Abstract
Some clinical studies have identified potential adverse patient outcomes associated with RBC storage length. This may in part be due to the release of potentially hazardous bioactive products that accumulate during storage and are delivered at high concentrations during transfusion. In this situation, a proinflammatory tissue microenvironment may be established that can alter immunoregulatory mechanisms. This review highlights some of the potential immunomodulatory effects of stored RBCs that may be responsible for adverse transfusion reactions.
Introduction
RBC transfusions are lifesaving, being administered to approximately 5 million patients per year in the United States. In the United States, RBCs can be stored under refrigeration in preservative solutions such as such as AdSol solution 1, 3, or 5 for up to 42 days.1 These storage solutions have met the US licensure requirements mandating that RBCs transfused at the end of the approved storage period should have at least 75% of the cells remaining in the circulation 24 hours after infusion, and that the hemolysis in the stored bag be < 1%. It is nevertheless known that, upon storage, RBCs undergo numerous biochemical and physiological changes with the concomitant release of potentially hazardous bioactive products referred to as RBC storage lesions. The clinical relevance of the storage lesion as it affects patient outcomes is currently under much debate.2 Several retrospective studies have shown increased mortality, higher risk of infections, and multiorgan failure in certain patient populations transfused with older blood, although these studies had various limitations that have been extensively reviewed elsewhere.2–4
To gain better clinical evidence on whether the length of RBC storage affects morbidity and mortality, several randomized clinical trials have been initiated. These include the National Heart, Lung, and Blood Institute (NHLBI) Red Cell Storage Duration Study (RECESS) comparing the effects of fresher RBC units (≤ 10 days of storage) with older RBC units (≥ 21 days of storage) in 1800 cardiac surgery patients; the Cleveland Clinic's Red Cell Storage Duration and Outcomes in Cardiac Surgery comparing postoperative morbid outcomes in recipients (N = 2800) of fresher (< 14 days) compared with older (> 20 days) RBCs; the Research Age of Blood Evaluation (ABLE) study being undertaken by the Canadian Institutes of Health comparing the effects of fresher RBC units (< 8 days of storage) with standard-issue RBC units (2-42 days of storage) in 2500 critically ill patients; and the Age of Red Blood Cells in Premature Infants (ARIPI) trial comparing fresher RBC units (< 7 days of storage) with standard-issue RBC units (< 42 days) in 450 low-birth-weight infants. In addition, the NHLBI is currently funding several research programs to mechanistically understand the immunomodulatory and vasoregulatory effects of the storage lesion.5 This review highlights some of the possible relationships between the RBC storage lesion and the immune system6 and presents a possible model of how transfusion of stored blood may result in the breakdown of immunoregulatory mechanisms that can ultimately have adverse pathologic effects.
Effect of WBC contamination and leukoreduction
Adverse immune effects due to presence of WBCs in nonleukoreduced RBC concentrates have been documented and include microbial risks, transfusion-associated GVHD, and recipient immune reactions such as alloimmunization and nonhemolytic febrile transfusion reactions.7–9 It is believed that these WBCs, upon exposure to the acidic conditions of storage and refrigeration, become activated and release cytokines, which can lead to their delivery at high concentrations during transfusion.10 In addition, during routine storage of RBCs, lipids accumulate in the plasma fraction that can prime neutrophils, causing neutrophil-mediated cytotoxicity of human pulmonary endothelial cells, which has been implicated as the mechanism of lung injury in transfusion-related acute lung injury (TRALI).11 Leukoreduction of RBC concentrates after collection results in the removal of WBCs by greater than 3 logs and a decrease in platelets by 4 to 5 logs, as well as marked reduction in some but not all lipids, although it did not prevent neutrophil priming or the ability to induce TRALI in a mouse model.12 Similarly, removal of WBCs by filtration and/or inactivation by gamma irradiation is associated with reduction but not elimination of immunologic adverse events consequences, although leukoreduction appears to reduce the percentage of hemolysis by almost half after RBC storage.13
Heme and heme oxygenase system
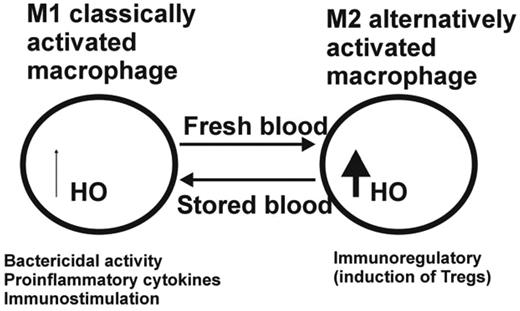
Based on the observation that decreased RBC recovery 24 hours after transfusion is associated with increased storage time, Hod and Spitalnik have argued that the reticuloendothelial system is burdened with an acute, large dose of RBCs after transfusion of stored RBCs with potential adverse immunological consequences for the transfused recipient.14 These investigators also pointed out that most RBC clearance of stored human units occurs within the first hour after transfusion15 and showed that transfusion of stored, but not fresh, mouse leukoreduced RBCs results in rapid clearance (< 2 hours) of 16% of transfused blood by the monocyte/macrophage system.14 Delivering such large amounts of hemoglobin to monocytes/macrophages is likely to affect macrophage plasticity, which is viewed as a spectrum of activation states ranging from the classic proinflammatory (M1) state, which is induced by the Th1 cytokine IFN-γ and bacterial components such as lipopolysaccharides, and the alternative activated (M2) state, which is associated with the resolution phase of inflammation and driven by IL-4, IL-10, TGFβ, and glucocorticoids.16 In response to the hemoglobin breakdown product heme, which catalyzes the formation of reactive oxygen species (ROS), the heme-degrading enzyme heme oxygenase 1 (HO-1) is up-regulated in macrophages and acts as a negative feedback regulator to break down heme into CO, which can act as a vasodilator; the oxidant iron; and biliverdin, which is quickly converted to the antioxidant bilirubin.17 HO-1 is considered immunosuppressive because it was shown to block the maturation of dendritic cells and to inhibit proinflammatory and allogeneic immune responses.18,19 Dendritic cells treated with HO-1 and CO can inhibit ROS and the production of proinflammatory cytokines such as IL-12, IL-6, TNF-α, and IFN type I.19,20 Conversely, the antiinflammatory IL-10 cytokine is increased after HO-1 overexpression or CO, and CO alone has been shown to inhibit T-lymphocyte proliferation.21 This has led to the proposal that HO-1 and CO may mediate immune tolerance by favoring the induction of master immunosuppressor cells, the so-called regulatory T cells (Tregs).22,23 Therefore, the activation of HO-1 expression can be thought of as a critical parameter to switch the classical proinflammatory activity of macrophages into an immunoregulatory one. It remains to be determined whether, at high concentrations of intracellular heme, as encountered after transfusion of stored blood, the HO system becomes overwhelmed and therefore is unable to neutralize the oxidative and inflammatory effects. Macrophages may thus remain cytotoxic, producing high levels of ROS, reactive nitrogen species, and proinflammatory cytokines, and act as classically activated macrophages (Figure 1).16 Conversely, low concentrations of heme may lead to the up-regulation of HO-1 and the induction of anti-inflammatory responses.21 Twenty-four-hour in vivo recovery of RBCs in storage shows a large standard deviation,24 and some units consistently store better than others.25 One can hypothesize that the transfusion of units that have the highest 24-hour recovery rates can induce an anti-inflammatory macrophage activation through the induction of HO, whereas the units with the lower 24-hour recovery rates may be associated with a pro-inflammatory state. The patient's underlying disease most likely affects the ultimate outcome, with the sum of the antioxidative, pro-oxidative, and inflammatory signals within the surrounding tissue tilting the balance to either pro- or anti-inflammatory macrophage activation. Taking this one step further, the differential macrophage polarization state may even explain why transfusion has been associated with both immune activation and immunosuppression.
Hypothetical model for the effect of stored versus fresh blood transfusion on macrophage differentiation. Under inflammatory conditions such as after transfusion of stored RBCs containing lipids and microparticles, macrophages are polarized toward the classical M1 macrophage activation pathway associated with bactericidal activity and proinflammatory cytokine production conducive of immunostimulation. The transfusion of fresh blood under noninflammatory conditions is associated with less RBC clearance and therefore less loading of macrophages with heme, as well as up-regulation of HO and a shift toward the M2 differentiation pathway, which is associated with immunoregulation through the induction of Tregs.
Hypothetical model for the effect of stored versus fresh blood transfusion on macrophage differentiation. Under inflammatory conditions such as after transfusion of stored RBCs containing lipids and microparticles, macrophages are polarized toward the classical M1 macrophage activation pathway associated with bactericidal activity and proinflammatory cytokine production conducive of immunostimulation. The transfusion of fresh blood under noninflammatory conditions is associated with less RBC clearance and therefore less loading of macrophages with heme, as well as up-regulation of HO and a shift toward the M2 differentiation pathway, which is associated with immunoregulation through the induction of Tregs.
RBC alloimmunization and stored blood
In a small retrospective study, Yazer and Triulizi found no correlation between the rate of anti-D alloimmunization among D-negative recipients of D-positive RBCs with various storage lengths, although as noted by the investigators, these results need to be confirmed in larger studies randomized with respect to the age of the transfused RBCs.26 Using a novel mouse model, Hendrickson et al showed that transfusion of leukoreduced stored RBCs led to a more robust alloimmune response compared with the transfusion of fresh RBCs.27 We have previously shown that Tregs control RBC alloimmunization in a mouse model,28 and that alloimmunized recipients have weaker Treg suppressive activity compared with their non-alloimmunized counterparts.29 Our hypothesis is that the transfusion of older blood is associated with the loss of immunosuppressive mechanisms. In this scenario, the proinflammatory environment14 may overwhelm the HO system in macrophages that would otherwise be induced and prevent Treg induction (Figure 1). Consistent with this hypothesis, mixing fresh with stored RBCs resulted in reduced immunogenicity in the mouse model described by Hendrickson et al, and the investigators suggested that fresh RBCs may have immunoregulatory activity that is lost upon storage.30 As mentioned above, central to our hypothesis is that the patient's underlying disease affects the pro- not the anti-inflammatory microenvironment, and ultimately dictates the macrophage polarization state at the time RBCs are being removed from the circulation.
Iron
A potential immunomodulatory effect of iron has been postulated because iron can alter the proliferation and activation of T, B, or natural killer cells, affecting Th1 effector functions and shifting the ratio of helper CD4+ T cells to CD8+ cytotoxic T cells toward the latter.31 In addition, macrophages are dependent on iron for enzymes involved in antimicrobial effector functions, including NADPH-dependent oxidative burst and NO production by inducible NO synthase.31 Iron is critical for most bacterial and parasite growth. In response to inflammation, the reticuloendothelial system retains iron and represses iron export mechanisms, leading to iron sequestration in macrophages, potentially as a means of starving invading pathogens from iron.32 Indeed, iron deficiency results in lower infections and skewing toward Th1 immune responses, whereas susceptibility to bacterial infections increases with increased iron concentration.33 However, iron loading of macrophages inhibits IFN-γ–mediated secretion of proinflammatory cytokines, reduces expression of MHC II and indoleamine-2,3 dioxygenase, and impairs NO synthesis, all of which are consistent with a compromised phagocytic and microbicidal macrophage activity and a shift toward Th2 effector functions.33 It would therefore appear that the iron-loaded macrophages are switched from a pro-inflammatory toward an anti-inflammatory alternatively activated (M2) state, which is thought to be associated with the resolution of inflammation.16 Interestingly, M2 macrophages, unlike their M1 counterparts, have been demonstrated to actively export non-heme-associated iron.16
In an elegant study by Hod et al, it was shown that stored, but not fresh, mouse leukoreduced RBCs resulted in rapid RBC clearance by the monocyte/macrophage system and a concomitant induction of an acute-phase inflammatory response and cytokine production.14 Furthermore, plasma non-transferrin-bound iron (NTBI) was detected 2 hours after transfusion of stored or washed stored RBCs, and plasma from these animals caused proliferation of E coli in vitro.14 Transfusion of supernatants from stored RBCs or from RBC ghosts prepared from stored RBCs did not have any of these effects, indicating that the cellular components of stored blood mediate the harmful effects of the transfused stored blood. The characteristics of the cleared stored RBCs that enables them to be specifically recognized by the macrophage/monocyte system remains unknown. In addition, the macrophage activation state (M1 or M2) was not determined and whether HO-1 was up-regulated was not examined. Hod et al hypothesized that the pro-oxidant effects of iron released after acute clearance of stored RBCs may be responsible for some of the harmful effects of RBC transfusion after prolonged storage. Consistent with this hypothesis, pretreatment with the Food and Drug Administration–approved iron chelator deferoxamine before transfusion reduced cytokine production, possibly through its antioxidant activity. The same investigators found increased plasma NTBI after transfusion of autologous, leukoreduced stored blood in normal healthy volunteers,34 similar to the studies in their mouse model. Although the healthy volunteers did not have any clinically evident adverse events, the investigators pointed out that in critically ill patients, it was possible that increased plasma NTBI could lead to increased risk of sepsis and even mortality. Interestingly, transfusion of stored blood was shown to increase cancer progression in a rat model.35 Although these investigators did not measure NTBI, it is possible that the growth of cancer cells, which generally proliferate more rapidly than normal cells and therefore require more iron, was supported by iron released from macrophages.16
Microparticles and C-mediated regulation
RBC membrane loss resulting in the release of microparticles into the supernatant is considered a potential procoagulant and pro-inflammatory component of the storage lesion.36 Many complement system components and immunoglobulins are enriched in RBC microparticles, which is consistent with a proinflammatory potential.37 In addition, microparticles express the procoagulant phosphatidylserine and the number of phosphatidylserine-enriched RBC microparticles increases with storage time.38,39 Xiong et al showed that RBC microparticles present in stored blood possess inflammatory chemokine binding via the Duffy protein and release ligand upon interaction with platelets in vitro.39 These investigators postulated that the interaction between the RBC microparticles in the transfusate and the recipient's platelets can affect binding and release of chemokines, causing alteration in the local inflammatory microenvironments in vivo. Although it is not known whether direct interactions between RBC microparticles and platelets occur in vivo to propagate or amplify inflammation, it has been shown that prolonged RBC storage can promote lung injury in a mouse model and that a compromised Duffy chemokine scavenging appears to play a role.40 Interestingly, others have reported that RBC microparticles can down-regulate lipopolysaccharide-induced TNF-α and CXCL8 responses in macrophages in vitro.41 Therefore, whether microparticles from RBC units alter innate immune responses of mononuclear phagocytes to perpetuate or curtail inflammation remains unclear.
Summary
RBC degradation clearly occurs during storage, and the breakdown products may have pathological consequences after transfusion—especially in the case of massive transfusions. Ongoing NHLBI-funded research programs are exploring the immunomodulatory and vasoregulatory effects associated with storage duration.5 It may be that the transfusion of stored blood can create an inflammatory microenvironment due to the delivery of high concentrations of lipids and microparticles accumulated during storage. It remains to be determined whether these breakdown products have an effect on the monocyte/macrophage system. Monocytes and macrophages possess plasticity of the differentiation potential, switching between proinflammatory and anti-inflammatory states depending on the microenvironment. Stored RBC clearance by macrophages may reinforce a pro-inflammatory immune response due to the effects of iron and/or the lack of HO up-regulation (Figure 1). It is also possible that Tregs cannot be induced under these conditions, or if they are, they may not possess the immunosuppressive properties to resolve the inflammation that is often encountered in chronic inflammatory disease states.
Randomized controlled trials are ultimately needed to provide clinical evidence of whether RBC storage duration affects morbidity and mortality, and several such trials are ongoing. The combined knowledge from basic and clinical studies on the immunomodulatory effects of stored blood will undoubtedly help with development of better blood products and strategies to eliminate storage-associated adverse effects. These insights may eventually enable the development of testing/screening assays that can match individual patients' immunological profiles with blood products to enhance or suppress their immune system.
Disclosures
Conflict-of-interest disclosure: The authors declare no competing financial interests. Off-label drug use: None disclosed.
Correspondence
Karina Yazdanbakhsh, Laboratory of Complement Biology, New York Blood Center, 310 East 67th St, New York, NY 10065; Phone: (212) 570-3383; Fax: (212) 737-4506; e-mail: kyazdanbakhsh@nybloodcenter.org.