germinal center B-cell types.4 These subtypes also predict for outcomes at diagnosis even when adjusted for clinical features, with inferior outcomes observed with activated B-cell type disease.5 Alternative strategies of organizing gene expression data emphasize this heterogeneity of DLBCL, with subsets characterized by signatures of host response, oxidative phosphorylation, and B-cell receptor pathway elements.6
With this appreciation of biological heterogeneity, significant progress has been made toward the development of subset-specific therapies. With this progress, the pathology report needs to provide more information than a simple bottom line diagnosis of DLBCL. This review will define the key information which should be contained within the pathology report, and how this information should be used to define a precision approach to curative therapy of DLBCL.
Differential diagnosis and treatment approaches for histologically-defined subgroups
The initial biopsy of DLBCL needs to be adequate to make a definitive diagnosis. Fine-needle aspiration is inadequate to render a diagnosis of DLBCL,7 as other entities such as follicular lymphoma grade 3 have identical cytological features. Generally speaking, the accuracy of diagnosing DLBCL in community practice is reasonably high. In a study conducted within the NCCN lymphoma database, concordance between community and referral pathologists was highest for DLBCL (95%).8 Nevertheless, it is important to confirm that indeed the pathology report is specifically diagnostic for DLBCL, and not simply describing a large-cell lymphoma.
Follicular lymphoma grade 3 may mimic DLBCL, particularly on core needle biopsy where there is inadequate tissue to appreciate a nodular architecture. Treatment for this entity may be different from DLBCL, particularly with consideration of the use of maintenance rituximab,9 and various novel approaches particularly in the relapsed setting.
B-cell lymphoma, unclassified, with features intermediate between DLBCL and Burkitt lymphoma has been used to describe biopsies that contain features of, but are not diagnostic of Burkitt lymphoma. This entity is one of the grey zone lymphomas, and often clinically approached as DLBCL, and frequently has double-hit genetic features, as detailed below.
Burkitt lymphoma is essential to differentiate from DLBCL, as outcomes of Burkitt lymphoma are excellent when treated appropriately, and R-CHOP is insufficient therapy for Burkitt lymphoma.10
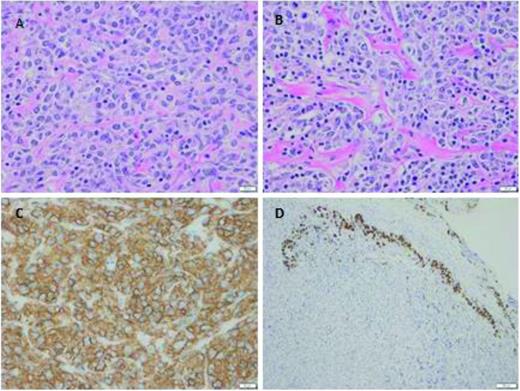
Primary mediastinal large B-cell lymphoma is a subtype of DLBCL which often presents in young women (median age 30-35) with a bulky mediastinal mass deriving from thymic tissue. Gene expression studies have revealed that primary mediastinal large B-cell lymphoma is indeed a unique disease, which has low levels of expression of multiple components of the B-cell receptor signaling cascade, a profile resembling that of classical Hodgkin lymphoma. Several other highly expressed genes are similar between Hodgkin lymphoma and primary mediastinal lymphoma, suggesting a molecular link between these two entities.11 Several additional unique features within the pathology report are present to identify primary mediastinal DLBCL. Fibrosis and the presence of so-called clear cells are distinctive morphologic features of primary mediastinal large B-cell lymphoma as indicated in Figure 1.12 Tumor necrosis factor-alpha-inducible protein-2 (TNFAIP2) appears to be a novel immunohistochemistry marker with high sensitivity and specificity to differentiate primary mediastinal DLBCL from other DLBCLs.13
Primary mediastinal large B-cell lymphoma (photos courtesy of Andrew Evans, Department of Pathology, University of Rochester, Rochester, NY). The tumor was a 5.2 cm isolated anterior mediastinal mass from a 23 year old female. (A-B) Tumor cells are medium-to-large in size, with round-to-irregular nuclei, open chromatin, and abundant pale cytoplasm. As commonly seen, tumor cells are infiltrating through a background of compartmentalizing alveolar or “packeted” fibrosis. (C) Membranous CD20 is abundantly and strongly expressed. (D) Remnant thymic epithelium (positive for cytokeratin) is commonly seen at the tumor edge infiltrated by malignant cells.
Primary mediastinal large B-cell lymphoma (photos courtesy of Andrew Evans, Department of Pathology, University of Rochester, Rochester, NY). The tumor was a 5.2 cm isolated anterior mediastinal mass from a 23 year old female. (A-B) Tumor cells are medium-to-large in size, with round-to-irregular nuclei, open chromatin, and abundant pale cytoplasm. As commonly seen, tumor cells are infiltrating through a background of compartmentalizing alveolar or “packeted” fibrosis. (C) Membranous CD20 is abundantly and strongly expressed. (D) Remnant thymic epithelium (positive for cytokeratin) is commonly seen at the tumor edge infiltrated by malignant cells.
It is essential to recognize this clinicopathologic entity, as the treatment of primary mediastinal large B-cell lymphoma may differ from other DLBCLs.14 A detailed discussion on treatment of this entity is beyond the scope of this article and has been recently reviewed previously.15 Although R-CHOP with consolidative radiation provides excellent outcomes in the majority of cases, single arm retrospective studies suggest that more aggressive chemotherapy induction options may provide improved progression-free survival. For example, a population registry series from British Columbia suggests that dose-intensified chemotherapy with MACOPB or VACOPB demonstrated a trend to superior outcome over CHOP-type chemotherapy; however very few patients received rituximab.16 More recently, the group from the National Cancer Institute in collaboration with Stanford has published outstanding results using the dose-adjusted (DA) R-EPOCH regimen without planned radiotherapy in patients with primary mediastinal DLBCL. After follow-up ranging from 10 months to 14 years, 96% of patients who received DA-EPOCH-R alone were in complete remission. There were no long-term cardiac or pulmonary toxicities reported on this trial.17 Based on these results of a single arm, nonrandomized trial, DA-R-EPOCH may be considered a standard option for many patients as it avoids radiotherapy in the vast majority of patients, which is often desirable given the epidemiology of the disease (young women), where secondary breast cancers from radiation therapy have emerged as a major cause of late morbidity after curative therapy.
Defining double-hit/double-protein DLBCL, and treatment approaches for this subgroup
Approximately 10% of patients with DLBCL have a translocation involving the myc locus on chromosome 8. Over the past 5 years, several groups have correlated the presence of myc translocation with poor outcome in DLBCL.18-21 There are two major ways to assess myc status. Historically, FISH has been used to determine the presence of a myc rearrangement, using break-apart probes at chromosome 8 myc locus. FISH is expensive, time-consuming, and may not be available for rapid routine use in all laboratories. Three years ago, the first experiences using immunohistochemical approach to assess MYC protein expression in formalin-fixed paraffin-embedded tissue were described, and this has been rapidly translated into practice in many academic centers across the United States.22
Cases with a myc breakpoint and bcl-2 rearrangement (FISH-defined) are the most common of the double-hit lymphomas defined as a myc abnormality with another abnormality.23 In a recently published comprehensive analysis of the Mitelman database, 62% of double-hit lymphomas involve bcl-2; 18% involved bcl-6, the remaining cases were triple hit lymphomas involving all 3 abnormalities.24 When interpreting the literature in myc positive lymphomas, it is critically important to understand the frequency of additional abnormalities, particularly bcl-225 , given the impact on outcome. These double-hit lymphomas generally are of germinal center cell-of-origin.
More recently, a larger group of patients with DLBCL with increased expression of MYC and BCL-2 measured by immunohistochemistry techniques referenced above has been described, referred to in this article as double-protein lymphoma. This group includes double-hit cases, but has additional cases with alternative mechanisms leading to overexpression of MYC and BCL-2. Most double-protein lymphomas that are not double-hit are of activated B-cell origin. Several groups have validated the double-protein status as a viable biomarker in R-CHOP treated patients. A group from Denmark evaluated 193 cases of DLBCL uniformly treated with R-CHOP therapy, and found that 29% of these de novo DLBCL cases had high expression of MYC and BCL-2 on immunohistochemistry evaluation, with poor outcome.26 Johnson et al from British Columbia used a similar platform to evaluate prospective cases of DLBCL with immunohistochemical stains for BCL-2 and MYC.27 Importantly, increased MYC was only prognostic of outcome if increased BCL-2 was also present in this series, defining the double-protein group, and these results were also validated in an independent cohort after adjusting for clinical and molecular high-risk features. The double-hit group has inferior prognosis compared with the double-protein group, which has been confirmed by other groups as well.28
Subsequently, the R-CHOP consortium group performed a comprehensive gene expression analysis of 893 patients DLBCL treated with R-CHOP.29 Interestingly, there was no difference in gene expression signatures between the germinal center B-cell (GCB) and activated B-cell (ABC) subtypes in the absence of double-hit or double-protein status, suggesting that the poor prognosis of ABC subtype is largely driven by double-protein status, and that heterogeneous molecular pathways are responsible for myc deregulation.30 Although ABC status has remained prognostic despite MYC status in other datasets,31 based up these observations, one may conclude that MYC/BCL2 coexpression, rather than cell-of-origin classification, is the best predictor of prognosis in patients with DLBCL treated with R-CHOP because the majority of treatment failures after R-CHOP are in double-protein DLBCL.32
Although the prognoses of both double-hit lymphoma, defined either by FISH or double-protein lymphoma defined by immunohistochemistry, are clearly poor with R-CHOP, there are limited data evaluating alternative therapy, and no published prospective data focused on this patient population. In the clinic, when possible, it is important to assess double-hit status by FISH on all cases, as some double-hit cases are below the IHC thresholds for defining double-protein lymphoma.
As myc translocations occur in Burkitt lymphoma, which responds to more aggressive chemotherapy platforms incorporating high dose alklylating agent therapy, and CNS active drugs, several have advocated using Burkitt-type regimens for double-hit DLBCL. In a recently published series from Vancouver, outcomes in double-hit DLBCL using the CODOX-M IVAC Burkitt regimen were improved, but induction failures remained a problem, and limited the ability of patients to have a planned consolidative ASCT.33 A subset analysis of the SWOG 9704 study,3 which randomized patients with DLBCL to either 8 cycles of R-CHOP or 6 cycles of R-CHOP followed by high-dose therapy and autologous stem cell transplant (AS CT) demonstrated that 14% were positive for myc, and these cases were morphologically and phenotypically heterogeneous and were associated with poor progression-free and overall survival in multivariate analysis.34 We have subsequently identified a subset of these patients with double-hit histology. Early progression and refractory disease limited the efficacy of ASCT for patients with double-hit DLBCL.
In the aforementioned Vancouver and Denmark series, the median age of patients with double-hit DLBCL by IHC exceeded 65 years, making dose escalation a challenge for the majority of patients with this high risk feature. The United States Intergroup has evaluated DA R-EPOCH therapy, which historically has demonstrated excellent activity in GCB DLBCL, for myc driven DLBCL, including patients with double-hit DLBCL, in a pilot phase 2 trial. Preliminary results of the myc-positive DLBCL group suggest a higher overall response rate with DA R-EPOCH than was observed in historic studies with R-CHOP, but the frequency of patients with true double-hit disease in this study was low, and follow-up to date is short, limiting definitive conclusions.35
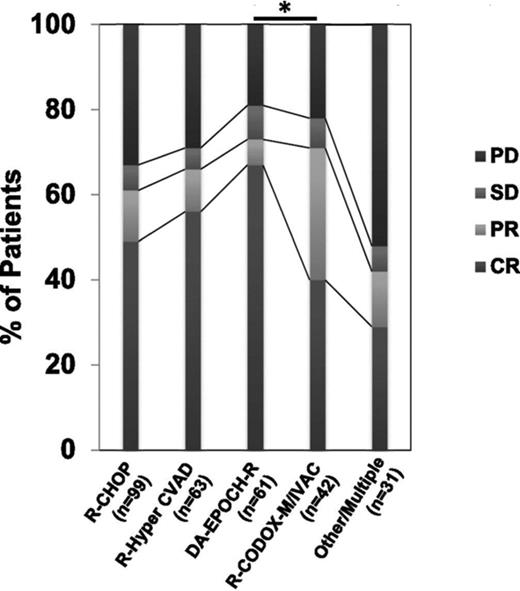
Recently, a group of investigators pooled data for a retrospective analysis addressing the impact of induction regimen and stem cell transplantation on outcomes in double-hit DLBCL.36 Intensive induction, including DA R-EPOCH and R-HyperCVAD/methotrexate/cytarabine was associated with higher response rates (Figure 2) and improved progression-free survival, and with adjustment for clinical risk factors, intensive induction appeared to be associated with improved overall survival. ASCT consolidation after various induction regimens did not appear to impact overall survival in this experience.36
Response rates of various regimens in a retrospective multicenter series of double-hit DLBCL (adapted from Petrich et al with permission36 (Fig 1)).
Similarly, the MD Anderson group has recently published a retrospective experience of 129 patients with double-hit lymphoma treated with a variety of regimens.37 Patients receiving DA-R-EPOCH therapy had superior outcomes compared with standard R-CHOP and other approaches. The cumulative incidence of CNS involvement was 13% at 2 years, emphasizing the poor prognosis of this subgroup of patients.
In conclusion, patients with double-hit lymphoma have poor outcome with R-CHOP and need alternative therapy. Outside of a clinical trial, there is evidence that more aggressive regimens, including the DA R-EPOCH regimen may confer improved outcomes. The larger group of patients with double-protein lymphoma (MYC and BCL-2 or BCL-6 positivity by immunohistochemistry) should be considered for clinical trials of novel therapeutic strategies. The United States National Clinical Trials Network has identified double-protein lymphoma, (including double-hit lymphoma), as the highest priority in DLBCL, and is currently developing an intergroup randomized trial in this subset of DLBCL.
Identifying cell-of-origin and treatment implications
As previously mentioned, gene-expression profiling has revealed two unique cell-of-origin subsets of DLBCL, including activated B-cell type and germinal center B-cell type. There is a smaller group deemed “intermediate”, which shares characteristics of both types. Establishing cell-of-origin at diagnosis of DLBCL is important for 2 reasons: first, it provides prognostic information, even in the R-CHOP era, as ABC subtype has inferior prognosis compared with GCB.5 More importantly, as detailed below, there are unique treatment strategies in the relapsed setting, and potentially the induction setting, for these specific subtypes of DLBCL.
Various immunohistochemical algorithms have been developed to replicate these microarray results in patients with DLBCL, as gene expression profiling requires fresh tissue, and is not routinely available in practice. In a study of 262 patients with newly diagnosed DLBCL treated with R-CHOP therapy, comparison of these algorithms confirmed ability to divide patients into two groups with significantly different overall and event-free survivals, but with different hazard ratios.38 The reproducibility between these algorithms is variable, and on the order of 80% concordance with gene expression profiling confirmatory studies. A major limitation of these algorithms is that patients with intermediate gene expression signatures are forced into either GCB or nongerminal center subtype as these are dichotomous algorithms. However, these algorithms offer ease of use and can be easily performed in any immunohistochemical laboratory with a rapid turnaround time. The Hans et al39 algorithm has gained the most clinical acceptance, and are most frequently used in practice.
Moving forward, a novel assay Lymph2Cx, using nanostring technology, has significant promise to overcome the limitations of these immunohistochemical algorithms. This assay uses a digital gene expression-based test for cell of origin assignment that can be performed in formalin-fixed paraffin-embedded tissue. A 20 gene assay has been demonstrated to be highly concordant with conventional gene expression profiling, and reproducible among laboratories.40 Additionally, the assay offers a rapid turnaround time. Confirmatory validation studies using this assay are underway, and it is likely this assay, when widely commercially available, will replace immunohistochemistry algorithms for cell of origin assignment in DLBCL.
For patients with GCB DLBCL, the prognosis is generally favorable with R-CHOP treatment, particularly when double-hit lymphomas are separated from this group. At present, these patients should be treated with R-CHOP therapy. In the relapsed setting, there is evidence that R-DHAP salvage chemotherapy is superior to R-ICE based upon a subset analysis of the CORAL study in patients with GCB DLBCL.41
For patients with ABC DLBCL, the prognosis is inferior with R-CHOP therapy, therefore there has been significant investigation of novel chemotherapy platforms and rational targeted therapy for this subset of patients. The LySA group conducted a randomized trial demonstrating superiority of the aggressive ACVBP regimen over R-CHOP in younger patients with low-risk DLBCL; a correlative study demonstrated equivalent outcomes in GCB and nongerminal center subsets indicating a potential benefit of the aggressive regimen in the nongerminal center subgroup.42 Lack of vindesine availability in the United States limits the ability to confirm these results prospectively, but they serve as an example on how, in the future, chemoimmunotherapy regimens for DLBCL may differ depending upon cell of origin.
Several novel agents have demonstrated promise in the nongerminal center subgroup of patients with DLBCL. For example, lenalidomide is a immunomodulatory drug with pleotropic mechanisms of action.43 An early study of single agent lenalidomide in relapsed/refractory DLBCL revealed a response rate of 28% in unselected patients.44 Subsequent analysis using the Hans immunohistochemistry algorithm demonstrated a higher response rate with longer durability in patients with ABC subtype DLBCL.45 Based upon this experience, a randomized trial in relapsed/refractory DLBCL comparing lenalidomide to investigators choice single-agent chemotherapy was performed. Preliminary results confirmed activity of lenalidomide in this setting, and suggest that patients with nongerminal center DLBCL have superior outcomes, particularly when cell-of-origin is defined by gene expression profiling (to determine true ABC subtype) rather than by an immunohistochemistry algorithm.46
Two recently published studies have combined lenalidomide with R-CHOP.47,48 Both of these studies show equivalent outcome of patients with GCB and nongerminal center genotypes as defined by immunohistochemistry, suggesting a benefit in the nongerminal center subset, as that group normally has inferior outcome. These promising results have led to an intergroup NCTN randomized trial led by ECOG comparing R-CHOP to R-CHOP with lenalidomide, which is powered to demonstrate benefit in the nongerminal center subgroup of patients, defined at diagnosis by immunohistochemistry.
Bortezomib is a proteasome inhibitor, which has effects on NFkappa-B signaling, which is enhanced in ABC-type DLBCL. In a proof of principle study, bortezomib combined with DA R-EPOCH had superior outcomes in ABC-type relapsed DLBCL compared with GCB-type.49 Similar to the lenalidomide situation, a phase 2 study has demonstrated safety of R-CHOP with bortezomib combination in de novo DLBCL, and suggested that nongerminal center subtype defined by immunohistochemistry had similar outcome as GCB subtype.50 Two large randomized trials comparing R-CHOP with R-CHOP and bortezomib are ongoing powered to show improvement in outcome in the nongerminal subset of patients.
Finally, ibrutinib is an inhibitor of Bruton's tyrosine kinase, which is a proximal component of the B-cell receptor pathway. The B-cell receptor pathway is preferentially active in ABC-subtype DLBCL, however GCB lines also may rely on this pathway.6 Preliminary results of a single-agent ibrutinib study in relapsed/refractory DLBCL demonstrated that almost all responses (generally of short duration) occurred in ABC-type disease. Again, based upon these data, a trial evaluating R-CHOP with ibrutinib in newly diagnosed DLBCL was performed, and demonstrated excellent outcome in the small number of patients demonstrated to have nongerminal center DLBCL.51 An ongoing randomized trial is comparing R-CHOP to R-CHOP with ibrutinib in patients nongerminal center DLBCL defined by immunohistochemistry.
Other novel approaches and future directions
ABT-199, a specific and safe bcl-2 inhibitor is currently in clinical trials combined with chemotherapy in DLBCL.52 This agent has been shown to have in vivo efficacy against aggressive Myc-driven mouse lymphomas.53 Additionally, bromodomains are conserved protein regions that recognize specific histone modifications. Bromodomain inhibition reduces tumor growth in lymphomas, largely through the disruption of transcriptional networks driven by oncogenic MYC.54 The small molecule JQ1 suppresses c-myc expression and significantly suppressed growth of DLBCL cells engrafted in a murine model.55 These two agents may have particular promise in double-hit DLBCL. There is great enthusiasm for checkpoint blockade in lymphoid malignancies, including relapsed/refractory DLBCL in the setting of autologous stem cell transplantation,56 and one might speculate that Host-response cluster of DLBCL may preferentially respond to this treatment.
DLBCL may be positive for Epstein-Barr virus (EBV) in the setting of immunosuppression, and this occurs more frequently in elderly patients. Autologous T cells directed to various EBV antigens can induce durable complete responses without significant toxicity in EBV-expressing DLBCL.57 To the degree that future research will provide guidance on optimal use of this therapy, it may be important to evaluate DLBCL for EBV-associated antigens.58
Antibodies and antibody drug conjugates are under development for a variety of antigens expressed in a subset of DLBCL. Brentuximab vedotin (anti-CD30 ADC) has demonstrated activity in DLBCL with a response rate of 40% in one study, without significant correlation of CD30 expression.59 Other targetable cell-surface molecules include CD19, CD22, CD70, and CD79b. Therefore, determining the expression of these antigens may guide DLBCL treatment options in the future.
Finally, whole-genome sequencing has revealed a multiple opportunities for future therapeutic intervention. Most mutations in DLBCL occur at low frequency and in various combinations, making drug development challenging.60 However, there is significant promise in this personalized approach, and as one example there is an ongoing trial of a novel TLR-antagonist in patients with DLBCL harboring the MYD88 L265P mutation. To what degree specific mutations will ultimately drive treatment decisions in DLBCL is not yet clear, but as technology evolves to make mutation testing able to be performed in real-time as part of routine diagnostics, it is likely that this will become a key component of the pathology report.
Conclusions: a precision approach to DLBCL
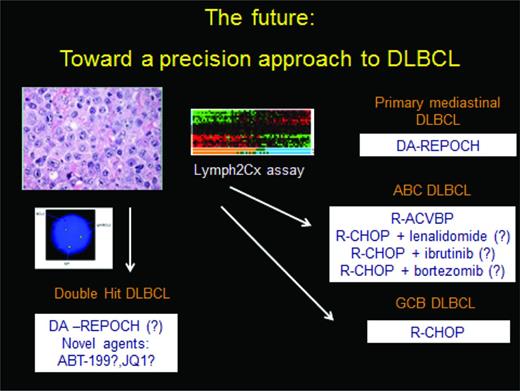
Treatment decisions in DLBCL depend on both patient and tumor factors. The pathology report needs to provide critical information which guides therapy, as no longer is “all” DLBCL treated uniformly with R-CHOP. A summary diagnostic algorithm is outlined in Table 1, and a treatment approach is outlined in Figure 3. Primary mediastinal DLBCL is a unique clinicopathologic entity that may have improved outcomes with more aggressive regimens than R-CHOP. Double-hit biology represents the greatest unmet need in DLBCL. It is clear that R-CHOP needs to be replaced for this group of patients; and prospective trials are ongoing to define appropriate treatment. DA-R-EPOCH is one appealing chemoimmunotherapy platform on which to build for this subset of patients. Cell-of-origin, defined more than a decade ago, is now ready to be used in treatment decisions, as is evidenced by numerous ongoing clinical trials segregating patients based on rational therapeutics given unique biology. The optimal diagnostic platform for this analysis may move beyond immunohistochemistry to the Lymph2Cx platform soon. Whether whole-genome sequencing or alternative gene expression signatures evolve to become standard remains to be seen, but it is clear that we have entered the age of precision medicine for patients with DLBCL.
A precision approach to DLBCL. Double-hit status should be assessed on all patients and treated accordingly on a clinical trial or using the DA R-EPOCH platform. Assessment of cell of origin, using either immunohistochemistry, gene expression profiling, or in the future the Lymph2Cx assay then should allow patients to be separated into ABC and GCB subtype; there are several phase 3 trials evaluating unique approaches to the former. R-CHOP is adequate therapy for the GCB subtype, which is not double-hit. Primary mediastinal DLBCL is a unique clinicopathologic entity with specific treatment recommendations.
A precision approach to DLBCL. Double-hit status should be assessed on all patients and treated accordingly on a clinical trial or using the DA R-EPOCH platform. Assessment of cell of origin, using either immunohistochemistry, gene expression profiling, or in the future the Lymph2Cx assay then should allow patients to be separated into ABC and GCB subtype; there are several phase 3 trials evaluating unique approaches to the former. R-CHOP is adequate therapy for the GCB subtype, which is not double-hit. Primary mediastinal DLBCL is a unique clinicopathologic entity with specific treatment recommendations.
Correspondence
Jonathan W. Friedberg, Samuel Durand Professor of Medicine, University of Rochester, 601 Elmwood Ave, Box 704, Rochester, NY 14642; Phone: (585)275-4911, Fax: (585)276-2743; e-mail: jonathan_friedberg@urmc.rochester.edu.
References
Competing Interests
Conflict-of-interest disclosure: The author declares no competing financial interests.
Author notes
Off-label drug use: New treatments for diffuse large B-cell lymphoma.