Abstract
A 63-year-old male patient without siblings is treated for acute myeloid leukemia with poor prognostic cytogenetics. Despite achieving a first complete remission, he relapsed within the first year of diagnosis. He then achieved a second complete remission. A search for an HLA-identical unrelated donor identified a 10/10 possible match. The patient has several comorbidities (hematopoietic stem cell comorbidity index = 3) and it is recommended that he undergo a reduced-intensity conditioning regimen for allogeneic peripheral blood stem cell transplantation. The patient is well-read on allogeneic stem cell transplantation and asks you the merits of antithymocyte globulin that you propose to include in the conditioning regimen.
Introduction
Reduced-intensity conditioning (RIC) regimens have led to a dramatic reduction of early transplantation mortality after allogeneic hematopoietic stem cell transplantation (allo-HSCT), thereby allowing patients who are otherwise ineligible for myeloablative conditioning to undergo allo-HSCT. The concept of RIC is to deliver adequate immunosuppression so that there is successful engraftment of donor hematopoietic cells, with manageable graft-versus-host disease (GVHD) and the eventual development of a potent graft-versus-tumor effect.1 With this goal in mind, many immunosuppressive strategies have been incorporated into various RIC regimens and studied, but no particular RIC regimen has emerged as the optimal one. Generally, most RIC regimens combine fludarabine with low-dose total body irradiation or intermediate doses of alkylating agents. In addition, antithymocyte globulin (ATG) is regularly incorporated to minimize the risk of GVHD. The main mechanism of action of ATG is in vivo T-cell depletion in blood and lymphoid tissues,2,3 but it also has other important immunomodulatory effects and is thought to be important in limiting the incidence and severity of GVHD in allo-HSCT while hastening engraftment. However, these effects can potentially lead to serious infections such as CMV and EBV and disease relapse.4–6 Indeed in the late 1980s, ex vivo or in vivo T-cell depletion of donor graft after myeloablative conditioning led to lower acute GVHD (aGVHD) but higher relapse rates compared with T-cell replete transplantations.7 Given the various combinations of immunosuppressive agents used in RIC regimens, it is unclear whether ATG itself is responsible for these undesirable effects. At present, the type, dose, and schedule of ATG treatment still need to be more precisely defined, and for all of these reasons, the use of ATG in allo-HSCT remains controversial. The scope of this chapter is to review the current place and role of ATG in RIC regimens.
Methods
A comprehensive PubMed search was performed using the terms “ATG” AND “reduced intensity conditioning” as well as “antithymocyte globulin” AND “reduced intensity conditioning” and “thymoglobulin” AND “reduced intensity conditioning.“ All references of relevant articles were further scanned to identify studies that should be included in this mini-review. Pediatric and nonhuman studies have been excluded. Studies pertaining to myeloablative conditioning were also excluded.
Results
To date, only a few randomized trials have been reported using ATG in the myeloablative conditioning setting.8–11 These trials showed that ATG-containing regimens lowered the risk of GVHD significantly compared with no ATG, but there was no improvement in transplant-related mortality or survival, possibly due to the increased risk of infections or decreased disease control. In the RIC setting, however, there are no adequate phase 3 randomized controlled trials to address the precise role of ATG, which is an authentic weakness. Indeed, to date, a single comparative study of 139 patients has partially addressed this question by prospectively comparing 2 commonly used RIC regimens with or without ATG.12 The first regimen consisted of fludarabine (30 mg/m2/d for 5 days), oral busulfan (4 mg/kg/d for 2 days), and low-dose rabbit ATG (rATG; 2.5 mg/kg on day −1). Cyclosporine alone was administered for postgraft immunosuppression. The second regimen included fludarabine (30 mg/m2/d for 3 days) and 2 Gy of total body irradiation on day 0. Cyclosporine and mycophenolate mofetil were administered for postgraft immunosuppression. In addition to ATG, each strategy obviously differed from the other in terms of myeloablation (intermediate dose busulfan vs low-dose total body irradiation) and postgraft immunosuppression (cyclosporine alone vs cyclosporine and mycophenolate mofetil). With a median follow-up of 5 years, overall survival is similar in both arms. The use of low-dose of ATG administered on day −1 was not associated with higher relapse or infectious death rates for either myeloid or lymphoid malignancies.
Champlin et al compared cyclophosphamide alone with cyclophosphamide plus equine ATG conditioning in a randomized controlled trial and did not find a difference in outcomes.13 However, the study was closed early and was not adequately powered to detect significant differences between the 2 treatment groups. The remaining data evaluating the dose, schedule, and outcomes of ATG in RIC come from small phase 2 or retrospective studies14–25 (Table 1). With respect to dose, the data seem to favor an intermediate dose of ATG as offering a balance between GVHD prevention and relapse incidence in the situation of RIC regimens. For example, Devillier et al23 showed in a recent retrospective study that 5 mg/kg of rATG significantly decreased grade II-IV aGVHD and extensive chronic GVHD compared with the 2.5 mg/kg dose. Malard et al,24 also in a retrospective study, showed that 5 mg/kg of rATG significantly reduced grade III-IV aGVHD and extensive chronic GVHD. In another retrospective study, Hamadani et al22 showed that 6 mg/kg of rATG was more effective in decreasing infectious complications and improving nonrelapse mortality compared with the 7.5 mg/kg dose, with no difference in GVHD incidence.
Studies reporting on ATG in RIC for allo-HSCT
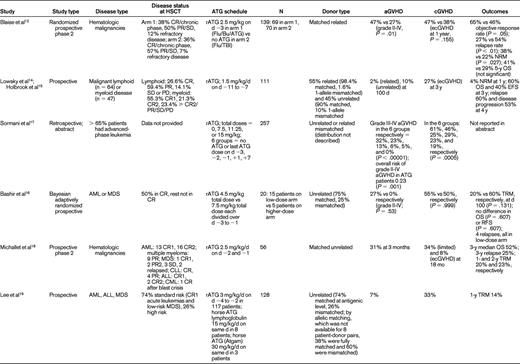
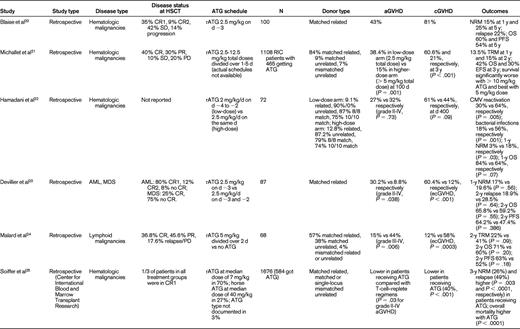
CR indicates complete remission; PR, partial remission; SD, stable disease; PD, progressive disease; Flu, fludarabine; Bu, busulfan; TBI, total body irradiation; NRM, nonrelapse mortality; OS, overall survival; PFS, progression-free survival; TRM, transplantation-related mortality; RFS, relapse-free survival; AML, acute myelogenous leukemia; MDS, myelodysplastic syndrome; CLL, chronic lymphocytic leukemia; CML, chronic myeloid leukemia; ALL, acute lymphoblastic leukemia; ecGVHD, extensive chronic GVHD; and d, days.
Where schedule is concerned, Lowsky et al14 in a nonmyeloablative conditioning setting using total lymphoid irradiation and rATG, started rATG 1.5 mg/kg/d 11 days before graft infusion and continued it for 5 days. They reported very low aGVHD (2 of 37 patients) for patients with myeloid and lymphoid malignancies. The results of this study were updated in 200915 and are shown in Table 1. The investigators concluded that it was unlikely that the low incidence of aGVHD could be explained by the persistence of ATG causing in vivo depletion of donor T cells. Biologically active ATG was not detectable in the system after day +7 of transplantation. The more important effect of ATG was thought to be depletion of recipient T cells without depletion of regulatory T cells. Sormani et al17 presented an abstract in 2007 describing 257 patients undergoing allo-HSCT. The dose of ATG varied from 7.5 mg/kg (n = 62), 11.25 mg/kg (n = 44), and 15 mg/kg (n = 83). Sixty-eight patients received no ATG, 17 received the last dose of ATG on day −3, 90 patients on day −2, 32 patients on day −1, 19 patients on day +1, and 31 patients on day +7. In these 6 groups, grade III-IV aGVHD occurred in 32%, 23%, 13%, 6%, 5%, and 0% of patients, respectively. Chronic GVHD (cGVHD) occurred in 61%, 46%, 25%, 29%, 23%, and 19% respectively. Patients receiving ATG had a significantly lower risk of developing aGVHD (P = .001). Timing and dose of ATG were significant predictors of GVHD, and the investigators concluded that it may be useful to use small doses of ATG close to transplantation. Most studies have indeed used ATG in the immediate vicinity of graft infusion.
The paucity of randomized prospective trials limits the value of all of these data. In fact, they are challenged by the results from a large retrospective Center for International Blood and Marrow Transplant Research study by Soiffer et al.25 Seventy percent of patients had acute myeloid leukemia, myelodysplastic syndrome, and non-Hodgkin lymphoma. A total of 797 patients (48%) received anti–T-cell antibodies: 584 (35%) received ATG, and 213 (13%) received alemtuzumab. Eight hundred seventy-nine (52%) received no in vivo T-cell depletion. For patients receiving ATG, approximately 70% received rATG at a median dose of 7 mg/kg and 27% received horse ATG (hATG) at a median dose of 40 mg/kg. The type of ATG was not reported in 3% of patients. Compared with T cell–replete regimens, there were no differences in grades II-IV and III-IV aGVHD with ATG-containing regimens overall, although grade II-IV (P = .03) and grade III-IV (P = .01) aGVHD rates were lower in patients receiving rATG compared with those receiving hATG. The rate of cGVHD was lower with all ATG-containing regimens (P < .001). Compared with T cell–replete regimens, 3-year nonrelapse mortality was higher (P = .003), as was the 3-year relapse rate (P < .0001), with ATG-based RIC. Nonrelapse mortality was lower (P = .004) and relapse risk was higher (P = .02) after rATG compared with those receiving hATG. Disease-free survival was lower in T-cell–depleted patients, and there was no difference in the rATG versus the hATG groups or in the lower-dose (< 7 mg/kg of rATG or < 40 mg of hATG) versus higher-dose (≥ 7 mg/kg of rATG vs ≥ 40 mg of hATG) ATG groups. The 3-year overall survival was lower in ATG-containing regimens compared with the T-cell–replete regimens (38% vs 46%, P = .008). Results appeared to be similar to the above analysis for transplantations performed from HLA-matched siblings or unrelated donors except that ATG seemed to be less effective in reducing aGVHD in the unrelated donor setting compared with the related donor setting. Overall, these results suggested that the use of in vivo T-cell depletion with RIC regimens reduces cGVHD but significantly increases the likelihood of disease relapse, which negatively affects disease-free survival after both related and unrelated donor allo-HSCT. Once again, the retrospective nature of the study limits definitive conclusions and emphasizes the need for future prospective evaluations.
Conclusions
Based on this review, and despite the limitations of existing data, we conclude that ATG may reduce GVHD in patients with hematological malignancies undergoing RIC for allo-HSCT. The dose and timing of ATG seem to be important. Low doses (< 3 mg/kg) appear not to curb GVHD adequately and high doses (> 7 mg/kg) seem to be associated with unacceptable incidence of infection and relapse, the latter occurring despite (or because of) a major reduction in GVHD incidence. Intermediate doses of rATG are able to efficiently decrease the incidence of both aGVHD and cGVHD and to limit infectious complications. Based on the data reviewed herein, we suggest that a dose of 4.5-6 mg/kg of rATG administered on the last few days before transplantation is the best schedule to incorporate ATG into a RIC regimen (grade 2C), and this dose should be used in future prospective evaluations to confirm the potential benefit of rATG in RIC regimens for GVHD.
Disclosures
Conflict-of-interest disclosure: The authors declare no competing financial interests. Off-label drug use: None disclosed.
Correspondence
Tanya Siddiqi, Department of Hematology/Hematopoietic Cell Transplantation, City of Hope National Medical Center, 1500 E Duarte Rd, Duarte, CA 91010; Phone: 626-256-4673; Fax: 626-301-8256; e-mail: tanyasiddiqi@coh.org.
