Abstract
Passive immune therapy consists of several different therapies, convalescent plasma, hyperimmune globulin, and severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) neutralizing monoclonal antibodies. Although these treatments were not part of any pandemic planning prior to coronavirus disease 2019 (COVID-19), due to the absence of high-quality evidence demonstrating benefit in other severe respiratory infections, a large amount of research has now been performed to demonstrate their benefit or lack of benefit in different patient groups. This review summarizes the evidence up to July 2021 on their use and also when they should not be used or when additional data are required. Vaccination against SARS-CoV-2 is the most important method of preventing severe and fatal COVID-19 in people who have an intact immune system. Passive immune therapy should only be considered for patients at high risk of severe or fatal COVID-19. The only therapy that has received full regulatory approval is the casirivimab/imdevimab monoclonal cocktail; all other treatments are being used under emergency use authorizations. In Japan, it has been licensed to treat patients with mild to moderate COVID-19, and in the United Kingdom, it has also been licensed to prevent infection.
Learning Objectives
Summarize current evidence on the use of passive immune therapy to prevent infection—whether it reduces risk of death or hospitalization
Summarize current evidence on the use of passive immune therapy for high-risk patients who have mild COVID-19 symptoms—risk of death
Can state whether passive immune therapy reduces all-cause mortality for hospitalized patients and whether any subgroups will benefit
CLINICAL CASE 1
A 74-year-old man (case) had a test for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) after a family gathering for the christening of his granddaughter. He had had the test because he was a close contact for the index case at the christening. His 49-year-old nephew (index case) had been admitted to hospital the day after the christening and was found to be SARS-CoV-2 positive. The nephew had had some symptoms at the time of the christening but had not thought that these were due to coronavirus disease 2019 (COVID-19). The 74-year-old man self-isolated with his wife while awaiting the results of the test.
Introduction
Passive antibody therapy is one of the oldest treatments for infectious diseases that is still in use. Emil von Behring (1854-1917) was awarded the first Nobel Prize in Medicine “for his work on serum therapy, especially its application against diphtheria.”1 Today, passive antibody therapy involves treatment with polyclonal antibodies derived from humans (convalescent plasma [CP] or hyperimmune globulin), animals (antisera), or antigen-specific monoclonal antibodies (mAbs).2 In this review, I focus on CP and neutralizing mAbs and the current evidence for their use. There are no published trials on the use of hyperimmune globulin in COVID-19.3
CP after infection, particularly after severe illness, may contain high levels of polyclonal pathogen-specific antibodies. These antibodies may confer passive immunity to recipients and in viral diseases are thought to have their main action via neutralization of viral particles.4 Collection and transfusion of CP can occur rapidly after the onset of a pandemic, with collection of plasma occurring from 14 days after a patient has recovered from the infection. The antibody response in CP donors adapts with the virus, either due to donors becoming infected with new variants of the virus or due to vaccination after an initial natural infection. However, the levels of neutralizing antibody can vary significantly from 1 unit to the next, and a minimum threshold of antibody is required within each unit to ensure that the transfusion contains a sufficient level of neutralizing antibody (Table 1).
Comparison between different passive antibody therapies
| Characteristic . | CP . | Human hyperimmune globulin . | mAb . |
|---|---|---|---|
| Speed of production after start of pandemic | Rapid—weeks Can be produced once patients have recovered from infection (14 to 28 days after recovery) | Slow—months Needs time to collect plasma from a large number of people who have recovered from infection | Slowest—months Need to identify potential antibodies that would be useful to develop as a mAb and then manufacture antibody |
| Can adapt to viral variants | Yes | Yes—more slowly than CP | No |
| Number of anti–SARS-CoV-2 antibodies product contains | Many—polyclonal | Many—polyclonal | One to 2 antibodies |
| Amount of antibody contained within the product | Very variable. Variability can be reduced by using mini-pools—used in Argentina but not all countries allowed to produce mini-pools | Fixed amount of total antibody | Fixed amount of neutralizing SARS-CoV-2 neutralizing antibody |
| Route of administration | Intravenous | Subcutaneous, intramuscular, or intravenous | Subcutaneous, intramuscular, or intravenous |
| Derived from blood* | Yes | Yes | No |
| Availability | Able to be produced in any country in which people have recovered from the infection and are able to produce plasma | Not able to be produced in every country but manufacturing requirements are not complex | Limited supply due to complex manufacturing requirements |
| Cost | Relatively cheap—$100 to $200 per dose Can be used in low- and middle-income countries | More expensive than CP but cheaper than mAb—may be able to be produced locally, in low- and middle-income countries | Expensive—$1000s per dose Cannot be afforded by low- and middle-income countries Can only be produced at a limited number of manufacturing sites |
| Characteristic . | CP . | Human hyperimmune globulin . | mAb . |
|---|---|---|---|
| Speed of production after start of pandemic | Rapid—weeks Can be produced once patients have recovered from infection (14 to 28 days after recovery) | Slow—months Needs time to collect plasma from a large number of people who have recovered from infection | Slowest—months Need to identify potential antibodies that would be useful to develop as a mAb and then manufacture antibody |
| Can adapt to viral variants | Yes | Yes—more slowly than CP | No |
| Number of anti–SARS-CoV-2 antibodies product contains | Many—polyclonal | Many—polyclonal | One to 2 antibodies |
| Amount of antibody contained within the product | Very variable. Variability can be reduced by using mini-pools—used in Argentina but not all countries allowed to produce mini-pools | Fixed amount of total antibody | Fixed amount of neutralizing SARS-CoV-2 neutralizing antibody |
| Route of administration | Intravenous | Subcutaneous, intramuscular, or intravenous | Subcutaneous, intramuscular, or intravenous |
| Derived from blood* | Yes | Yes | No |
| Availability | Able to be produced in any country in which people have recovered from the infection and are able to produce plasma | Not able to be produced in every country but manufacturing requirements are not complex | Limited supply due to complex manufacturing requirements |
| Cost | Relatively cheap—$100 to $200 per dose Can be used in low- and middle-income countries | More expensive than CP but cheaper than mAb—may be able to be produced locally, in low- and middle-income countries | Expensive—$1000s per dose Cannot be afforded by low- and middle-income countries Can only be produced at a limited number of manufacturing sites |
Any product derived from human blood requires viral testing to ensure that the transfused product does not cause a transfusion-transmitted infection. In high-income countries with good screening systems, transfusion-transmitted infection is very rare. In the United Kingdom, there were no transfusion-transmitted infections reported to the national hemovigilance system in 2020 (https://www.shotuk.org/wp-content/uploads/myimages/TTI-Supplementary-material-2020.pdf).
Hyperimmune globulin is currently used to protect vulnerable individuals from other viral infections, including varicella zoster.5 It is produced by pooling thousands of donations from people who have recovered from an infection or have been vaccinated against an infection and have high levels of antibodies. It produces a consistent product that always has a defined level of antibody within it. However, it takes time to produce, so it cannot be used as early in a pandemic. It will also evolve with changes with the viral variant but not as rapidly as CP.
Neutralizing monoclonal antibody therapy can be derived from humans who have had an infection, or been vaccinated, or from humanized mice that have been exposed to SARS-CoV-2 antigens.2 Monoclonal antibody production can identify antibodies with a high level of neutralizing activity and can be produced without the need for blood donors. mAbs will, however, not adapt to viral variants, and so over time, the virus can become resistant to the monoclonal antibody. This has already happened with the SARS-CoV-2 virus (Table 2). Monotherapy with bamlanivimab has had its emergency use authorization (EUA) withdrawn due to development of viral resistance.6 This may mean that monoclonal cocktails are effective and less likely to lead to resistance than use of a single monoclonal. However, even a monoclonal cocktail could become ineffective in the future, as shown by the prospective mapping of viral variants that detected a potential mutation (E406W) that could escape neutralization by both components of the casirivimab/imdevimab monoclonal cocktail, as well as both components of the bamlanivimab/etesevimab cocktail (Table 2).7 Monoclonal therapy is expensive and requires very specialized manufacturing units. It is therefore a treatment that most low- and middle-income countries cannot afford and will find more difficult to manufacture locally. CP can be produced in many countries and is much more affordable.8 Therefore, when thinking about whether to use passive immunization therapy, consideration needs to be made not just on its effectiveness but also on its accessibility.
Different types of mAb in clinical use
| Name of monoclonal . | Site of action . | Route of administration . | Clinical trial results* . | In clinical use (outside of clinical trials) . | Viral resistance detected . | Competent authority approval . |
|---|---|---|---|---|---|---|
| Bamlanivimab | Blocks binding of SARS-CoV-2 to the ACE2 receptor by targeting the RBD on the spike protein of SARS-CoV-2 | IV | Yes | Previously—FDA EUA revoked April 2021 | Yes Marked—gamma (P.1) and beta (B.1.351) VoCs Modest—delta (B.1.617.2) VoC | EUA (now revoked in United States) |
| Bamlanivimab plus etesevimab | Blocks binding of SARS-CoV-2 to the ACE2 receptor by targeting different but overlapping epitopes on the spike protein RBD of SARS-CoV-2 | IV | Yes | Yes—use paused in United States | Yes Marked—gamma (P.1) and beta (B.1.351) VoC Modest—Delta (B.1.617.2) VoC | EUA† |
| Casirivimab plus imdevimab | Blocks binding of SARS-CoV-2 to the ACE2 receptor by targeting different epitopes on the spike protein RBD of SARS-CoV-2 (do not overlap) | IV or S/C | Yes | Yes | Yes—only to casirivimab Marked—beta (B.1.351) VoC | Yes—MHRA and Japanese regulatory agency† |
| Sotrovimab | Blocks binding of SARS-CoV-2 to the ACE2 receptor by targeting an epitope on the RBD of the spike protein that is conserved between SARS-CoV and SARS-CoV-2 | IV, trialing IM | Yes | Yes | No | EUA† |
| Regdanvimab | Blocks binding of SARS-CoV-2 to the ACE2 receptor by targeting an epitope on the RBD of the spike protein of SARS-CoV-2 | IV | Yes | Yes | Yes Marked—beta (B.1.351) VoC | EUA‡ |
| Name of monoclonal . | Site of action . | Route of administration . | Clinical trial results* . | In clinical use (outside of clinical trials) . | Viral resistance detected . | Competent authority approval . |
|---|---|---|---|---|---|---|
| Bamlanivimab | Blocks binding of SARS-CoV-2 to the ACE2 receptor by targeting the RBD on the spike protein of SARS-CoV-2 | IV | Yes | Previously—FDA EUA revoked April 2021 | Yes Marked—gamma (P.1) and beta (B.1.351) VoCs Modest—delta (B.1.617.2) VoC | EUA (now revoked in United States) |
| Bamlanivimab plus etesevimab | Blocks binding of SARS-CoV-2 to the ACE2 receptor by targeting different but overlapping epitopes on the spike protein RBD of SARS-CoV-2 | IV | Yes | Yes—use paused in United States | Yes Marked—gamma (P.1) and beta (B.1.351) VoC Modest—Delta (B.1.617.2) VoC | EUA† |
| Casirivimab plus imdevimab | Blocks binding of SARS-CoV-2 to the ACE2 receptor by targeting different epitopes on the spike protein RBD of SARS-CoV-2 (do not overlap) | IV or S/C | Yes | Yes | Yes—only to casirivimab Marked—beta (B.1.351) VoC | Yes—MHRA and Japanese regulatory agency† |
| Sotrovimab | Blocks binding of SARS-CoV-2 to the ACE2 receptor by targeting an epitope on the RBD of the spike protein that is conserved between SARS-CoV and SARS-CoV-2 | IV, trialing IM | Yes | Yes | No | EUA† |
| Regdanvimab | Blocks binding of SARS-CoV-2 to the ACE2 receptor by targeting an epitope on the RBD of the spike protein of SARS-CoV-2 | IV | Yes | Yes | Yes Marked—beta (B.1.351) VoC | EUA‡ |
Results available in Table 2 for all-cause mortality and need for hospitalization (by day 30) in Table 3 for outpatients.
EUA in the United States as well as other countries. In the United States, the indication is for mild or moderate COVID-19 not requiring oxygen therapy.
EUA in South Korea and Indonesia, but indications for use differ.
FDA, Food and Drug Administration (United States); IM, intramuscular; IV, intravenous; MHRA, Medicines and Healthcare products Regulatory Agency (United Kingdom); RBD, receptor-binding domain; S/C, subcutaneous; VoC, variant of concern.
Prophylactic therapy
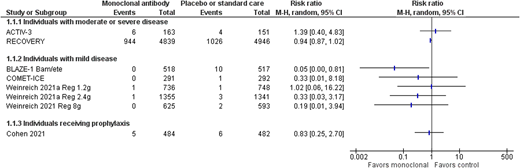
The mainstay of preventing infection in people with an intact immune system is vaccination. Active immunization against SARS-CoV-2 has been shown to be effective at preventing severe and fatal COVID-19, as well as reducing the risk of symptomatic COVID-19.9 In the United States, by the end of June 2021, vaccination had averted an estimated 279 000 deaths and up to 1.25 million hospitalizations.10 However, vaccination is not as effective in patients with an impaired immune system, either due to immunosuppressive therapy or an underlying disease that affects the immune system. Patients with hematologic malignancies mount blunted antibody responses to SARS-CoV-2 vaccination, and those most at risk appear to be patients who are actively treated with Bruton tyrosine kinase inhibitors, ruxolitinib, venetoclax, or anti-CD20 antibody therapies.11 Consideration therefore needs to be given to preventing infection in people who are unvaccinated or partially vaccinated and are at high risk of developing severe and fatal COVID-19 due to comorbidities or those who have been fully vaccinated but are unable to mount an effective immune response to vaccination. There have been 2 completed trials assessing monoclonal therapy12,13 (Table 3), with 1 trial assessing the effect of 1.2 g of the casirivimab/imdevimab cocktail (subcutaneously via 4 injections) given to household contacts of a positive case.13 However, most of these individuals were not at high risk of severe disease, with fewer than 10% older than 65 years and only 1% with immune suppression. The other trial used bamlanivimab, which showed a benefit in nursing home residents who received the intervention in progression to moderate or severe disease but no evidence of a difference in mortality (Figure 1). However, bamlanivimab is not effective against many of the viral variants currently in circulation. Ongoing studies of CP, hyperimmune globulin, and mAbs assessing high-risk prophylaxis will be assessed within living systematic reviews, so additional information may be available over the next few months.23,24 It is especially important to know their additional impact over and above vaccination for those individuals who have a poor response to vaccination, for example, the immunosuppressed population.11
Completed randomized controlled trials of anti-SARS-CoV-2 mAb therapy
| Study . | Country . | Intervention(s) . | Number randomized up to data cutoff . | Number analyzed . | Patient population . | Primary outcome . | Type of analysis . | Viral variants considered in analyses* . | Mortality at 30 days (GRADE)† . | Need for hospitalization at 30 days or death (GRADE)† . |
|---|---|---|---|---|---|---|---|---|---|---|
| Prophylaxis | ||||||||||
| O'Brien et al‡13 NCT04452318 | Moldova, Romania, United States | S/C casirivimab/imdevimab 1.2 g (0.6 g + 0.6 g) | 753 | 1505 | Age ≥12 years Household contact of confirmed SARS-CoV-2 case SARS-CoV-2 PCR and antibody negative | Incidence of symptomatic COVID-19 | Interim Planned recruitment 3750 | No Recruited ??-January 2021 | NR | NR |
| Placebo | 752 | |||||||||
| BLAZE-212 NCT04497987 | United States | Bamlanivimab (4.2 g) | 588 | 966 | Residents and staff at 74 skilled nursing and assisted living facilities Within 7 days of a reported confirmed SARS-CoV-2 case | Incidence of COVID-19—virological or clinical | Final | No Recruited August- November 2020 | RR, 0.83 95% CI, 0.25-2.70 (low) | NR |
| Placebo | 587 | |||||||||
| Outpatients (asymptomatic) | ||||||||||
| O'Brien et al14 NCT04452318‡ | United States | S/C casirivimab/imdevimab 1.2 g (0.6 g + 0.6 g) | 155 | 311 | Age ≥12 years Confirmed SARS-CoV-2 SARS-CoV-2 antibody negative Asymptomatic | Incidence of symptomatic COVID-19 | Interim Planned recruitment 3750 | July 2020 to January 2021 | NR | RR, 0.14 95% CI, 0.01-2.76 (low) |
| Placebo | 156 | |||||||||
| Outpatients (mild disease) | ||||||||||
| Weinreich et al (phase 3)15 NCT04425629‡ | Chile, Mexico, Romania, United States | Casirivimab/imdevimab 1.2 g (0.6 g + 0.6 g) | 838 | 4057 | Adult Confirmed SARS-CoV-2 ≤7 days from onset symptoms ≥1 risk factor COVID-19 Mild symptoms (WHO 2-3) Excluded Hospitalized (any reason) Vaccinated or plan to be vaccinated | Clinical—COVID-related hospitalization or death (day 29) | Interim Planned recruitment 6420 Subgroup—antibody negative | No Recruited September 2020 to January 2021 | RR, 1.02 95% CI, 0.06-16.22 (low) | RR, 0.30 95% CI, 0.13-0.68 (low) |
| Casirivimab/imdevimab 2.4 g (1.2 g + 1.2 g) | 1529 | RR, 0.33 95% CI, 0.01-3.94 (low) | RR, 0.29 95% CI, 0.17-0.48 (moderate) | |||||||
| Placebo | 1500 | — | — | |||||||
| COMET-ICE‡16 NCT04545060 | Brazil, Canada, Spain, United States | Sotrovimab (0.5 g) | 291 | 583 | Adult Confirmed SARS-CoV-2 ≤5 days from onset symptoms >55 years or comorbidities Mild symptoms (WHO 2-3) Excluded Likely to require hospitalization ≤24 h Likely to die ≤7 days Severely immunocompromised Vaccinated or plan to be vaccinated within 4 weeks | Clinical—disease progression | Interim Stopped recruitment due to efficacy 1057 participants | No Recruited August 2020 to March 2021 | RR, 0.33 95% CI, 0.01-8.18 (low) | RR, 0.14 95% CI, 0.04-0.48 (low) |
| Placebo | 292 | |||||||||
| BLAZE-117 NCT04427501 | Puerto Rico, United States | Bamlanivimab (0.7 g) | 104 | 577 | Adult Confirmed SARS-CoV-2 Mild symptoms (WHO 2-3) Excluded Requires oxygen therapy Any serious concomitant systemic disease Pregnant or breastfeeding | Virological—viral clearance | Interim Planned recruitment 3160 | No Recruited June-September 2020 | No events (low) | RR, 0.17 95% CI, 0.02-1.33 (low) |
| Bamlanivimab (2.8 g) | 109 | RR, 0.32 95% CI, 0.07-1.47 (low) | ||||||||
| Bamlanivimab (7.0 g) | 104 | RR, 0.34 95% CI, 0.08-1.56 (low) | ||||||||
| Bamlanivimab/etesevimab (2.8 g + 2.8 g) | 114 | See updated results below | See updated results below | |||||||
| Placebo | 161 | — | — | |||||||
| BLAZE-118 NCT04427501 | United States | Bamlanivimab/etesevimab (2.8 g + 2.8 g) | 518 | 1035 | Adult Confirmed SARS-CoV-2 Mild symptoms (WHO 2 to 3) Excluded Requires oxygen therapy Any serious concomitant systemic disease Pregnant or breastfeeding | Clinical—COVID-19–related hospitalization (acute care for ≥24 hours) or death from any cause by day 29 | Interim Planned recruitment 3160 | No September- December 2020 | RR, 0.05 95% CI, 0.00-0.81 (low) | RR, 0.30 95% CI, 0.16-0.59 (low) |
| Placebo | 517 | |||||||||
| Eom et al‡19 NCT04602000 | Romania, South Korea, Spain, United States | Regdanvimab 0.04 g/kg | 101 | 327 | Adult Confirmed SARS-CoV-2 ≤7 days from onset symptoms Excluded Hospitalized | Clinical—time to clinical recovery Virological—viral clearance | Interim Planned recruitment 1172 | No Recruited October-December 2020 | No events (low) | RR, 0.45 95% CI, 0.14-1.42 (low) |
| Regdanvimab 0.08 g/kg | 103 | RR, 0.56 95% CI, 0.19-1.60 (low) | ||||||||
| Placebo | 103 | — | ||||||||
| Weinreich et al (phase 1/2)20 NCT04425629 | Chile, Mexico, Romania, United States | Casirivimab/imdevimab 2.4 g (1.2 g + 1.2 g) | 92 | 275 | Adult Confirmed SARS-CoV-2 ≤7 days from onset symptoms Excluded Hospitalized | Virological—viral clearance | Interim | No Recruited June- August 2020 | NR | RR, 0.43 95% CI, 0.08-2.19 (low) |
| Casirivimab/imdevimab 8 g (4 g + 4 g) | 90 | RR, 0.21 95% CI, 0.02-1.79 (low) | ||||||||
| Placebo | 93 | — | ||||||||
| Inpatients (moderate or severe disease) | ||||||||||
| RECOVERY‡21 NCT04381936 | United Kingdom | Casirivimab/imdevimab 8 g (4 g + 4 g) | 4839 | 9185 | Any age Suspected or confirmed COVID-19 Median 9 days symptom onset >90% requiring oxygen therapy (WHO score ≥5) | Clinical— all-cause mortality day 28 | Complete Subgroup— antibody negative | No Recruited September 2020 to May 2021 | RR, 0.94 95% CI, 0.87-1.02 (moderate) | NA |
| Standard care | 4946 | |||||||||
| ACTIV-322 NCT04501978 | Denmark, India, Poland, Singapore, Spain, Switzerland, United Kingdom, United States | Bamlanivimab (7 g) | 314 | Inpatients, moderate disease | Adult Confirmed SARS-CoV-2 ≤12 days from onset symptoms Excluded Requiring organ support Pregnant/breast feeding | Clinical—time to sustained recovery | Interim analysis, bamlanivimab arm stopped for futility, recruitment ongoing for other arms | No Recruited August to October 2020 | RR, 1.39 95% CI, 0.07-1.47 (low) | NA |
| Study . | Country . | Intervention(s) . | Number randomized up to data cutoff . | Number analyzed . | Patient population . | Primary outcome . | Type of analysis . | Viral variants considered in analyses* . | Mortality at 30 days (GRADE)† . | Need for hospitalization at 30 days or death (GRADE)† . |
|---|---|---|---|---|---|---|---|---|---|---|
| Prophylaxis | ||||||||||
| O'Brien et al‡13 NCT04452318 | Moldova, Romania, United States | S/C casirivimab/imdevimab 1.2 g (0.6 g + 0.6 g) | 753 | 1505 | Age ≥12 years Household contact of confirmed SARS-CoV-2 case SARS-CoV-2 PCR and antibody negative | Incidence of symptomatic COVID-19 | Interim Planned recruitment 3750 | No Recruited ??-January 2021 | NR | NR |
| Placebo | 752 | |||||||||
| BLAZE-212 NCT04497987 | United States | Bamlanivimab (4.2 g) | 588 | 966 | Residents and staff at 74 skilled nursing and assisted living facilities Within 7 days of a reported confirmed SARS-CoV-2 case | Incidence of COVID-19—virological or clinical | Final | No Recruited August- November 2020 | RR, 0.83 95% CI, 0.25-2.70 (low) | NR |
| Placebo | 587 | |||||||||
| Outpatients (asymptomatic) | ||||||||||
| O'Brien et al14 NCT04452318‡ | United States | S/C casirivimab/imdevimab 1.2 g (0.6 g + 0.6 g) | 155 | 311 | Age ≥12 years Confirmed SARS-CoV-2 SARS-CoV-2 antibody negative Asymptomatic | Incidence of symptomatic COVID-19 | Interim Planned recruitment 3750 | July 2020 to January 2021 | NR | RR, 0.14 95% CI, 0.01-2.76 (low) |
| Placebo | 156 | |||||||||
| Outpatients (mild disease) | ||||||||||
| Weinreich et al (phase 3)15 NCT04425629‡ | Chile, Mexico, Romania, United States | Casirivimab/imdevimab 1.2 g (0.6 g + 0.6 g) | 838 | 4057 | Adult Confirmed SARS-CoV-2 ≤7 days from onset symptoms ≥1 risk factor COVID-19 Mild symptoms (WHO 2-3) Excluded Hospitalized (any reason) Vaccinated or plan to be vaccinated | Clinical—COVID-related hospitalization or death (day 29) | Interim Planned recruitment 6420 Subgroup—antibody negative | No Recruited September 2020 to January 2021 | RR, 1.02 95% CI, 0.06-16.22 (low) | RR, 0.30 95% CI, 0.13-0.68 (low) |
| Casirivimab/imdevimab 2.4 g (1.2 g + 1.2 g) | 1529 | RR, 0.33 95% CI, 0.01-3.94 (low) | RR, 0.29 95% CI, 0.17-0.48 (moderate) | |||||||
| Placebo | 1500 | — | — | |||||||
| COMET-ICE‡16 NCT04545060 | Brazil, Canada, Spain, United States | Sotrovimab (0.5 g) | 291 | 583 | Adult Confirmed SARS-CoV-2 ≤5 days from onset symptoms >55 years or comorbidities Mild symptoms (WHO 2-3) Excluded Likely to require hospitalization ≤24 h Likely to die ≤7 days Severely immunocompromised Vaccinated or plan to be vaccinated within 4 weeks | Clinical—disease progression | Interim Stopped recruitment due to efficacy 1057 participants | No Recruited August 2020 to March 2021 | RR, 0.33 95% CI, 0.01-8.18 (low) | RR, 0.14 95% CI, 0.04-0.48 (low) |
| Placebo | 292 | |||||||||
| BLAZE-117 NCT04427501 | Puerto Rico, United States | Bamlanivimab (0.7 g) | 104 | 577 | Adult Confirmed SARS-CoV-2 Mild symptoms (WHO 2-3) Excluded Requires oxygen therapy Any serious concomitant systemic disease Pregnant or breastfeeding | Virological—viral clearance | Interim Planned recruitment 3160 | No Recruited June-September 2020 | No events (low) | RR, 0.17 95% CI, 0.02-1.33 (low) |
| Bamlanivimab (2.8 g) | 109 | RR, 0.32 95% CI, 0.07-1.47 (low) | ||||||||
| Bamlanivimab (7.0 g) | 104 | RR, 0.34 95% CI, 0.08-1.56 (low) | ||||||||
| Bamlanivimab/etesevimab (2.8 g + 2.8 g) | 114 | See updated results below | See updated results below | |||||||
| Placebo | 161 | — | — | |||||||
| BLAZE-118 NCT04427501 | United States | Bamlanivimab/etesevimab (2.8 g + 2.8 g) | 518 | 1035 | Adult Confirmed SARS-CoV-2 Mild symptoms (WHO 2 to 3) Excluded Requires oxygen therapy Any serious concomitant systemic disease Pregnant or breastfeeding | Clinical—COVID-19–related hospitalization (acute care for ≥24 hours) or death from any cause by day 29 | Interim Planned recruitment 3160 | No September- December 2020 | RR, 0.05 95% CI, 0.00-0.81 (low) | RR, 0.30 95% CI, 0.16-0.59 (low) |
| Placebo | 517 | |||||||||
| Eom et al‡19 NCT04602000 | Romania, South Korea, Spain, United States | Regdanvimab 0.04 g/kg | 101 | 327 | Adult Confirmed SARS-CoV-2 ≤7 days from onset symptoms Excluded Hospitalized | Clinical—time to clinical recovery Virological—viral clearance | Interim Planned recruitment 1172 | No Recruited October-December 2020 | No events (low) | RR, 0.45 95% CI, 0.14-1.42 (low) |
| Regdanvimab 0.08 g/kg | 103 | RR, 0.56 95% CI, 0.19-1.60 (low) | ||||||||
| Placebo | 103 | — | ||||||||
| Weinreich et al (phase 1/2)20 NCT04425629 | Chile, Mexico, Romania, United States | Casirivimab/imdevimab 2.4 g (1.2 g + 1.2 g) | 92 | 275 | Adult Confirmed SARS-CoV-2 ≤7 days from onset symptoms Excluded Hospitalized | Virological—viral clearance | Interim | No Recruited June- August 2020 | NR | RR, 0.43 95% CI, 0.08-2.19 (low) |
| Casirivimab/imdevimab 8 g (4 g + 4 g) | 90 | RR, 0.21 95% CI, 0.02-1.79 (low) | ||||||||
| Placebo | 93 | — | ||||||||
| Inpatients (moderate or severe disease) | ||||||||||
| RECOVERY‡21 NCT04381936 | United Kingdom | Casirivimab/imdevimab 8 g (4 g + 4 g) | 4839 | 9185 | Any age Suspected or confirmed COVID-19 Median 9 days symptom onset >90% requiring oxygen therapy (WHO score ≥5) | Clinical— all-cause mortality day 28 | Complete Subgroup— antibody negative | No Recruited September 2020 to May 2021 | RR, 0.94 95% CI, 0.87-1.02 (moderate) | NA |
| Standard care | 4946 | |||||||||
| ACTIV-322 NCT04501978 | Denmark, India, Poland, Singapore, Spain, Switzerland, United Kingdom, United States | Bamlanivimab (7 g) | 314 | Inpatients, moderate disease | Adult Confirmed SARS-CoV-2 ≤12 days from onset symptoms Excluded Requiring organ support Pregnant/breast feeding | Clinical—time to sustained recovery | Interim analysis, bamlanivimab arm stopped for futility, recruitment ongoing for other arms | No Recruited August to October 2020 | RR, 1.39 95% CI, 0.07-1.47 (low) | NA |
Was the type of virus (eg, alpha, beta, gamma, delta) detected at baseline taken into consideration as a subgroup analysis?
GRADE assessment—assesses certainty of the evidence. High certainty: very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect.
Pre–peer review.
NA, not applicable; NR, not reported; PCR, polymerase chain reaction; WHO, World Health Organization.
SARS-CoV-2 mAb vs placebo or standard care—all-cause mortality at 28 days or hospital discharge.
SARS-CoV-2 mAb vs placebo or standard care—all-cause mortality at 28 days or hospital discharge.
CLINICAL CASE (Continued)
The patient and his wife received the results of the polymerase chain reaction test the next day, and both had tested positive for SARS-CoV-2. In total, 15 of the 45 people who attended the christening tested positive for SARS-CoV-2 within the days following the christening. He started to develop symptoms of fever and a dry cough the day after his positive test, but his symptoms were mild, and he treated the fever with paracetamol and rested at home.
Treatment with asymptomatic infection or mild symptoms
Vaccination is known to decrease the risk of progression to moderate or severe COVID-19 disease, although it has less of an impact on the development of asymptomatic or mild symptoms. All of the current evidence for passive immune therapy is prior to vaccination, or patients who were vaccinated or about to be vaccinated were excluded from the trials. Data from Public Health England have shown that vaccination decreases the risk of hospitalization by over 90%, even with the delta variant. It is therefore more difficult to know the real additional effect of passive immune therapy over and above vaccination (Tables 3 and 4).
Completed randomized controlled trials of CP and hyperimmune globulin therapy
| Study . | Country . | Intervention(s) . | Randomized per arm . | Number analyzed . | Patient population . | Primary outcome . | Neutralizing antibody titer . | Viral variants considered in analyses* . |
|---|---|---|---|---|---|---|---|---|
| Prophylaxis | ||||||||
| No completed studies | ||||||||
| Outpatients (asymptomatic) | ||||||||
| No completed studies | ||||||||
| Outpatients (mild disease) | ||||||||
| Libster et al8 NCT04479163 | Argentina | 250 mL CP on day 1 | 80 | 160 | Age >74 or 65 to 74 and comorbidity Confirmed SARS-CoV-2 Mild illness, not requiring hospitalization ≤48 h from symptom onset | Development of severe disease—defined as RR ≥30 breaths/min or oxygen saturations <93% on air | Anti–S IgG SARS-CoV-2 (COVIDAR IgG) minimum titer 1:1000 | No Recruited June-October 2020 |
| Saline (Placebo) | 80 | |||||||
| Inpatients (moderate disease (WHO 4 or 5) | ||||||||
| Agarwal et al25 CTRI/2020/04/024775 | India | Two doses 200 mL CP, 24 h apart, preferably different donors | 235 | 464 | Adult Confirmed SARS-CoV-2 SpO2 ≤93% and RR >24/min or PaO2/FiO2 200-300 Excluded Critically ill (PaO2/FiO2 <200 or shock requiring vasopressors) | Composite all- cause mortality or progression to severe disease (PaO2/FiO2 <100) within day 28 | NAb not used to select plasma, tested at end of study—63% of donors had NAb titer >1:20 with median titer 1:40 | No Recruited April-July 2020 |
| Standard care | 229 | |||||||
| Simonovich et al26 NCT04383535 | Argentina | 10-15 mL/kg Mini-pools (5-10 donors) | 228 | 333 | Adult Confirmed SARS-CoV-2, requiring hospitalization, pneumonia, plus SpO2 <93% or PaO2/FiO2 <300 Excluded MV or NIV | Clinical status at day 30 ordinal categories 1: death 2: invasive ventilatory support 3: hospitalized with supplemental oxygen requirements 4: hospitalized without supplemental oxygen requirements 5: discharged without full return of baseline physical function 6: discharged with full return of baseline physical function | Anti–S IgG SARS-CoV-2 (COVIDAR IgG) Median titer of 1:3200 (IQR, 1:800 to 1:3200) | No Recruited May-August 2020 |
| Saline (placebo) | 105 | |||||||
| Avendaño-Solà et al†27 NCT04345523 | Spain | 250-300 mL CCP on day 1 | 38 | 81 | Adult Confirmed SARS-CoV-2 Radiological changes or clinical features plus SpO2 <94%, <12-day symptom onset Excluded MV, high-flow O2 | Proportion of patients in category 5, 6, or 7 of 7-category COVID-19 ordinal scale at day 15 | NAb not available to select plasma, all donation on subsequent testing had VMNT-ID50 > 1:80 | No Recruited April-July 2020 |
| Standard care | 43 | |||||||
| AlQahtani et al28 NCT04356534 | Bahrain | Two doses 200 mL CCP, 24 h apart | 20 | 40 | Age ≥21 Confirmed SARS-CoV-2 Pneumonia, plus SpO2 <92% or PaO2/FiO2 <300 Excluded MV or MOF | Requirement for ventilation (NIV or MV) | NR | No Recruited April-June 2020 |
| Standard care | 20 | |||||||
| Inpatients (moderate or severe disease—WHO 4 to 7) | ||||||||
| RECOVERY 202129 NCT04381936 | United Kingdom | Two doses 275 mL CCP, 24 h apart | 5795 | 11 558 | Any age Suspected or confirmed COVID-19 Median 9 days symptom onset >90% requiring oxygen therapy (WHO score ≥5) | Clinical—all-cause mortality day 28 | Minimum Euroimmun 6 | Partially Used surrogate of randomized before/after December 2020 for WT/alpha variant Recruited May 2020 to January 2021 |
| Standard care | 5763 | |||||||
| CONCOR-1†30 NCT04348656 | Brazil, Canada, United States | 500 mL CCP | 614 | 921 | Adult Confirmed SARS-CoV-2 Requiring oxygen therapy (WHO score ≥5) Excluded Symptoms >12 days MV | Intubation or death by day 30 | Viral neutralizing antibodies titer >1:160 or antibodies against the RBD of the SARS-CoV-2 spike protein titer >1:100 | No Recruited May 2020 to January 2021 |
| Standard care | 307 | |||||||
| O'Donnell et al31 NCT04359810 | Brazil, United States | 200-250 mL CCP | 150 | 223 | Adult Confirmed SARS-CoV-2 Requiring oxygen therapy (WHO score ≥5) | Clinical status at day 28 following randomization (7-point ordinal scale based on WHO) | Anti–SARS-CoV-2 antispike IgG antibody titer ≥1:400 | No Recruited April-November 2020 |
| 200-250 mL nonimmune plasma | 73 | |||||||
| CAPSID†32 NCT04433910 | Germany | CCP (250-325 mL) on days 1, 3, and 5 | 53 | 105 | Age 18-75 years Confirmed SARS-CoV-2 RR >30 or requiring oxygen therapy (WHO score ≥5) | Composite end point of survival and no longer fulfilling criteria of severe COVID-19 (day 21) | Median PRNT50 neutralization titer 1:160 (IQR, 1:80 to 1:320) | No Recruited August-December 2020 |
| Standard care, crossover to CCP at day 14 if not improved | 52 | |||||||
| Gharbharan et al33 NCT04342182 | The Netherlands | 300 mL CCP on day 1 | 43 | 86 | Adult Confirmed SARS-CoV-2 within 96 h Excluded Patients on MV >96 h | Mortality until discharge or maximum of 60 days | Minimum of PRNT50 titer of ≥1:80 | No Recruited April-June 2020 |
| Standard care | 43 | |||||||
| Ray et al†34 CTRI/2020/05/025209 | India | Two doses 200 mL CCP, 24 h apart | 40 | 80 | Adult Confirmed SARS-CoV-2 RR >30, SpO2 <90%, PaO2/FiO2 <300 Excluded pregnant, MV | All-cause mortality at 30 days | Euroimmun ≥1.5 | |
| Standard care | 40 | |||||||
| Bennett-Guerrero et al35 NCT04344535 | United States | 480 mL (2 units) CCP | 59 | 74 | Adult Confirmed SARS-CoV-2 Excluded Pregnant/breastfeeding | Number of days patient remained ventilator free (up to 28 days) | Ideally >1:320, but meeting minimum titer per FDA | No Recruited April-August 2020 |
| 480 mL (2 units) nonimmune plasma | 15 | |||||||
| Pouladzadeh et al36 IRCT20200310046736N1 | Iran | 500 mL CCP | 30 | 60 | Confirmed SARS-CoV-2 WHO score >4 Excluded Pregnant/breastfeeding Comorbidities (eg, heart, liver, kidney disease) Smokers | Improvement in the levels of cytokine storm indices | NR | No Recruited March-May 2020 |
| Standard care | 30 | |||||||
| Hamdy Salman and Ail Mohamed37 NCT04530370 | Egypt | 250 mL CP on day 1 | 15 | 30 | Adult Confirmed SARS-CoV-2 2 or more of RR ≥24, SpO2 ≤93%, PaO2/FiO2 <300, pulmonary infiltrates Excluded MOF, septic shock | At least 50% improvement of the severity of illness at any time during 5-day study period | NAb not used to select plasma | No Recruited June-August 2020 |
| Saline (placebo) | 15 | |||||||
| Bajpai et al†38 NCT04346446 | India | Two doses 250 mL CCP, 24 h apart | 14 | 29 | Age 18-65 years Confirmed SARS-CoV-2 Pneumonia, plus SpO2 <93% or PaO2/FiO2 <300 Excluded Comorbidities (kidney, heart or liver disease, COPD) | Proportion of patients remaining free of mechanical ventilation day 7 | Variable | No Recruited April-May 2020 |
| Nonimmune plasma | 15 | |||||||
| Inpatients (severe disease—WHO 6 to 7) | ||||||||
| REMAP-CAP†39 NCT02735707 | Australia, Canada, United Kingdom, United States | Two doses 275 mL CCP, 24 h apart | 1084 | 2000 | Adult Confirmed SARS-CoV-2 ≤48 h since admission to ICU | Organ support–free days at day 21 | Minimum Euroimmun 6 | No Recruited May 2020 to January 2021 |
| Standard care | 916 | |||||||
| Gonzalez et al†40 NCT04381858 | Mexico | Two doses 200 mL CCP, 24 h apart | 130 | 90 | Adult (16-90 years) Suspected or confirmed COVID-19 Severe respiratory failure | Duration of hospitalization All-cause mortality day 28 | NAb not used to select plasma | No Recruited May-October 2020 |
| IVIg 0.3 g/kg daily for 5 days | 60 | |||||||
| Li et al41 ChiCTR2000029757 | China | 4-13 mL/kg of CP | 52 | 103 | Confirmed SARS-CoV-2 Excluded high-titer S-RBD–specific IgG (≥1:640) | Time to clinical improvement (patient discharge or reduction 2 points on 6-point disease severity scale) | Minimum of S-RBD–specific IgG of 1:640 (approximately equivalent to NAb of 1:40) | No Recruited February-April 2020 |
| Standard care | 51 | |||||||
| Study . | Country . | Intervention(s) . | Randomized per arm . | Number analyzed . | Patient population . | Primary outcome . | Neutralizing antibody titer . | Viral variants considered in analyses* . |
|---|---|---|---|---|---|---|---|---|
| Prophylaxis | ||||||||
| No completed studies | ||||||||
| Outpatients (asymptomatic) | ||||||||
| No completed studies | ||||||||
| Outpatients (mild disease) | ||||||||
| Libster et al8 NCT04479163 | Argentina | 250 mL CP on day 1 | 80 | 160 | Age >74 or 65 to 74 and comorbidity Confirmed SARS-CoV-2 Mild illness, not requiring hospitalization ≤48 h from symptom onset | Development of severe disease—defined as RR ≥30 breaths/min or oxygen saturations <93% on air | Anti–S IgG SARS-CoV-2 (COVIDAR IgG) minimum titer 1:1000 | No Recruited June-October 2020 |
| Saline (Placebo) | 80 | |||||||
| Inpatients (moderate disease (WHO 4 or 5) | ||||||||
| Agarwal et al25 CTRI/2020/04/024775 | India | Two doses 200 mL CP, 24 h apart, preferably different donors | 235 | 464 | Adult Confirmed SARS-CoV-2 SpO2 ≤93% and RR >24/min or PaO2/FiO2 200-300 Excluded Critically ill (PaO2/FiO2 <200 or shock requiring vasopressors) | Composite all- cause mortality or progression to severe disease (PaO2/FiO2 <100) within day 28 | NAb not used to select plasma, tested at end of study—63% of donors had NAb titer >1:20 with median titer 1:40 | No Recruited April-July 2020 |
| Standard care | 229 | |||||||
| Simonovich et al26 NCT04383535 | Argentina | 10-15 mL/kg Mini-pools (5-10 donors) | 228 | 333 | Adult Confirmed SARS-CoV-2, requiring hospitalization, pneumonia, plus SpO2 <93% or PaO2/FiO2 <300 Excluded MV or NIV | Clinical status at day 30 ordinal categories 1: death 2: invasive ventilatory support 3: hospitalized with supplemental oxygen requirements 4: hospitalized without supplemental oxygen requirements 5: discharged without full return of baseline physical function 6: discharged with full return of baseline physical function | Anti–S IgG SARS-CoV-2 (COVIDAR IgG) Median titer of 1:3200 (IQR, 1:800 to 1:3200) | No Recruited May-August 2020 |
| Saline (placebo) | 105 | |||||||
| Avendaño-Solà et al†27 NCT04345523 | Spain | 250-300 mL CCP on day 1 | 38 | 81 | Adult Confirmed SARS-CoV-2 Radiological changes or clinical features plus SpO2 <94%, <12-day symptom onset Excluded MV, high-flow O2 | Proportion of patients in category 5, 6, or 7 of 7-category COVID-19 ordinal scale at day 15 | NAb not available to select plasma, all donation on subsequent testing had VMNT-ID50 > 1:80 | No Recruited April-July 2020 |
| Standard care | 43 | |||||||
| AlQahtani et al28 NCT04356534 | Bahrain | Two doses 200 mL CCP, 24 h apart | 20 | 40 | Age ≥21 Confirmed SARS-CoV-2 Pneumonia, plus SpO2 <92% or PaO2/FiO2 <300 Excluded MV or MOF | Requirement for ventilation (NIV or MV) | NR | No Recruited April-June 2020 |
| Standard care | 20 | |||||||
| Inpatients (moderate or severe disease—WHO 4 to 7) | ||||||||
| RECOVERY 202129 NCT04381936 | United Kingdom | Two doses 275 mL CCP, 24 h apart | 5795 | 11 558 | Any age Suspected or confirmed COVID-19 Median 9 days symptom onset >90% requiring oxygen therapy (WHO score ≥5) | Clinical—all-cause mortality day 28 | Minimum Euroimmun 6 | Partially Used surrogate of randomized before/after December 2020 for WT/alpha variant Recruited May 2020 to January 2021 |
| Standard care | 5763 | |||||||
| CONCOR-1†30 NCT04348656 | Brazil, Canada, United States | 500 mL CCP | 614 | 921 | Adult Confirmed SARS-CoV-2 Requiring oxygen therapy (WHO score ≥5) Excluded Symptoms >12 days MV | Intubation or death by day 30 | Viral neutralizing antibodies titer >1:160 or antibodies against the RBD of the SARS-CoV-2 spike protein titer >1:100 | No Recruited May 2020 to January 2021 |
| Standard care | 307 | |||||||
| O'Donnell et al31 NCT04359810 | Brazil, United States | 200-250 mL CCP | 150 | 223 | Adult Confirmed SARS-CoV-2 Requiring oxygen therapy (WHO score ≥5) | Clinical status at day 28 following randomization (7-point ordinal scale based on WHO) | Anti–SARS-CoV-2 antispike IgG antibody titer ≥1:400 | No Recruited April-November 2020 |
| 200-250 mL nonimmune plasma | 73 | |||||||
| CAPSID†32 NCT04433910 | Germany | CCP (250-325 mL) on days 1, 3, and 5 | 53 | 105 | Age 18-75 years Confirmed SARS-CoV-2 RR >30 or requiring oxygen therapy (WHO score ≥5) | Composite end point of survival and no longer fulfilling criteria of severe COVID-19 (day 21) | Median PRNT50 neutralization titer 1:160 (IQR, 1:80 to 1:320) | No Recruited August-December 2020 |
| Standard care, crossover to CCP at day 14 if not improved | 52 | |||||||
| Gharbharan et al33 NCT04342182 | The Netherlands | 300 mL CCP on day 1 | 43 | 86 | Adult Confirmed SARS-CoV-2 within 96 h Excluded Patients on MV >96 h | Mortality until discharge or maximum of 60 days | Minimum of PRNT50 titer of ≥1:80 | No Recruited April-June 2020 |
| Standard care | 43 | |||||||
| Ray et al†34 CTRI/2020/05/025209 | India | Two doses 200 mL CCP, 24 h apart | 40 | 80 | Adult Confirmed SARS-CoV-2 RR >30, SpO2 <90%, PaO2/FiO2 <300 Excluded pregnant, MV | All-cause mortality at 30 days | Euroimmun ≥1.5 | |
| Standard care | 40 | |||||||
| Bennett-Guerrero et al35 NCT04344535 | United States | 480 mL (2 units) CCP | 59 | 74 | Adult Confirmed SARS-CoV-2 Excluded Pregnant/breastfeeding | Number of days patient remained ventilator free (up to 28 days) | Ideally >1:320, but meeting minimum titer per FDA | No Recruited April-August 2020 |
| 480 mL (2 units) nonimmune plasma | 15 | |||||||
| Pouladzadeh et al36 IRCT20200310046736N1 | Iran | 500 mL CCP | 30 | 60 | Confirmed SARS-CoV-2 WHO score >4 Excluded Pregnant/breastfeeding Comorbidities (eg, heart, liver, kidney disease) Smokers | Improvement in the levels of cytokine storm indices | NR | No Recruited March-May 2020 |
| Standard care | 30 | |||||||
| Hamdy Salman and Ail Mohamed37 NCT04530370 | Egypt | 250 mL CP on day 1 | 15 | 30 | Adult Confirmed SARS-CoV-2 2 or more of RR ≥24, SpO2 ≤93%, PaO2/FiO2 <300, pulmonary infiltrates Excluded MOF, septic shock | At least 50% improvement of the severity of illness at any time during 5-day study period | NAb not used to select plasma | No Recruited June-August 2020 |
| Saline (placebo) | 15 | |||||||
| Bajpai et al†38 NCT04346446 | India | Two doses 250 mL CCP, 24 h apart | 14 | 29 | Age 18-65 years Confirmed SARS-CoV-2 Pneumonia, plus SpO2 <93% or PaO2/FiO2 <300 Excluded Comorbidities (kidney, heart or liver disease, COPD) | Proportion of patients remaining free of mechanical ventilation day 7 | Variable | No Recruited April-May 2020 |
| Nonimmune plasma | 15 | |||||||
| Inpatients (severe disease—WHO 6 to 7) | ||||||||
| REMAP-CAP†39 NCT02735707 | Australia, Canada, United Kingdom, United States | Two doses 275 mL CCP, 24 h apart | 1084 | 2000 | Adult Confirmed SARS-CoV-2 ≤48 h since admission to ICU | Organ support–free days at day 21 | Minimum Euroimmun 6 | No Recruited May 2020 to January 2021 |
| Standard care | 916 | |||||||
| Gonzalez et al†40 NCT04381858 | Mexico | Two doses 200 mL CCP, 24 h apart | 130 | 90 | Adult (16-90 years) Suspected or confirmed COVID-19 Severe respiratory failure | Duration of hospitalization All-cause mortality day 28 | NAb not used to select plasma | No Recruited May-October 2020 |
| IVIg 0.3 g/kg daily for 5 days | 60 | |||||||
| Li et al41 ChiCTR2000029757 | China | 4-13 mL/kg of CP | 52 | 103 | Confirmed SARS-CoV-2 Excluded high-titer S-RBD–specific IgG (≥1:640) | Time to clinical improvement (patient discharge or reduction 2 points on 6-point disease severity scale) | Minimum of S-RBD–specific IgG of 1:640 (approximately equivalent to NAb of 1:40) | No Recruited February-April 2020 |
| Standard care | 51 | |||||||
Was the type of virus (e.g., alpha, beta, gamma, delta, etc.) detected at baseline taken into consideration as a subgroup analysis?
Pre–peer review.
CCP, COVID-19 convalescent plasma; COPD, chronic obstructive pulmonary disease; FDA, Food and Drug Administration; FiO2, fraction of inspired oxygen; ICU, intensive care unit; IQR, interquartile range; IVIg, intravenous immunoglobulin; MOF, multiorgan failure; MV, mechanical ventilation; NAb, neutralizing antibody; NIV, non-invasive ventilation; PaO2, partial pressure of oxygen; PRNT50, 50% reduction in plaque count using the plaque reduction neutralization test; RR, respiratory rate; SpO2, oxygen saturation; VMNT, virus microneutralization test; WT, wild type.
In the studies that assessed bamlanivimab alone or regdanvimab, there were no deaths in any of the study arms; therefore, any effect on all-cause mortality cannot be assessed.42 The only study that showed a reduction in mortality was the BLAZE-1 trial17 arm that used the bamlanivimab/etesevimab monoclonal cocktail (Figure 1); the casirivimab/imdevimab cocktail trial showed a trend in the direction of effect, but it was not clinically significant,15,20 nor was the effect of high-dose CP (Figure 2).3,8 Therefore, although it is suggestive that passive immune therapy if given early could save lives and the reduce risk of severe disease, additional data are required before it can be used in routine practice. None of the studies have performed a cost-effectiveness analysis, but it is likely that those patients who do not respond to vaccination and are at high risk of severe or fatal COVID-19 are the group that will demonstrate a benefit.
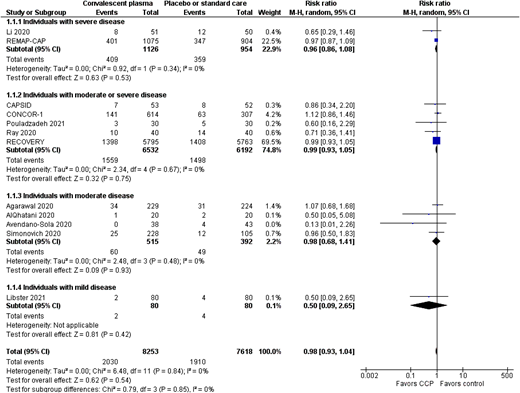
CP vs placebo or standard care—all-cause mortality at 28 days or hospital discharge.
CP vs placebo or standard care—all-cause mortality at 28 days or hospital discharge.
CLINICAL CASE (Continued)
Seven days after the christening, he developed increasing shortness of breath and called an ambulance. On examination by the paramedics, he was pyrexial (temperature 38.9°C), had a respiratory rate of 25 breaths per minute, and was hypoxic with an oxygen saturation of 91% on room air. He was given oxygen by the paramedics and taken to the local emergency room. In the emergency room, he was given steroids and admitted to the hospital for oxygen therapy.
Treatment with moderate or severe symptoms
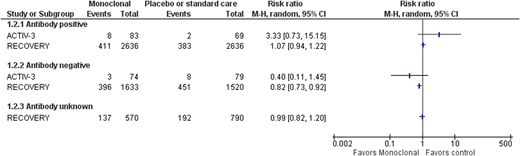
Two trials have assessed monoclonal antibody use in moderately or severely unwell patients; neither showed a benefit for patients overall (Figure 1), but there was a benefit for patients who had not yet developed a detectable antibody response (Figure 3). Those patients who did not develop an antibody response had a much higher mortality rate than those who did not.19,21 This is partly because patients who have a delayed antibody response are older and have more comorbidities. This does mean that reducing mortality in this group will mean a larger absolute reduction in mortality; for example, based on the RECOVERY data, 54 lives per 1000 patients treated would be saved (95% CI, 24-80 lives saved per 1000 patients treated). Some SARS-CoV-2 variants are resistant to bamlanivimab monotherapy, whereas the current major variants in circulation are still sensitive to neutralization by the casirivimab/imdevimab cocktail. However, this may change with the development of new variants.7 As variant screening cannot be done in real time, passive antibody treatments need to be used that are effective against all current variants in a particular region of the world. CP use does not show a benefit overall for patients with moderate or severe COVID-19.29,30,39 CP has also been assessed in antibody-negative patients in 2 major trials, RECOVERY and REMAP-CAP.29,39 This shows a similar trend in the direction of a possible effect in CP, but it does not reach statistical significance (risk ratio [RR], 0.93; 95% CI, 0.86-1.01). This may partly be because CP is a much more variable product, with some units having much lower antibody levels than others, so even if a minimum titer is used within the trials, this may not have been sufficient. Several trials have shown an effect in a subgroup of participants who received a higher titer product.8,32
SARS-CoV-2 mAb vs placebo or standard care—all-cause mortality at 28 days or hospital discharge by antibody status at baseline.
SARS-CoV-2 mAb vs placebo or standard care—all-cause mortality at 28 days or hospital discharge by antibody status at baseline.
CLINICAL CASE (Continued)
Over the next 2 days, the 74-year-old man continued to deteriorate and was admitted to intensive care for noninvasive ventilatory support. An emergency use access request for CP was made, and he received CP on day 10 after the christening.
Treatment of critically unwell participants
Fewer trials have specifically assessed interventions for the critically unwell patients (requiring respiratory or cardiovascular organ support) with an intensive care level of care (Tables 1 and 2). Several trials exclude patients requiring mechanical ventilation or organ support of any type. One of the trials that has focused on this patient group is the REMAP-CAP trial.39 It showed no benefit of CP overall (Figure 2), but a prespecified subgroup showed potential benefit of CP. This trial, based on Bayesian statistics, showed an 89.9% posterior probability of benefit in this subgroup. This was a broad group of immunosuppressed patients based on the Acute Physiology and Chronic Health Evaluation (APACHE) score definition of immunosuppression.39 However, as it was a small subgroup, additional research is required to confirm whether or not this is a true finding. The RECOVERY trial did include patients receiving noninvasive and invasive ventilation, and no evidence of a difference was seen for those patients receiving invasive (RR, 0.71; 95% CI, 0.35-1.47; 70 participants) or noninvasive ventilation (RR, 0.86; 95% CI, 0.68-1.08; 673 participants), but the CIs are wide.
CLINICAL CASE (Continued)
Unfortunately, the 74-year-old man continued to deteriorate and subsequently required invasive ventilation. Fourteen days after the christening, he died due to COVID-19. In total, 5 of the guests at the christening were admitted to hospital, including all 4 of the grandparents of the baby and the uncle (the index case), who had diabetes and hypertension. Both grandfathers died of COVID-19.
Key points (see Visual abstract)
Up to now (July 2021), most of the evidence is based on trials performed prior to the introduction of vaccination. Vaccination is likely to be the most cost-effective strategy for preventing infection in the general population, including healthy individuals with high-risk exposure (eg, health care workers). There are insufficient data on the outcomes of prophylactic passive antibody therapy in immunosuppressed individuals.
Passive immune therapy (monoclonal therapy and CP) may be beneficial for high-risk patients who have mild COVID-19 symptoms, but more data are required. Some countries, including the United States, are using passive monoclonal therapy for this indication under EUA.
High-dose passive immune therapy (casirivimab/imdevimab) reduces all-cause mortality for hospitalized patients who have not yet developed a detectable antibody response (SARS-CoV-2 IgG antibody). This monoclonal cocktail has just been approved by the Medicines and Healthcare products Regulatory Agency based on this evidence, but indications for its use are currently not available.
CP may be beneficial for immunosuppressed individuals who are severely or critically unwell, but more data are required.
The virus continues to change, and so although treatments may be effective against current SARS-CoV-2 viral variants of concern, this may not be true in the future. Passive immune therapies will either have to be very broad spectrum or adapt with the virus.
Conflict-of-interest disclosure
Lise J. Estcourt: author on Cochrane living systematic reviews of monoclonal therapies and CP. Investigator on the RECOVERY and REMAP-CAP trials.
Off-label drug use
Lise J. Estcourt: nothing to declare.