Abstract
Co-incident venous thromboembolism and thrombocytopenia are frequent in patients with active malignancies. The optimal approach for anticoagulation in patients with cancer and thrombocytopenia is not established. Different strategies are often utilized including dose-reduced anticoagulation dictated by degree of thrombocytopenia or transfusing platelets in order to facilitate therapeutic anticoagulation. This minireview provides an overview of the data and we outline our approach toward anticoagulation in patients with venous thromboembolism and thrombocytopenia in the setting of cancer.
Learning Objectives
Understand factors to consider when determining thrombosis and bleeding risk in patients with cancer who have thrombosis and concomitant thrombocytopenia
Compare strategies of anticoagulation (full dose vs modified dose) in patients with active cancer-associated thrombosis and concomitant thrombocytopenia
CLINICAL CASE
A 75-year-old man with acute myeloid leukemia being treated with decitabine and venetoclax presents to the emergency room with left lower extremity edema and pain. Vascular ultrasound shows extensive acute noncompressible thrombus involving the left common femoral vein. Platelet count on admission is 28 000/µL and believed to be a combination of marrow suppression from underlying disease and treatment toxicity. How would you approach anticoagulation in this patient?
Introduction
Thrombosis and thrombocytopenia are both common complications in patients with active malignancy. Thrombocytopenia may be secondary to the primary malignancy itself (especially in hematologic cancers) or as a consequence of cancer-directed therapies. Thrombocytopenia is not protective for thrombosis but significantly increases the risk of bleeding, especially with anticoagulation. Data are limited as patients with thrombocytopenia are often excluded from randomized clinical trials evaluating anticoagulation efficacy. Thus, clinicians frequently face a number of challenging treatment decisions regarding whether to anticoagulate, at what dose, and for how long.
Scope of the problem
Data describing frequency of thrombocytopenia and thrombosis are limited. We recently conducted a retrospective cohort analysis at Beth Israel Deaconess Medical Center and identified 3635 unique cancer-associated thrombosis (CAT) events over 10 years. Approximately 1 in 4 episodes of venous thromboembolism (VTE) occurred in the setting of thrombocytopenia, and approximately 1 in 10 had platelet counts less than 50 000/µL. The prevalence was much higher among the group with hematologic malignancies.1 It is clear that thrombocytopenia is a common complication in patients with CAT.
Balancing risk and benefit of anticoagulation
A systematic review in 2018 that evaluated outcomes in patients with CAT and thrombocytopenia identified 2 relatively small retrospective studies with a total of 121 patients, of whom 27%, regardless of treatment strategy, experienced recurrent VTE and 15% experienced a major bleeding episode.2
In balancing decisions around anticoagulation management in CAT with concomitant thrombocytopenia, one must balance the risks of thrombosis and its progression with the risk of bleeding. Patients with cancer are at particularly increased risk of clinically significant hemorrhage, which is modulated by several factors. In the COMMAND VTE registry, 527 patients with CAT were analyzed, and cumulative incidence of major bleeding was 2.7% at 3 months. In multivariable analysis, active cancer, history of major bleeding, anemia, thrombocytopenia, and age ≥75 years were independently associated with a risk for major bleeding.3 With regard to thrombocytopenia, the depth and duration of thrombocytopenia can vary considerably in patients with cancer (depending on etiology) and are important practical considerations when making anticoagulation decisions; more severe and prolonged thrombocytopenia likely increases the risk of hemorrhage.
Decision-making must also account for the risk of progression and recurrence of thrombosis. Factors such as the location and extent of thrombosis, as well as acuity of the VTE, are important considerations. In a prospective cohort study of patients with acute thrombosis who received anticoagulation, risk of recurrence was 3-fold in patients with active cancer compared with those without cancer.4 Thus, anticoagulation is usually recommended for most patients with active malignancy and thrombosis. Specifically in patients with active cancer, the risk of CAT is considered especially high with bulky or metastatic tumors, certain tumor types (eg, pancreatic, gastric cancer, ovarian cancer, brain tumors, lymphoma), and specific cancer-directed therapies (eg, asparaginase, immunomodulatory drugs in myeloma or major cancer surgery). The type of index thrombotic event can influence the risk of recurrence of thrombosis. The risk of recurrence or progression on therapeutic anticoagulation (largely derived in nonthrombocytopenic cohorts) ranges from 10% to 15% for proximal lower extremity thrombosis and segmental pulmonary embolism to 4% to 8% for thrombotic events considered lower risk, such as catheter-associated thrombosis, distal lower extremity thrombosis, or subsegmental pulmonary embolism.5
Biomarkers and predictive models for recurrent thrombosis and bleeding in CAT are extremely limited. Post hoc analysis of patients with active cancer who received apixaban for thromboprophylaxis (AVERT study) showed that growth differentiation factor 15, initially validated in anticoagulated patients with atrial fibrillation, had predictive value for major bleeding.6
Anticoagulation strategies tailored to platelet counts
Although the correlation between thrombocytopenia and bleeding risk may not be linear, it is generally accepted that bleeding risk is higher in individuals with platelet counts below 50 000/µL. Thus, in patients with platelet counts ≥50 000/µL who require anticoagulation for VTE treatment, full-dose anticoagulation is generally appropriate, with the same bleeding considerations as nonthrombocytopenic populations. Patients with active malignancy may have cancer-specific risk factors that could modulate this risk-benefit balance and should be carefully considered. For patients with severe thrombocytopenia (platelets <50 000/µL) who require anticoagulation for acute thrombosis, 2 management strategies are often considered: (1) full-dose anticoagulation with higher target transfusion support or (2) dose-modified anticoagulation (either half-dose anticoagulation or prophylactic dose anticoagulation). Factors that influence anticoagulation strategies include thrombotic burden and risk of recurrence, the presence of bleeding risk factors (apart from thrombocytopenia), and severity or duration of thrombocytopenia. Data supporting the superiority of one treatment strategy over another are limited.
In 2017, a quality improvement initiative evaluated a strategy in which low-molecular-weight heparin (LMWH) was used at full therapeutic dosing for platelet counts above 50 000/µL, reduced to half-dose for those with platelet counts between 25 000 and 50 000/µL, and temporarily held for a platelet count below 25 000/µL. There were no observed instances of recurrent VTE or major bleeding among patients managed with this approach.7 In a recent prospective multicenter observational study (TROVE), among the 121 patients with cancer and acute VTE who were included, 62% received full-dose anticoagulation and 27% received modified-dose anticoagulation. The cumulative incidence of major hemorrhage was double in the group receiving full-dose anticoagulation compared with patients who received modified-dose anticoagulation (12.8% vs 6.6%) at 60 days. Moreover, the cumulative incidence of recurrent VTE at 60 days was 5.6% and 0% in the full-dose and modified-dose anticoagulation groups, respectively.8 Without evidence from a randomized trial data, there remains equipoise on the optimal anticoagulation strategy in this high-risk population. We note that the bulk of the evidence supporting the use of dose-modified LMWH in acute CAT with thrombocytopenia has been reported for the management of deep vein thrombosis (DVT) rather than pulmonary embolism (PE).
Strategies to increase platelet counts to support administration in patients with cancer and concomitant thrombocytopenia are limited. In most patients, the thrombocytopenia is related to the malignancy or its therapy. Platelet transfusions are often used to maintain platelet counts to “safer” levels, but this approach is often challenging from a resource utilization perspective, as well as practical aspects of alloimmunization and inability to reliably achieve target platelet goals. Thrombopoietin receptor agonists are effective agents in immune-mediated thrombocytopenia and are now used in patients with underlying liver disease to support invasive procedures as well as chemotherapy-induced thrombocytopenia to prevent dose reductions. However, experience in the setting of acute thrombosis is limited. The optimal threshold for platelets is also not clear; empirically, we usually target at least 25 000/µL but recognize that this often needs to be tailored to the individual patient, availability of platelet products, and individualized risk of bleeding or thrombosis. This is demonstrated in the prospective TROVE study, where in patients started on full-dose anticoagulation with platelet transfusion support, platelet transfusion thresholds ranged from 10 000 to 50 000/µL.8
Our recommendations for anticoagulation in the setting of acute VTE and thrombocytopenia in cancer are similar to those published by the International Society of Thrombosis and Hemostasis Subcommittee on Hemostasis and Malignancy.9 We provide additional recommendations favoring platelet transfusion with anticoagulation in the immediate post-VTE diagnosis in the setting of severe thrombocytopenia.
Special considerations
Choice of agent
Although direct oral anticoagulants have been shown to be effective and safe for the management of patients with CAT in large randomized studies, patients with significant thrombocytopenia were excluded from these studies. In the TROVE study, 3 of 16 patients receiving direct oral anticoagulants developed clinically relevant nonmajor bleeding events. Due to shorter half-life and ability to modify dosing along with minimal drug interactions, heparin (either low molecular weight or unfractionated) is the most commonly administered anticoagulant in the setting of CAT and thrombocytopenia.
Avoiding anticoagulation in select situations
For patients with low thrombotic burden (isolated distal DVT or subsegmental PE), reversible causes of thrombosis (thrombosis associated with central venous catheter with removal of the central catheter), or extremely high bleeding risk (particularly those with recent major bleeding or active bleeding), holding anticoagulation may be appropriate (Table 1). Decisions around anticoagulation strategy should incorporate patients' values and align with the goals of care delivered. Moreover, such patients should be closely monitored for progression of thrombosis or change in bleeding risk that may require reconsideration of anticoagulation.
Inferior vena cava filters
In certain scenarios, such as active high-risk bleeding with high clot burden, considerations for placement of an inferior vena cava filter may be necessary.10 The role of retrievable filters in patients with active malignancy should be considered limited, as patients with active malignancy may have higher rates of complications from use of a filter and have been shown to have lower rates of retrieval; thus, use of anticoagulation after the filter placement would be considered to prevent filter-associated complications.11
Recommendations
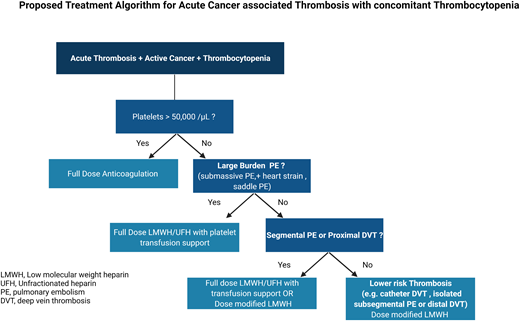
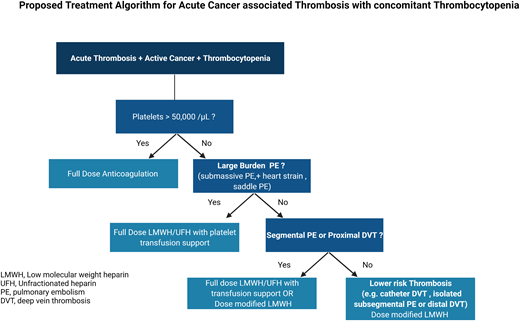
We recommend giving full therapeutic anticoagulation without platelet transfusion to patients with CAT and a platelet count of ≥50 000/µL. (Certainty of evidence, Very Low +)
For patients with acute CAT and severe thrombocytopenia (<50 000/µL) and large-burden PE (ie, submassive, evidence of heart strain, central pulmonary arteries), we suggest full-dose anticoagulation (LMWH or unfractionated heparin) with platelet transfusion support to maintain a platelet count of ≥40, 000 to 50 000/µL. (Certainty of evidence, Very Low +)
For patients with acute CAT and severe thrombocytopenia (<50 000/µL) and proximal lower DVT or segmental PE, we suggest either full-dose LMWH with higher platelet targets or dose-modified LMWH (as outlined below). For patients who are more than 30 days post acute VTE event, we favor dose-modified LMWH. (Certainty of evidence, Very Low +)
For patients with acute CAT and severe thrombocytopenia (<50 000/µL) and lower-risk DVT (ie, distal lower extremity, isolated subsegmental, or catheter related):
We suggest reducing the dose of LMWH to 50% of the therapeutic dose or using a prophylactic dose of LMWH in patients with a platelet count of 25 000 to 50 000/µL. (Certainty of evidence, Very Low +)
We suggest temporarily discontinuing anticoagulation in patients while the platelet count is <25 000/µL. We favor transfusing to a platelet threshold of 25 000/µL and lower- dose anticoagulation during the first 2 weeks of the thrombotic event. (Certainty of evidence, Very Low +)
We recommend resuming full-dose LMWH when the platelet count is >50 000/µL without transfusion support, in the absence of other contraindications. (Certainty of evidence, Very Low +)
For patients at high risk or active hemorrhage, observation without anticoagulation may be considered (with consideration for inferior vena caval filter placement). (Certainty of evidence, Very Low +)
Conflict-of-interest disclosure
Jeffrey I. Zwicker has received research funding from Incyte and Quercegen; consultancy services to Sanofi, CSL, and Parexel; and honoraria from/advisory board participation with Pfizer/Bristol Myers Squibb (BMS), Portola, and Daiichi.
Rushad Patell declares no competing financial interests.
Off-label drug use
Rushad Patell: nothing to disclose.
Jeffrey I. Zwicker: nothing to disclose.