Abstract
Hormonal contraceptive therapy (estrogens and/or progestogens) includes different formulations associated with varying venous thromboembolism (VTE) risks. The thrombogenicity of combined hormonal contraceptives (CHCs) is due at least in part to multiple changes in clotting factors and the vasculature and is dependent on both estrogen dose and type of progestin. Transdermal patch and vaginal ring users have similar or higher VTE risk as combined oral contraceptive users. Progestin-only agents have varying VTE risk. While depot medroxyprogesterone acetate appears to increase VTE risk, the levonorgestrel-based intrauterine system and low-dose progestin-only pills have no additional VTE risk. There are less data for the subdermal progestin-only implant. This article reviews contraceptive-related VTE risk by agent and by clinical scenario, including in patients with inherited thrombophilia, systemic lupus erythematosus with or without antiphospholipid antibodies or antiphospholipid syndrome, and sickle cell disease. Relevant clinical practice guidelines are reviewed. A multidisciplinary approach to counseling is needed for patient-focused decision-making.
Learning Objectives
Describe the pathophysiology and VTE risk of different hormonal contraceptive agents
Apply the available evidence to shared decision-making about hormonal contraceptives in patients with inherited or acquired VTE risk factors
CLINICAL CASE 1
An 18-year-old cisgender woman has been referred to review contraceptive options because a family member with heterozygous factor V Leiden (FVL) had an unprovoked venous thromboembolism (VTE).
CLINICAL CASE 2
A 25-year-old cisgender woman with sickle cell disease (SCD) has had an acute pain crisis requiring hospitalization, and she has noticed that her menstrual cycles are a common pain trigger. She wants to know what options are safe for hormonal therapy to reduce pain crises.
Background
Hormonal contraceptive therapy use is common, with 16% to 26% of individuals or an estimated 100 million women or more using these agents worldwide.1-3 Combined hormonal contraceptive (CHC) therapy includes estrogens and/or progestogens. Estrogens develop and maintain the female reproductive system and secondary sex characteristics, and progestogens induce a secretory endometrium to support gestation.4 Progestogens include physiologic progesterone and synthetic progestins. Most progestins developed for contraception are structurally related to testosterone.4 When used for contraception, progestins inhibit ovulation via negative feedback of the hypothalamic-pituitary-ovarian axis and have local cervical and uterine effects.4 Relating to contraception, estrogen helps to suppress gonadotropins, prevent follicle development, and reduce the irregular bleeding that progestin can cause.3,4
Different hormonal formulations exist and include either a combination of an estrogen and a progestin (oral contraceptives, transdermal patch, vaginal ring), or progestin-only agents (oral, injectable, intrauterine system [IUS], subdermal implant). Oral contraceptives usually include ethinyl estradiol (EE) with a different progestin, although newer estrogens exist (eg, 17-beta estradiol/nomestrol acetate, estrogen valerate/dienogest, sterols/drospirenone). Oral contraceptives are grouped by generation (ie, first, second, third, or fourth generation) according to progestin type. Drospirenone is related to spironolactone and has prominent antiandrogen and antimineralocorticoid properties.
A newer-generation oral contraceptive does not necessarily mean a lower VTE risk. For example, newer formulations contain lower EE doses (eg, 20-30 µg) that are associated with less thrombogenicity, but they may also have a more thrombogenic progestin type (eg, desogestrel, drosperinone).5
Thrombotic mechanisms
The thrombogenicity of CHCs is due, at least in part, to the combination of multiple changes seen in the clotting factor levels and the vasculature.3 CHCs increase procoagulant factors (prothrombin, factor VII, VIII, X, and fibrinogen) and decrease anticoagulant factors (protein S [PS] and antithrombin [AT]).3,6 An acquired activated protein C (APC) resistance develops, which is thought to be important to CHC thrombogenicity and is likely due to lower tissue factor pathway inhibitor and PS levels.6 Fibrinolytic system changes seen with CHC use include increased tissue plasminogen activator, reduced plasminogen activator inhibitor type 1, and decreased thrombin activatable fibrinolysis inhibitor and are of unknown clinical relevance.3,6 Once CHCs are stopped, most hemostatic changes resolve in 2 to 4 weeks.7
Compared to second-generation progestins, (eg, levonorgestrel [LNG]), later-generation progestins (eg, desogestrel, gestodene, drosperinone) have greater acquired APC resistance and other markers of hypercoagulability.3,5,8,9 Why does the type of progestin have an impact on thrombosis risk? One theory is that thrombogenicity is related to the total estrogenicity from the combined estrogen-progestin compound (dose of estrogen vs strength of progestin's antiestrogen effect, which varies among progestins).6,10 The sex-binding hormone globulin has been suggested as a surrogate for total estrogenicity and correlates with the level of acquired APC resistance among CHC users,6,11 although a causal relationship between the sex-binding hormone globulin and VTE has not been demonstrated.12
One mechanism of oral estrogen's hypercoagulability is “first-pass” hepatic metabolism, which leads to the increased hepatic synthesis of coagulation proteins.3 However, this is not the only mechanism because nonoral CHCs, such as the transdermal patch or vaginal ring, do not undergo first-pass metabolism or cause liver protein changes but do induce acquired APC resistance and elevated markers of hypercoagulability despite lower systemic estrogen levels.13-18 Thrombogenicity may, at least partially, relate to the third-generation progestins used in the transdermal patch and vaginal ring.3
There is no acquired APC resistance with the LNG IUS, the low-dose progestin-only pill (POP), or the progestin subdermal implant.10,19,20 With the implant, sustained increased factor VII levels have been seen in some but not all studies, and an unexpected opposite effect was seen with increased anticoagulant factors and decreased thrombin AT complex levels (a surrogate for less thrombin generation).19,21-25 Similarly, the POPs have favorable effects on blood coagulation and may have a net antithrombotic effect.3 Depot medroxyprogesterone acetate (DMPA) has few coagulation changes but has other effects on endothelial function.26-28
Combined hormonal therapy and VTE risk: general population
Oral contraceptives
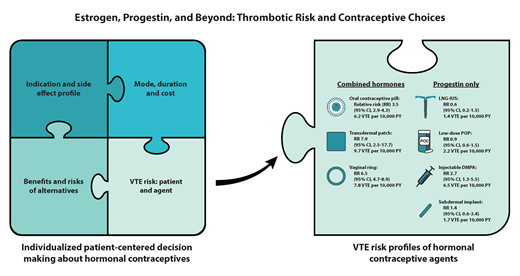
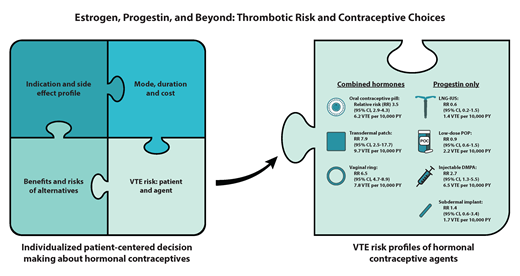
Most epidemiological studies report relative and not absolute VTE risk estimates with hormone exposure, which limits data interpretation and decision-making for clinicians, patients, and those involved in clinical practice guidelines. As a reference, for females aged 15 to 49 years without hormone exposure, the VTE incidence rate is 1.9 to 3.7 per 10 000 person-years (PYs).29,30 COCs have a relative risk (RR) of 3.5 (95% CI, 2.9-4.3),31,32 but this increase in VTE risk (baseline VTE risk multiplied by 3.5) is still considered low (∼0.05-0.15% PY) for most individuals. See Table 1 for differences by agent. The risk of VTE is highest in the first year of initiating COCs; however, the risk remains long-term (>5 years).33,34
Transdermal patch
In a database study, the adjusted RR for the transdermal patch was 7.9 (95% CI, 3.5-17.7) relative to nonusers and 2.5 (95% CI, 1.1-5.6) relative to the LNG COC.35 This corresponds to a VTE incidence of 9.7 per 10 000 exposure years, which is similar to third-generation COCs (Table 1).29,35 In a systematic review that identified 5 additional studies, VTE risk was either the same or higher than LNG or norgestimate-based COCs.36
Vaginal ring
In the same database study, the adjusted RR was 6.5 (95% CI, 4.7-8.9) compared to nonusers and 2.0 (95% CI, 1.4-2.9) compared to the LNG oral contraceptive pill (Table 1).35 In the systematic review, 2 additional studies evaluated the vaginal ring and found it had a similar risk to the LNG oral contraceptive pill.36
Progestin-only agents and VTE risk: general population
LNG intrauterine system
Low-dose POPs
Users of low-dose POPs have no increased VTE risk compared to nonusers (RR 0.9; 95% CI, 0.57-1.45).37 This risk estimate did not include users of the drosperinone POP. Because the drosperinone POP is newer, less data and clinical experience information are available, and the progestin type is one of the more thrombogenic progestogens. In a literature review, there were no VTE events out of more than 20 000 cycle exposures, including in individuals with VTE risk factors.39 One disadvantage of POPs is that the medication must be taken consistently every day, and there is decreased effectiveness if a dose is late or missed, although the drosperinone POP reports a longer missed pill safety window of 24 hours.39 Progestin-only oral medications for treatment of heavy menstrual bleeding carry a higher thrombotic risk of 5-6-fold,40,41 which likely relates to higher doses used and possibly the population under study (eg, older age, possible inflammation present).
Depot medroxyprogesterone acetate
Among 3 studies, 2 identified an increase in VTE risk (RR 2.67; 95% CI, 1.29-5.53).37,38 This risk may be explained by the higher peak hormone plasma concentrations, compared to the LNG IUS or progestin-only contraceptives.37 One advantage is adherence, and disadvantages include reduced bone mineral density and possible metabolic changes.
Implants
Less VTE data are available for the implants. In a database study evaluating the etonogestrel implant, the adjusted RR was 1.4 (95% CI, 0.6-3.4) compared to nonusers, with 5 confirmed VTE events over 29 497 PY, which corresponds to an incidence rate of 1.7 per 10 000 PY.35 In a case-control study that grouped subdermal implants into a “medium-dose” category with certain POPs, the odds ratio (OR) of VTE was 0.9 (95% CI, 0.5-1.6) compared to nonusers.34 Last, in a database study of 8369 individuals with the etonogestrel implant inserted immediately postpartum, the adjusted OR of VTE requiring readmission within 30 days was 1.81 (95% CI, 0.44-7.45) compared to nonusers.42 One advantage is adherence, and disadvantages include procedure risks and more variable vaginal bleeding.
It should be noted that variable vaginal bleeding can be a disadvantage of all progestin-only methods, particularly for the first few cycles after initiation. Rates of amenorrhea are higher with LNG IUS and DMPA.
Contraceptive hormonal therapy considerations for patients at VTE risk
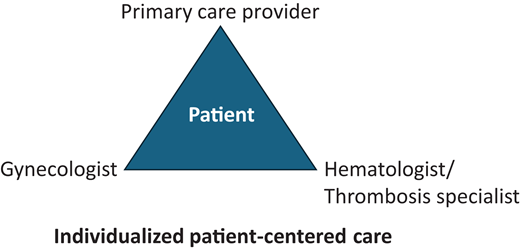
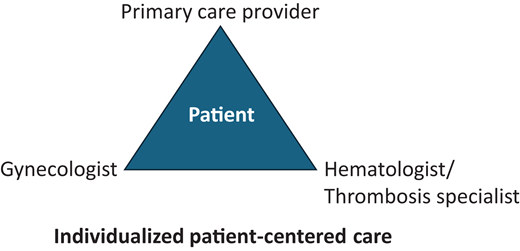
Even if a patient may be at increased risk of VTE, the contraceptive choice remains a patient-centered decision based on factors that include indication and side effect profile, cost, mode of administration, duration, VTE risk based on both the patient and contraceptive agent, and the risks and benefits of alternative options. While the focus of this article is on VTE risk, we must acknowledge the important benefits of CHCs that include avoiding unwanted pregnancy; menstrual suppression, including treatment of heavy menstrual bleeding and other gynecological disorders; and other indications that may outweigh the potential risks. No one health care provider may have all this information, so a multidisciplinary decision is often made between the patient and their primary care provider, gynecologist, and/or hematologist/thrombosis specialist (Figure 1).
Table 2 contains a summary of guideline recommendations for contraceptive therapy in individuals with underlying conditions associated with increased VTE risk. Below are VTE risk information and the authors' approach to a number of these conditions. Additional VTE risk factors, such as increased age, smoking, and elevated body mass index (BMI), may also influence decision-making.43
When the added VTE risk exceeds tolerable thresholds, a progestin-only IUS or POP may be suggested. In a systematic review that included 8 studies of women with medical conditions (hypertension, smoking, thrombophilia, or prior VTE), there was no additional VTE risk among those receiving progestin-only contraception, except for DMPA in 2 studies.38 Similarly, in an older cohort study evaluating the oral progestin chlormadinone acetate in individuals with older age, elevated BMI, inherited thrombophilia, or antiphospholipid antibodies (aPLs) there was no increase in VTE risk compared to a matched cohort of nonusers after adjusting for age, BMI, and thrombophilia (RR 0.8; 95% CI, 0.2-3.9).44 For those with VTE risk factors, there may be an added VTE risk with DMPA,38 but likely not with the subdermal implant.34 These statements, however, are based on little data.
Inherited thrombophilia and family history of VTE
Among CHC users, having an inherited thrombophilia increases VTE risk to varying degrees based on the thrombophilia type. In a systematic review, AT, PC, or PS deficiency was associated with an absolute VTE risk of 4.3 to 4.6 per 100 pill-years.45 In patients with heterozygous FVL or prothrombin gene mutation (PGM), the absolute VTE risk was lower, at 0.49 to 2.0 per 100 pill-years.45 Despite this difference in reported VTE risk, the US Medical Criteria for Contraceptive Use treats all thrombophilia types the same and states that CHCs are an unacceptable health risk and should not be used (Table 2).46 The authors of this article disagree and consider mild thrombophilia more of a relative and not absolute contraindication, where there is room for a patient-centered decision with all impacting factors considered, including management of menstrual disorders or prevention of unwanted pregnancy, especially as pregnancy carries a higher thrombotic risk.47
The 2023 American Society of Hematology (ASH) thrombophilia guidelines have a similar stance, suggesting CHCs are not strictly contraindicated for those with heterozygous FVL or PGM based on a smaller associated VTE risk. For women with a family history of a low-risk thrombophilia (heterozygous FVL or PGM) and VTE, the ASH panel suggested no testing for the known thrombophilia to guide contraceptive use.48 In contrast, the panel suggested testing for the known thrombophilia if there was a family history of high-risk thrombophilia (PC, PS, or AT deficiency) and VTE to guide contraceptive use (Table 2).48 However, the panel also acknowledged the importance of patient values and preferences and differing risk and benefit trade-offs, so an individualized discussion is needed.48
Having a family history of VTE, regardless of thrombophilia status, increases the VTE risk by 2-4-fold.49 For patients with a low tolerable threshold for VTE risk, a family VTE history alone may be enough to avoid contraceptives with increased VTE risk, whereas other individuals may accept a small increased absolute VTE risk. For those with a family history of VTE, the estimated number needed to test to prevent 1 VTE is shown in Table 3.50 The 2023 ASH guidelines recommend no testing for thrombophilia for contraceptive decision-making among individuals with a family history of VTE and no known thrombophilia.48
Systematic lupus erythematosus and/or aPLs
Contraception counseling is important for patients with systemic lupus erythematosus (SLE) and other autoimmune diseases to decrease the risk of immunosuppressant teratogenicity or a high-risk pregnancy if their disease is not adequately controlled.51 Combined or progestin-only oral contraceptives do not increase the risk of flare among SLE patients with stable disease, but trials excluded patients with aPLs.52,53 Drosperinone can increase potassium levels and should be used with caution in patients with SLE nephritis on angiotensin-converting enzyme or angiotensin receptor blocker inhibitors. DMPA decreases bone mineral density and has a metabolic risk; this may be important for patients on chronic steroids. In patients with active SLE or autoimmune disease, a progestin-only hormonal contraceptive that does not carry thrombotic risk, such as the LNG IUS, should be considered.51
There are minimal data on the risk of hormonal contraception in patients with aPLs or antiphospholipid syndrome (APS).54 In a case-control study of 378 women aged under 50 years presenting with ischemic stroke or myocardial infarction, the OR of stroke was higher with a positive lupus anticoagulant (LAC) and COC use (201.0; 95% CI, 22.1-1828.0) compared to COC use alone (2.9; 95% CI, 1.8-4.6).55 Similarly, the OR of myocardial infarction was higher with a positive LAC and COC use (21.6; 95% CI, 1.9-242.0) than COC use alone (OR, 2.3; 95% CI, 1.6-3.4), but conclusions are limited by small numbers.55
The previous 2016 US MEC guideline version stated for patients with aPL that all hormonal options had more risks than benefits. However, the 2024 US MEC guidelines have been updated and now have a similar approach to other “at risk” groups (Table 2). EULAR (European Alliance of Associations for Rheumatology) guidelines recommend progestin-only contraception for those with aPL and APS but also suggest that estrogen-based contraception may be considered for gynecological disorders not otherwise managed in patients who are fully anticoagulated with a low-risk aPL profile.51
Sickle cell disease
There is recent recognition that VTE risk with SCD is similar to that of other thrombophilias.56,57 Menstrual cycle–associated pain crises and dysmenorrhea reduce quality of life.58 Hormonal contraception does not increase SCD pain crises.59 Some randomized trials have shown a reduction in pain crises in SCD patients receiving DMPA, compared to placebo or CHCs, but there are methodological limitations.59 Two small cohort studies showed a reduction in pain crises and other symptoms with the progestin- only implant, compared to a control group, but patients were not randomized.59 From a VTE perspective, progestin-only contraception does not appear to increase VTE in SCD.59 There are less data for combined hormones, but there is a signal for increased VTE risk among sickle cell trait individuals (Table 4).60 Given the potential concerns for VTE risk with CHC or DMPA in other “at-risk” conditions, other formulations of progestins may be preferable.
The National Heart, Lung, and Blood Institute and British Journal of Haematology guidelines support contraception counseling and long-acting progestin-only contraception use; however, an individualized assessment is needed (Table 4).61,62 Women with SCD report low use and knowledge about long-acting progestin-only contraception, such as the LNG IUS or implant, and further awareness is needed.58 Similar to aPLs, SCD carries a risk of arterial thrombosis, so a discussion about optimizing cardiovascular risk factors is needed when considering CHCs.62
CLINICAL CASE 1 (continued)
After a discussion about the available evidence and guidelines, the patient decides to forgo testing and is comfortable using an oral contraceptive with a lower estrogen dose and second-generation progestin.
CLINICAL CASE 2 (continued)
While the LNG IUS would have been a good choice for VTE risk and decreased menstrual bleeding, the patient preferred to try a progestin-only implant because of the small number of studies that favored improved pain crises. She wanted to avoid DMPA because of potential VTE risk.
Acknowledgments
Leslie Skeith and Shannon M. Bates are members of the Canadian Venous Thromboembolism Research Network.
Dr. Bates receives unencumbered salary support through the Eli Lilly Canada/May Cohen Chair in Women's Health at McMaster University.
Conflict-of-interest disclosure
Leslie Skeith: no competing financial interests to declare.
Shannon M. Bates: no competing financial interests to declare.
Off-label drug use
Leslie Skeith: Off-label drug use is described relating to reducing pain crises in patients with SCD.
Shannon M. Bates: Off-label drug use is described relating to reducing pain crises in patients with SCD.