Abstract
Platelet endothelial cell adhesion molecule-1 (PECAM-1/CD31) is an immunoglobulin–immunoreceptor tyrosine-based inhibitory motif (Ig-ITIM) superfamily member that recruits and activates protein-tyrosine phosphatases, SHP-1 and SHP-2, through its intrinsic ITIMs. PECAM-1–deficient (PECAM-1−/− ) mice exhibit a hyperresponsive B-cell phenotype, increased numbers of B-1 cells, reduced B-2 cells, and develop autoantibodies. In the periphery, there are reduced mature recirculating B-2 cells and increased B-1a cells within the peritoneal cavity. In addition, PECAM-1−/− B cells display hyperproliferative responses to lipopolysaccharide and anti-IgM stimulation and showed enhanced kinetics in their intracellular Ca++ response following IgM cross-linking. PECAM-1−/− mice showed increased serum levels of IgM with elevated IgG isotypes and IgA antidinitrophenol antibody in response to the T-independent antigen, dinitrophenol-Ficoll. Finally, PECAM-1−/− mice developed antinuclear antibodies and lupuslike autoimmune disease with age.
Introduction
The development and maintenance of a diverse B-cell repertoire is governed by signals through the B-cell receptor (BCR) antigen complex, modulated by regulatory B-cell coreceptors. This process is dependent on both the strength and duration of signaling thresholds transduced by the BCR complex on B cells. Developing and activated B cells undergo selective mechanisms during positive and negative selection, maturation, and activation that determine their survival and functional capacity. Upon exposure to self-antigen, B cells can be physically eliminated by deletion in the bone marrow, undergo self-editing, be functionally impaired by anergy, or become activated leading to autoimmunity.1 Several factors influence the fate of developing and activated B cells, including their response to self-antigen, the microenvironment, and mechanisms of B-cell coreceptor ligation.
Platelet endothelial cell adhesion molecule-1 (PECAM-1/CD31) is a 130-kd glycoprotein and a member of the immunoglobulin (Ig) superfamily. In human beings, it is expressed at the lateral junctions of endothelial cells and on the surface of hematopoietic cells, including monocytes, neutrophils, natural killer cells, platelets, and naive T and B cells.2 There is emerging evidence to suggest that PECAM-1 may be an important regulator of antigen-induced cell activation in the context of lymphocytes. Inhibitory effects have been observed in response to PECAM-1 cross-linking. Specifically, induction of immune stimulation of the T-cell antigen receptor (TCR) complex on Jurkat T cells leads to tyrosine phosphorylation of PECAM-1 and recruitment of protein-tyrosine phosphatases (PTPs) SHP-2 and possibly SHP-1.3 In addition, coligation of the TCR complex with immunoreceptor tyrosine-based inhibitory motif (ITIM)–bearing PECAM-1 on Jurkat T cells leads to a transient block in calcium mobilization.4 At present, there has been little information available on the role of PECAM-1 in B-cell physiology. In human beings, PECAM-1 is expressed on naive follicular mantle zone B cells and plasma cells but not on germinal center–specific memory B cells.5 Signaling studies of PECAM-1 have suggested that PECAM-1 may function as an inhibitory receptor in vivo. Its cytoplasmic domain contains 2 ITIMs that upon phosphorylation of the putative tyrosine residues recruit and activate PTPs SHP-1 and SHP-2 under physiologically relevant conditions.6-8 Thus, PECAM-1 might negatively regulate BCR signaling by recruiting SHP-1 and/or SHP-2. Consistent with this hypothesis, functional analysis of chimeric receptors containing the extracellular and transmembrane domains of FcγRIIB1 and the cytoplasmic domain of PECAM-1 in the context of genetically modified chicken DT40 B cells revealed that the PECAM-1 cytoplasmic domain was capable of delivering an inhibitory signal that is dependent upon intact ITIMs and PTPs SHP-2 and SHP-1.9,10 These inhibitory effects were observed upon coligation of BCR with inhibitory receptor with negative regulation of BCR-mediated calcium mobilization responses and NF-AT (nuclear factor of activated T cells) transcriptional activation in DT40 B cells.9 Taken together, a number of in vitro functional and biochemical studies indicate that ligation of PECAM-1 leads to effects upon B-cell signaling.
The important regulatory role of B-cell coreceptors has been demonstrated by targeted disruption of coinhibitory receptor genes in mice, where the absence of an inhibitory receptor leads to uncoupling of signaling pathways responsible for control of B-cell tolerance and activation.11-14 Immunologic defects observed include alterations in B-cell development pathways, B-lymphocyte antigen receptor signal transduction and, in some cases, predisposition to development of high-affinity autoantibodies and spontaneous autoimmune disease. Because the primary function of PECAM-1 was originally thought to be in cell adhesion, preliminary characterization of PECAM-1–deficient mice focused on a functional evaluation of PECAM-1 in the context of inflammatory adhesion-dependent cascades and not in the context of an inhibitory coreceptor in immune cells.15 To elucidate the function of PECAM-1 during B-cell development and activation in vivo, we initially examined in vitro and in vivo functional B-cell responses of B6 PECAM-1–deficient mice compared with B6 wild-type PECAM-1+/+ mice. Subsequently, we examined whether B6 PECAM-1–deficient mice showed any evidence of spontaneous autoimmune disease with age.
Materials and methods
Mice
The construction of PECAM-1–deficient mice has been previously described.15 These mice were housed in a pathogen-free facility at the University of Adelaide Animal House Facility. PECAM-1–deficient mice were further backcrossed onto C57/BL6 background for 6 generations. Confirmation of the homozygous phenotype of these PECAM-1–deficient mice was determined by PECAM-1 staining of splenic lymphocytes.
Flow cytometric analysis
Single-cell suspensions from bone marrow, spleen, peritoneal cavity, and lymph nodes were single or double stained with antibodies directed against a range of murine lymphocyte surface antigens, including CD43 (S7), CD23 (B3B4), CD45R (B220, RA3-6B2), CD5 (B3B4) (all from PharMingen, San Diego, CA); CD3 (KT3) (Immunotech, Marseille, France); IgM (Jackson ImmunoResearch Laboratories, West Grove, PA); IgD (a gift from Professor Kensuke Miyake, Saga Medical School, Saga, Japan); and rat anti-mouse PECAM-1 (390, a gift from Dr Steve Albelda, University of Pennsylvania School of Medicine, Philadelphia). Antibodies were biotinylated and detected with streptavidin–R-phycoerythrin (R-PE) (Southern Biotechnology Associates, Birmingham, AL), directly labeled with fluorescein isothiocyanate (FITC) or R-PE, or detected with the labeled secondary antibodies, antigoat FITC (Santa Cruz Biotechnology, Santa Cruz, CA) and antirat R-PE (Southern Biotechnology Associates). Analysis was restricted to lymphocyte populations as defined by forward- and side-scatter characteristics. Data were collected on an EPICS XL-MCL (Beckman-Coulter, Fullerton, CA) and analyzed using WinMDI version 2.8 software (Joseph Trotter, http://facs.scripps.edu/software.html).
Cell proliferation assay
Splenocytes from wild-type and PECAM-1−/−6-week-old mice were depleted of T cells by incubating with Thy1.2 monoclonal antibodies and Low-Tox M rabbit complement (Cedarlane, Hornby, ON) for 1 hour at 37°C. Resting B cells were separated from nonlymphoid cells, dead T cells, and red blood cells by a discontinuous 50%/75% Percoll gradient (Amersham Pharmacia Biotech, Castle Hill, Australia). A total of 105 purified B cells (> 98% B220+) were plated into 96-well flat-bottom trays in complete RPMI 160 media containing 10% fetal calf serum. B cells were stimulated in triplicate with indicated concentrations of lipopolysaccharide (LPS) (Sigma Chemical, St Louis, MO), affinity-purified F(ab′)2 goat antimouse IgM (Jackson ImmunoResearch Laboratories), affinity-purified whole Ig goat antimouse IgM (Jackson ImmunoResearch Laboratories), and hampster antimouse CD40 (clone HM40-3) (PharMingen) with and without murine recombinant interleukin-4 (IL-4) (Peprotech, Rocky Hill, NJ) for 72 hours. Proliferation was assessed by the incorporation of a cell titer 96-assay reagent (Promega, Madison, WI) added during the last 2 hours of culture, followed by measuring OD values at 490 nm.
Apoptosis assays
To determine apoptosis levels in wild-type and PECAM-1−/− B cells, 5 × 105 purified B cells were cultured in complete RPMI in the presence or absence of 50 μg/mL whole anti-IgM. At times 0 and 17 hours, cells were washed twice in phosphate-buffered saline (PBS) and stained at 0°C with propidium iodide and FITC-conjugated annexin V (Immunotech) as per the manufacturer's instructions and analyzed on an EPICS XL-MLC flow cytometer.
Differential CFSE labeling to compare survival of PECAM-1+/+ and PECAM-1−/− lymphocytes after transfer
Splenic lymphocytes were isolated from 3 8-week-old B6 PECAM-1+/+ and B6 PECAM-1−/− mice using Percoll gradient separation as described above. Lymphocytes from PECAM-1+/+ and PECAM-1−/− mice were labeled with different intensities of the fluorescein-based vital dye 5 and 6 carboxy fluorescein diacetate succinimidylester (CFSE) using previously described methodology.16 This allows 2 different cell populations to be tracked independently and their survival in the recipient animals to be directly compared. Briefly, cells were suspended at 5 × 107 mL in PBS/0.1% bovine serum albumin (BSA), and CFSE (Molecular Probes, Eugene, OR) from a 5 mM stock dissolved in dimethyl sulfoxide was added to give a final concentration of 10 μM and 2.5 μM for the PECAM-1+/+and PECAM-1−/− lymphocytes, respectively, to give “fully” and a “quarter” labeled populations, respectively. After 10 minutes' incubation at 37°C, cells were washed in ice-cold PBS/0.1% BSA 3 times to remove unbound CFSE. Equal numbers of “fully” labeled PECAM-1+/+ and “quarter” labeled PECAM-1−/− lymphocytes were mixed, and a total of 3 × 107 per mouse was injected via the lateral tail vein of PECAM-1+/+ recipients. At days 7 and 30, 3 mice were killed, spleens removed, and splenic lymphocytes isolated. Single-cell suspensions were stained with antibodies directed against biotinylated CD5 (Ly-1) (T-cell marker) (PharMingen) and PECAM-1 (390, a gift from Dr Steve Albelda), with streptavidin-PE as a secondary label, and with CD45R-PE (B220) (B-cell marker) (PharMingen). Analysis was restricted to lymphocyte populations as defined by forward- and side-scatter characteristics. Data were collected on an EPICS XL-MCL and analyzed using WinMDI version 2.8 software. These data allowed the relative survival of PECAM-1−/− versus PECAM-1+/+ T and B lymphocytes to be compared by direct ratio. Because this approach internally controls for variation in injection volume, it is more accurate than injecting individual populations of cells into separate recipients.
Calcium mobilization assays
Wild-type and PECAM-1−/− purified splenic B cells (5 × 106/mL) were loaded with 3 μM Fura-2/AM (Molecular Probes) in medium at room temperature for 45 minutes. Cells were washed twice with PBS and resuspended in the same cell concentration in PBS supplemented with 1 mM CaCl2 and 1 mM MgCl2. A total of 2.0 mL of cell suspension was added to a cuvette with a small stirrer. Calcium mobilization profiles were recorded at 510-nm emission wavelength excited by 340 nm and 380 nm using a Luminescence Spectrophotometer LS-50B (Perkin Elmer, Bucks, England). After establishing a baseline, 1 to 10 μg/mL affinity-purified F(ab′)2 goat antimouse IgM (Jackson ImmunoResearch Laboratories) (cross-linking BCR alone) was added to Fura-2–loaded murine PECAM-1+/+ and PECAM-1−/− splenic B cells. Cumulative calcium mobilization was evaluated by integration for 4 minutes of calcium mobilization over the baseline given before stimulation.
Immunizations and serum immunoglobulin assays
Eight-week-old wild-type and PECAM-1−/− mice were bled for isolation of serum for Ig determination. These mice were then immunized intraperitoneally with either 100 μg alum-precipitated dinitrophenol–keyhole limpet hemocyanin (DNP-KLH) (Calbiochem-Novabiochem, San Diego, CA) or 20 μg alum-precipitated DNP-Ficoll (Biosearch Technologies, Novato, CA). Mice were bled at days 7, 14, and 21 after primary immunization. At day 21, mice were rechallenged with DNP-KLH and bled at day 35 to assess the secondary response. Sera were collected, and the anti-DNP antibodies were determined by DNP- and isotype-specific enzyme-linked immunosorbent assay (ELISA). In DNP-KLH experiments, sera isolated from PECAM-1+/+ and PECAM-1−/− mice at days 7, 14, and 21 were diluted from 1:1000, while at day 35 sera were diluted from 1:10 000. Briefly, Nunc Maxisorb ELISA plates were coated with DNP-BSA (5 μg/mL) (Sigma Chemical), blocked with 1% skim milk and 0.05% Tween 20, and incubated with sera serially diluted in PBS–Tween 20 plus 0.5% skim milk for 1 hour at 37°C. Bound antibody was detected with secondary horseradish peroxidase–conjugated rabbit antimouse isotype-specific antisera (Zymed Laboratories, San Francisco, CA) using 3,3′,5,5′-tetramyl benzidine (TMB) as a substrate. Isotype-specific Ig levels in unimmunized animals were determined using a mouse MonoAB ID kit from Zymed Laboratories.
Assays for autoantibodies
Sera were collected monthly for 16 months from wild-type and PECAM-1−/− mice and assayed for the presence of antinuclear antibodies (ANAs). Briefly, sera were diluted from an initial 1:50 dilution in PBS and applied to HEp-2000 slides (Immunoconcepts, Sacramento, CA) for 30 minutes at room temperature. Following 2 15-minute washes in PBS, bound antibodies were detected with fluorescein-conjugated antimouse IgG (Fab′2) 1:100 (Silenus Laboratories, Hawthorn, Australia) for 30 minutes and examined using an immunofluorescence microscope. Anti–double-stranded DNA (dsDNA) autoantibodies were determined using a commercial ELISA assay (Alpha Diagnostic International, San Antonio, TX) as per the manufacturer's instructions. Based upon the manufacturer's recommendations, a definitive positive ELISA reading was defined as an OD 450 nm reading of 0.2 above the negative control. Therefore, any ELISA readings above 0.48 were considered positive.
Pathology
Kidney, lungs, foot joints, and other organs were removed from aged PECAM-1+/+ (n = 8) and PECAM-1−/−(n = 8) mice and then were fixed in 10% formalin in PBS and embedded in paraffin. Sections were then cut and stained for hematoxylin and eosin and periodic acid–Schiff (PAS) reaction. Kidney portions were also embedded in Tissue-Tek OCT compound: (Sakura Finetek USA Inc., Torrance, CA) and snap-frozen. Frozen sections (4 μm) were cut and air-dried, fixed in cold acetone for 30 minutes, blocked in 10% normal sheep serum, washed in PBS, and stained with fluorescein-conjugated sheep antimouse IgG (Fab′2) 1:100 dilution (Silenus Laboratories). Proteinuria was detected in long-term PECAM-1+/+ (n = 8) and PECAM-1−/− mice (n = 8) using Multistix 9 Reagent Strips for Urinalysis (Bayer Australia, Pymble, Australia). Urinary protein levels of more than 1 g/L (100 mg/dL) were considered as positive evidence of proteinuria.
Results
PECAM-1 expression of wild-type and PECAM-1−/−mice
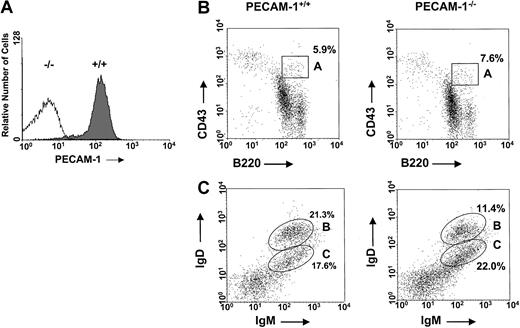
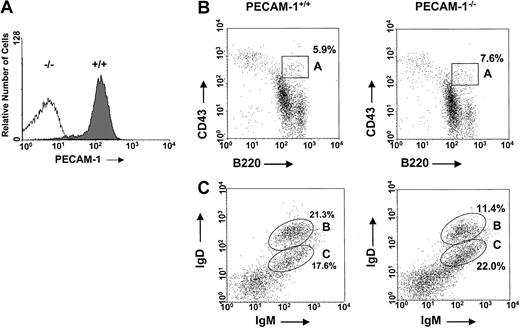
The generation of PECAM-1−/− mice has been previously reported.15 These mice have been subsequently backcrossed onto pure B6 background. To check the level of PECAM-1 expression on the surface of primary cells derived from PECAM-1+/+ and PECAM-1−/− mice, we routinely performed flow cytometry studies to confirm homozygous mutants of PECAM-1 and wild-type controls. Cell surface staining of mouse spleen cells derived from PECAM-1+/+ and PECAM-1−/−mice confirmed lack of expression in homozygous mutants of PECAM-1 compared with wild-type controls (Figure1A). In these studies, we observed that more than 95% of the splenic lymphocytes from wild-type mice are positive for surface expression of PECAM-1. This is in contrast to humans, where PECAM-1 expression is restricted to naive B and T cells and not memory B and T cells.5 17
Normal levels of pro-B cells and mature B cells in the bone marrow of PECAM-1−/− mice.
(A) PECAM-1 is expressed on more than 95% of wild-type C57/BL6 splenic lymphocytes. This expression is completely ablated in PECAM-1 knock-out animals. Spleen cells from PECAM-1+/+ and PECAM-1−/− mice were stained with biotinylated antimouse PECAM-1 390 antibody and binding detected with streptavidin–R-PE. Data analyses were performed on an EPICS XL-MCL flow cytometer and confined to the lymphocyte population, as defined by their forward- and side-scatter characteristics. (B,C) Representative scatterplots of bone marrow lymphocytes from 6-week-old PECAM-1+/+ (n = 15) and PECAM-1−/− mice (n = 15). Cells were isolated from a single femur, red blood cells were lysed, and the cells were dual stained for (B) pro-B lymphocytes using CD43/B220 and (C) mature B lymphocytes using IgM/IgD.
Normal levels of pro-B cells and mature B cells in the bone marrow of PECAM-1−/− mice.
(A) PECAM-1 is expressed on more than 95% of wild-type C57/BL6 splenic lymphocytes. This expression is completely ablated in PECAM-1 knock-out animals. Spleen cells from PECAM-1+/+ and PECAM-1−/− mice were stained with biotinylated antimouse PECAM-1 390 antibody and binding detected with streptavidin–R-PE. Data analyses were performed on an EPICS XL-MCL flow cytometer and confined to the lymphocyte population, as defined by their forward- and side-scatter characteristics. (B,C) Representative scatterplots of bone marrow lymphocytes from 6-week-old PECAM-1+/+ (n = 15) and PECAM-1−/− mice (n = 15). Cells were isolated from a single femur, red blood cells were lysed, and the cells were dual stained for (B) pro-B lymphocytes using CD43/B220 and (C) mature B lymphocytes using IgM/IgD.
Impaired B-cell development in the bone marrow of PECAM-1−/− mice
Because a deficiency of a B-cell coreceptor such as CD22 or CD72 has shown defects in B-cell development involving the bone marrow and periphery, we examined B-cell development in the bone marrow of young and adult PECAM-1−/− mice compared with wild-type (PECAM-1+/+) controls by flow cytometry.13,18,19 Evaluation of the total numbers of bone marrow cells derived from age- and sex-matched 5-week-old PECAM-1+/+ and PECAM-1−/− mice revealed comparable cell numbers (10.4 × 106 per femur ± 3.8 × 106 vs 10.9 × 106 per femur ± 3.4 × 106) (n = 17). Assessment of early pro-B cells in bone marrow defined by dual positivity for CD43+B220+ revealed comparable percentages of pro-B cells in 5-week-old PECAM-1+/+ and PECAM-1−/− mice (Figure 1B, Table1).
However, assessment of the later stages of B-cell development revealed a reduction in mature IgM+IgDhi B cells but not in immature IgM+IgDlo B cells in 5-week-old PECAM-1−/− mice compared with PECAM-1+/+ mice (Figure 1C). These data suggest that the absence of PECAM-1 diminishes the efficiency of transition from immature to mature B cell.
Transient block in B-cell maturation and increased B-1a cells in peripheral B-cell pools of PECAM-1−/− mice
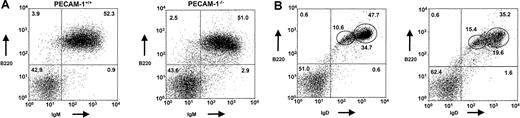
Age- and sex-matched 5-week-old PECAM-1−/− mice show decreased body and spleen weight and decreased numbers of cells in the spleen (8.5 × 107 vs 13.9 × 107) and peritoneum (0.52 × 106 vs 1.11 × 106) compared with wild-type PECAM-1+/+ control mice (Table2). Single staining experiments for the expression of IgM and IgD demonstrate that the decrease in spleen cellularity is mainly due to a reduction in mature IgD-expressing cells (44.7% vs 52.1%) (Table 1).
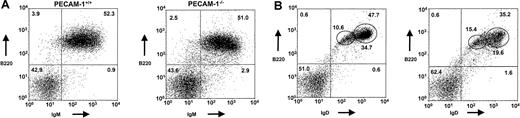
Dual staining with B220 and IgD/IgM supports this interpretation with the PECAM-1−/− mice showing markedly reduced levels of B220+IgDhi-expressing cells compared with wild-type controls (19.4% vs 34.2%) (Figure2A,B; Table 1). Immature B220+IgM+- and B220+IgDlow-expressing B cells and T-cell numbers show no statistical differences between PECAM-1−/− and PECAM-1+/+ mice (Table 1). In contrast, B220+-, IgD+-, and B220+IgDhigh-expressing B cells showed statistically significant reductions between PECAM-1−/−and PECAM-1+/+ mice. These results suggest a reduction in conventional B-2 cells in the periphery of PECAM-1−/−mice (Table 1).
PECAM-1−/− mice show a transient developmental block in B-cell maturation in the periphery.
The developmental block in B-cell maturation was defined by decreased percentage numbers of mature IgD+-expressing B cells in the spleen. Representative scatter plots of splenic lymphocytes from PECAM-1+/+ (n = 26) and PECAM-1−/−(n = 26) mice. Dissociated splenocytes were dual stained to identify (A) immature B cells (B220+/IgM+) and (B) mature B cells (B220+/IgD+). All dotplots contain data for this representative experiment, and oval boxes indicate low and high IgD expression levels.
PECAM-1−/− mice show a transient developmental block in B-cell maturation in the periphery.
The developmental block in B-cell maturation was defined by decreased percentage numbers of mature IgD+-expressing B cells in the spleen. Representative scatter plots of splenic lymphocytes from PECAM-1+/+ (n = 26) and PECAM-1−/−(n = 26) mice. Dissociated splenocytes were dual stained to identify (A) immature B cells (B220+/IgM+) and (B) mature B cells (B220+/IgD+). All dotplots contain data for this representative experiment, and oval boxes indicate low and high IgD expression levels.
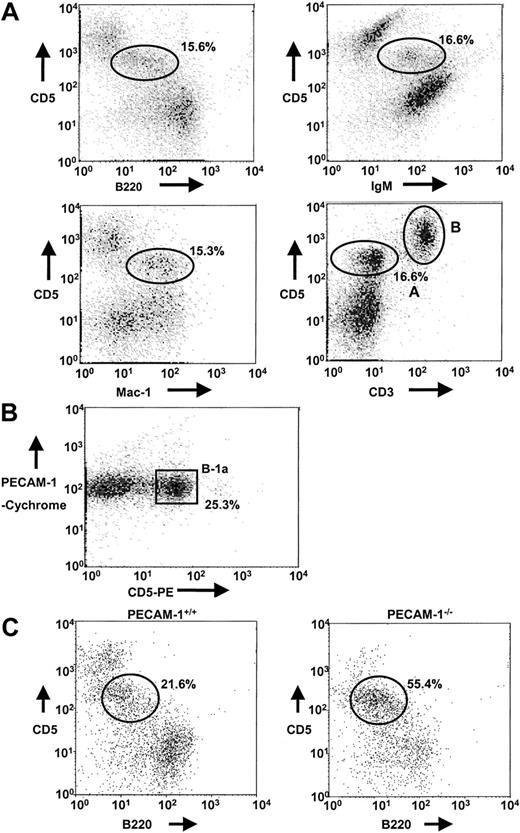
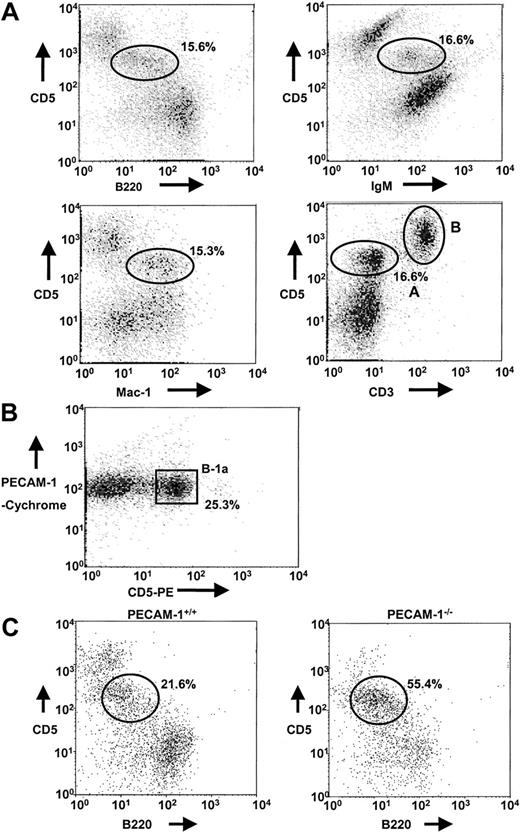
In preliminary experiments, we identified B-1a cells in the peritoneal lavage of PECAM-1+/+ mice by using dual staining of CD5/B220, CD5/IgM, CD5/Mac-1, and CD5/CD3 markers. In each case, a distinct population of cells was identified (15.3%-16.6%) as the B-1a cell population, defined as CD5+B220lowIgM+Mac-1+CD3−(CD5/CD3, region A), which is distinct from the T-cell population (CD5/CD3, region B) (Figure 3A). In addition, T-cell depletion with anti-Thy 1.2 and complement lysis and Percoll gradient separation of peritoneal cells revealed that PECAM-1 is expressed on B-1a cell population (Figure 3B). Using specific markers of the B-1a population, we examined the frequency of B-1a cells in lavages isolated from the peritoneal cavity of PECAM-1+/+ and PECAM-1−/− mice. As shown in Figure 3C, PECAM-1−/− mice show an overrepresentation of the B-1a pool in the peritoneal cavity (55.4% vs 21.6%) compared with wild-type control mice. This elevation in B-1 cells is consistent with an inhibitory receptor phenotype as has been observed in CD72,19 SHP-1,20 and CD22 mice.13,18 21 These data suggest that PECAM-1−/− mice had a reduction in conventional B-2 cells in the periphery and an overrepresentation of the B-1a pool.
Absence of PECAM-1 leads to increased B-1a cell population in the peritoneum.
(A) Peritoneal cells isolated from PECAM-1+/+ mice were collected by peritoneal lavage with 5 mL PBS and cells dual stained with a range of markers including CD5/B220, CD5/Mac-1, CD5/IgM, and CD5/CD3 to identify the B-1a cell population (CD5/CD3, region A) distinct from T cells (CD5/CD3, region B). B-1a cells stain CD5+B220lowIgM+Mac-1+CD3−. These flow profiles are representative of at least 6 experiments. (B) T-cell–depleted peritoneal cells isolated from PECAM-1+/+mice were dual stained with PECAM-1–Cychrome and CD5-PE. B-1a cells express PECAM-1. (C) Peritoneal cells were collected by peritoneal lavage with 5 mL PBS and cells dual stained with CD5 and B220 (CD45R) to identify the B-1a cell population. Representative scatterplots of peritoneal lymphocytes from PECAM-1+/+ (n = 25) and PECAM-1−/− (n = 25) mice.
Absence of PECAM-1 leads to increased B-1a cell population in the peritoneum.
(A) Peritoneal cells isolated from PECAM-1+/+ mice were collected by peritoneal lavage with 5 mL PBS and cells dual stained with a range of markers including CD5/B220, CD5/Mac-1, CD5/IgM, and CD5/CD3 to identify the B-1a cell population (CD5/CD3, region A) distinct from T cells (CD5/CD3, region B). B-1a cells stain CD5+B220lowIgM+Mac-1+CD3−. These flow profiles are representative of at least 6 experiments. (B) T-cell–depleted peritoneal cells isolated from PECAM-1+/+mice were dual stained with PECAM-1–Cychrome and CD5-PE. B-1a cells express PECAM-1. (C) Peritoneal cells were collected by peritoneal lavage with 5 mL PBS and cells dual stained with CD5 and B220 (CD45R) to identify the B-1a cell population. Representative scatterplots of peritoneal lymphocytes from PECAM-1+/+ (n = 25) and PECAM-1−/− (n = 25) mice.
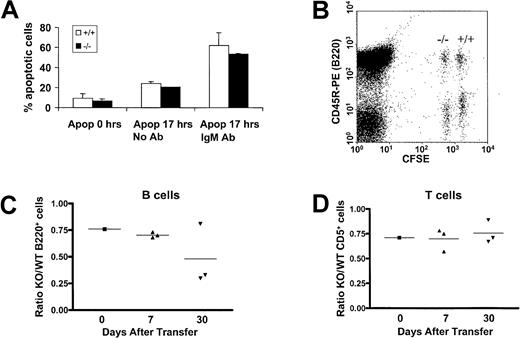
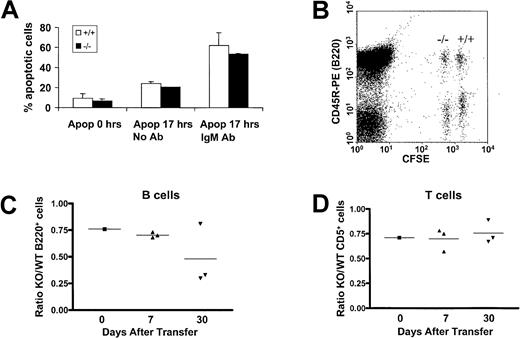
One possible explanation for the transitional defect from immature to mature B cells in PECAM-1−/− mice may be attributed to increased apoptosis of maturing B cells due to increased signaling through the BCR complex. To test this possibility, we determined apoptosis levels of wild-type and PECAM-1−/− purified splenic B cells following in vitro culturing with and without the presence of intact anti-IgM. As shown in Figure4A, at times 0 and 17 hours, no difference was observed between the apoptosis levels of wild-type compared with PECAM-1−/− B cells. These data are suggestive that PECAM-1 does not play a role in regulation of cell death pathways in B cells.
Evaluation of apoptotic responses of PECAM-1+/+ and PECAM-1−/− splenic B cells upon IgM cross-linking and in vivo transfer of CFSE-labeled lymphocytes.
(A) T-cell–depleted splenic B cells were purified through a Percoll gradient and cultured in the presence or absence of whole antimouse IgM antibody. Cells were washed and stained with annexin V and propidium iodide at 0 and 17 hours after purification and the percentage of apoptotic cells determined on an EPICS XL-MLC flow cytometer. (B) A 2-dimensional dot plot of splenic lymphocytes taken from an individual mouse injected 7 days previously with CFSE-labeled PECAM-1+/+ and PECAM-1 knock-out (−/−), which were “fully” or “one-quarter” labeled, respectively. In this example, simultaneous staining with the B-cell–specific antibody CD45R-PE allows calculation of the ratio of PECAM-1−/−compared with PECAM-1+/+ B cells. Similarly, CD5 staining was used to ratio T cells (data not shown). In addition, anti–PECAM-1 390 was used to confirm the identity of PECAM-1+/+ and PECAM-1−/− lymphocytes after transfer (data not shown). (C,D) Flow cytometric analysis allowed the calculation of the ratio of PECAM-1−/− to PECAM-1+/+ B and T cells prior to injection and at 7 and 30 days after transfer, demonstrating a decrease in PECAM-1−/− B cells but not T cells.
Evaluation of apoptotic responses of PECAM-1+/+ and PECAM-1−/− splenic B cells upon IgM cross-linking and in vivo transfer of CFSE-labeled lymphocytes.
(A) T-cell–depleted splenic B cells were purified through a Percoll gradient and cultured in the presence or absence of whole antimouse IgM antibody. Cells were washed and stained with annexin V and propidium iodide at 0 and 17 hours after purification and the percentage of apoptotic cells determined on an EPICS XL-MLC flow cytometer. (B) A 2-dimensional dot plot of splenic lymphocytes taken from an individual mouse injected 7 days previously with CFSE-labeled PECAM-1+/+ and PECAM-1 knock-out (−/−), which were “fully” or “one-quarter” labeled, respectively. In this example, simultaneous staining with the B-cell–specific antibody CD45R-PE allows calculation of the ratio of PECAM-1−/−compared with PECAM-1+/+ B cells. Similarly, CD5 staining was used to ratio T cells (data not shown). In addition, anti–PECAM-1 390 was used to confirm the identity of PECAM-1+/+ and PECAM-1−/− lymphocytes after transfer (data not shown). (C,D) Flow cytometric analysis allowed the calculation of the ratio of PECAM-1−/− to PECAM-1+/+ B and T cells prior to injection and at 7 and 30 days after transfer, demonstrating a decrease in PECAM-1−/− B cells but not T cells.
An alternative explanation for the deficiency of mature recirculating B cells in the periphery of PECAM-1−/− mice may be due to decreased B-cell survival. This feature has been reported in CD22-deficient mice.17 To test this possibility, we examined the in vivo survival of CFSE-labeled purified wild-type and PECAM-1−/− splenic lymphocytes following in vivo transfer to wild-type PECAM-1+/+ mice. Splenic lymphocytes were isolated from age- and sex-matched recipient mice at days 7 and 30 after transfer, and surviving PECAM-1−/−and PECAM-1+/+ B and T cells were identified based on their differential CFSE fluorescence and measured by staining for CD45R and CD5 prior to flow cytometry as demonstrated in Figure 4B. In these experiments dual staining with CD5 and CD3 identified T cells as a distinct population separate from the CD45R (B220) B-cell pool (data not shown). PECAM-1 staining was also performed to confirm the identity of the PECAM-1+/+ and PECAM-1−/− injected cells (data not shown). There was no spontaneous division of transferred cells in the time frame of the experiment (data not shown); therefore, injected cells were identifiable as wild-type and PECAM-1 knock-out based on their CFSE intensity. As shown in Figure 4C, the ratio of PECAM-1−/− compared with wild-type B220+ splenic B cells showed reduced survival over time with a 50% reduction by day 30 after transfer. In contrast, in Figure4D, the ratio of PECAM-1−/− compared with wild-type CD5+ splenic T cells showed no significant difference over time. These results support the hypothesis that the deficiency of mature recirculating B cells in the periphery of PECAM-1−/− mice can be attributed to decreased B-cell survival.
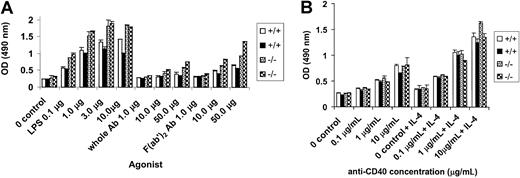
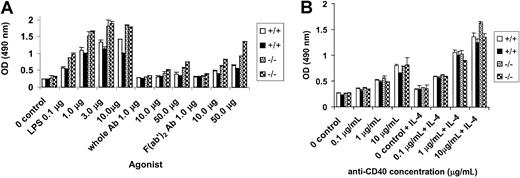
Hyperproliferation of PECAM-1−/− B cells in vitro
We next determined the proliferative ability of PECAM-1−/− B cells in response to B-cell antigen receptor stimulation by anti-IgM cross-linking, mitogen stimulation by LPS, and CD40 stimulation using an anti-CD40 antibody in the presence and absence of IL-4. In these experiments, T-cell–depleted splenic B cells from wild-type and PECAM-1−/− mice were obtained by T-cell depletion with anti-Thy1.2 and complement lysis and purified through Percoll gradient centrifugation. Resting B cells were obtained from the 50% to 75% interface of the Percoll gradient and used for proliferation assays. These resting B cells from PECAM-1−/− mice showed increased proliferation to all doses of F(ab′)2 anti-IgM and to stimulation with LPS compared with wild-type controls (Figure5A). In contrast, the resting B cells from PECAM-1−/− mice did not show increased proliferation to varying doses of anti-CD40 with or without IL-4 compared with wild-type controls (Figure 5B). These results are consistent with the concept that PECAM-1 is a negative regulator of activation-induced B-cell proliferation involving a BCR-dependent pathway and not the CD40 receptor pathway. To test if PECAM-1 expression is important for negative signaling through Fc receptors, we examined B-cell proliferation in response to varying doses of intact anti-IgM to determine effects on BCR-FcγRIIB1 cross-linking. As shown in Figure5A, resting B cells from PECAM-1−/− mice showed increased proliferation to all doses of intact anti-IgM stimulation. However, a reduction in proliferation was observed by intact anti-IgM stimulation compared with F(ab′)2 anti-IgM stimulation. These results are suggestive that PECAM-1 expression is not required for FcγRIIB1-mediated inhibition of B-cell proliferation.
Hyperproliferative responses of splenic B cells from PECAM-1−/− mice.
T-cell–depleted splenic B cells were purified through Percoll gradient centrifugation and resting B cells recovered from the 75% interface. A total of 105 lymphocytes were cultured in triplicate in microtiter plates in the presence of (A) varying concentrations of LPS, antimouse IgM, and F(ab′)2 fragments of antimouse IgM and (B) varying concentrations of anti-CD40 with or without IL-4 (2 ng/mL) for 72 hours. Proliferation was assessed by the addition of Cell Titre 96 (Promega, Madison, WI) dye reagent for the last 2 hours of culture and absorbance defined at 490 nm.
Hyperproliferative responses of splenic B cells from PECAM-1−/− mice.
T-cell–depleted splenic B cells were purified through Percoll gradient centrifugation and resting B cells recovered from the 75% interface. A total of 105 lymphocytes were cultured in triplicate in microtiter plates in the presence of (A) varying concentrations of LPS, antimouse IgM, and F(ab′)2 fragments of antimouse IgM and (B) varying concentrations of anti-CD40 with or without IL-4 (2 ng/mL) for 72 hours. Proliferation was assessed by the addition of Cell Titre 96 (Promega, Madison, WI) dye reagent for the last 2 hours of culture and absorbance defined at 490 nm.
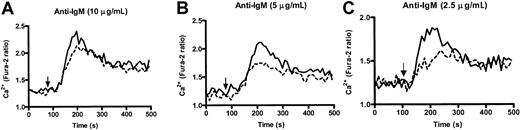
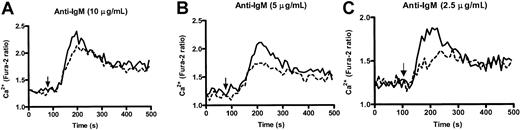
Enhanced kinetics in calcium responses of PECAM-1−/− B cells in vitro
Altered calcium responses have been observed in moth-eaten SHP-1 B cells20 and inhibitory coreceptors, CD22−/− 13,18,21 and CD72−/− B cells.19 Because PECAM-1 can recruit and activate PTPs, principally involving SHP-2 and to a lesser extent SHP-1, we wanted to test the ability of wild-type and PECAM-1−/− B cells to increase their intracellular Ca++ levels after anti-IgM F(ab′)2 cross-linking. As shown in Figure6, PECAM-1−/− B cells showed increased levels of free Ca++ following varying doses of anti-IgM stimulation compared with wild-type controls, particularly at subthreshold concentrations. This increase in free Ca++ levels was observed over all concentrations of anti-IgM tested. These results indicate that PECAM-1 negatively regulates BCR-mediated activation pathways upstream of Ca++ release.
PECAM-1−/− mice show enhanced kinetics of intracellular Ca++ responses upon IgM cross-linking.
Purified splenic B cells from wild-type and PECAM-1−/−mice were loaded with Fura-2/AM, washed, and resuspended in PBS containing 1 mM CaCl2 and 1 mM MgCl2. Calcium mobilization profiles were measured for PECAM-1+/+(---) and PECAM-1−/− (___) B cells after cross-linking BCR by stimulation with varying concentrations of anti-IgM F(ab′)2 (2.5-10 μg/mL) and calcium responses monitored in a luminescence spectrophotometer.
PECAM-1−/− mice show enhanced kinetics of intracellular Ca++ responses upon IgM cross-linking.
Purified splenic B cells from wild-type and PECAM-1−/−mice were loaded with Fura-2/AM, washed, and resuspended in PBS containing 1 mM CaCl2 and 1 mM MgCl2. Calcium mobilization profiles were measured for PECAM-1+/+(---) and PECAM-1−/− (___) B cells after cross-linking BCR by stimulation with varying concentrations of anti-IgM F(ab′)2 (2.5-10 μg/mL) and calcium responses monitored in a luminescence spectrophotometer.
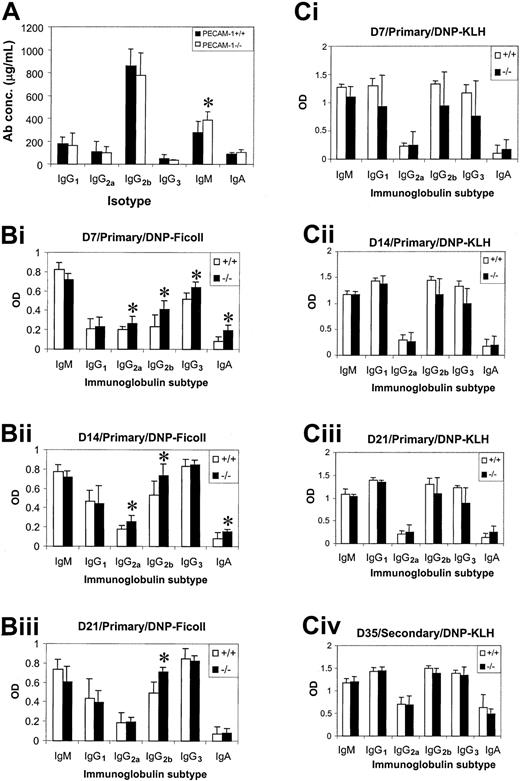
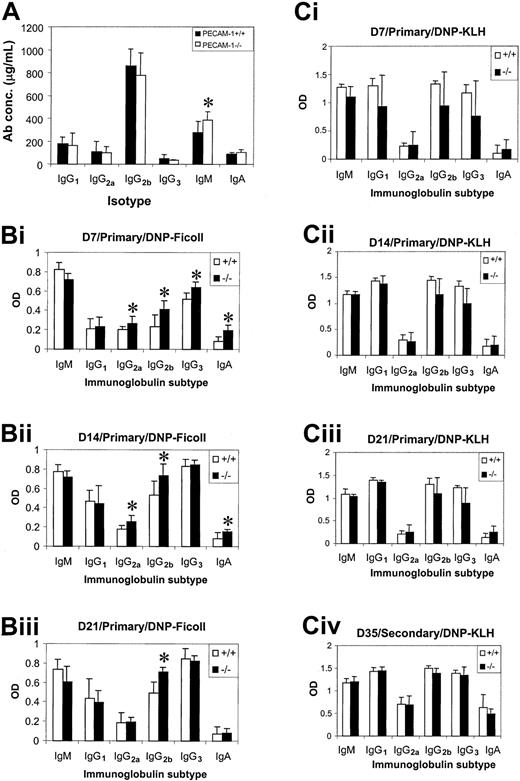
Humoral immune responses of PECAM-1−/− mice
Serum Ig levels were determined in nonimmunized PECAM-1+/+ and PECAM-1−/− mice using an isotype-specific capture ELISA to assess any functional defect caused by loss of PECAM-1 expression in B-cell function. At 8 weeks of age, PECAM-1−/− mice showed a significant increase (Student t test; P < .001; n = 8) in the level of serum IgM compared with wild-type control mice, while all other Ig isotypes were comparable (Figure7A). To test the ability of PECAM-1−/− mice to mount normal humoral immune responses to T-cell–dependent and T-cell–independent antigens, 8-week-old wild-type and PECAM-1−/− mice were immunized with DNP-KLH or DNP-Ficoll. Mice immunized with DNP-Ficoll were bled at days 7, 14, and 21 and sera tested for DNP-specific antibodies using an isotype-specific ELISA. As shown in Figure 7B, PECAM-1−/−mice showed elevated IgG2a, IgG2b, IgG3, and IgA responses to DNP-Ficoll at day 7 and to a lesser extent at day 14 compared with wild-type control mice (Student t test; P < .001; n = 8). By day 21, only the IgG2b response was still elevated. This alteration in response to T-cell–independent antigen is of potential significance, particularly because all of the mice had been housed in a clean barrier-protected environment.
Humoral immune responses in PECAM-1−/−mice upon challenge with T-cell–independent antigen, DNP-Ficoll, and T-cell–dependent DNP-KLH.
(A) Serum Ig levels were determined in 8-week-old nonimmunized wild-type (PECAM-1+/+) (n = 10) and PECAM-1−/− (n = 10) animals using a capture antibody ELISA (asterisk denotes statistical significance; paired Studentt test; P < .001). (B) Eight-week-old PECAM-1−/− mice produced increased levels of IgG2a, IgG2b, IgG3, and IgA upon antigen challenge with a T-cell–independent antigen, DNP-Ficoll. Mice were immunized with 20 μg alum-precipitated DNP-Ficoll intraperitoneally on day 0 and bled on days 7, 14, and 21. DNP-specific antibodies were determined using a DNP-BSA capture ELISA and isotype-specific antisera (asterisk denotes statistical significance; paired Student t test; P < .001; n = 8). (C) T-cell–dependent antigen DNP-KLH induces comparable antibody responses in 8-week-old wild-type (PECAM-1+/+) and PECAM-1−/− mice. Mice were immunized with 100 μg alum-precipitated DNP-KLH intraperitoneally on days 0 and 21 and bled on days 7, 14, 21, and 35. DNP-specific antibodies were determined using a DNP-BSA capture ELISA and isotype-specific antisera (paired Student t test; P > .05; n = 8). Sera isolated from PECAM-1+/+ and PECAM-1−/− mice at days 7, 14, and 21 were diluted from 1:1000, while at day 35 sera were diluted from 1:10 000 before assay.
Humoral immune responses in PECAM-1−/−mice upon challenge with T-cell–independent antigen, DNP-Ficoll, and T-cell–dependent DNP-KLH.
(A) Serum Ig levels were determined in 8-week-old nonimmunized wild-type (PECAM-1+/+) (n = 10) and PECAM-1−/− (n = 10) animals using a capture antibody ELISA (asterisk denotes statistical significance; paired Studentt test; P < .001). (B) Eight-week-old PECAM-1−/− mice produced increased levels of IgG2a, IgG2b, IgG3, and IgA upon antigen challenge with a T-cell–independent antigen, DNP-Ficoll. Mice were immunized with 20 μg alum-precipitated DNP-Ficoll intraperitoneally on day 0 and bled on days 7, 14, and 21. DNP-specific antibodies were determined using a DNP-BSA capture ELISA and isotype-specific antisera (asterisk denotes statistical significance; paired Student t test; P < .001; n = 8). (C) T-cell–dependent antigen DNP-KLH induces comparable antibody responses in 8-week-old wild-type (PECAM-1+/+) and PECAM-1−/− mice. Mice were immunized with 100 μg alum-precipitated DNP-KLH intraperitoneally on days 0 and 21 and bled on days 7, 14, 21, and 35. DNP-specific antibodies were determined using a DNP-BSA capture ELISA and isotype-specific antisera (paired Student t test; P > .05; n = 8). Sera isolated from PECAM-1+/+ and PECAM-1−/− mice at days 7, 14, and 21 were diluted from 1:1000, while at day 35 sera were diluted from 1:10 000 before assay.
Mice immunized with DNP-KLH were bled at days 7, 14, and 21 and were rechallenged with DNP-KLH at day 21 and bled at day 35 to determine their ability to mount a secondary antibody response. As shown in Figure 7C, PECAM-1−/− mice showed no difference in levels of primary and secondary antibody responses compared with wild-type control mice (Student t test; P > .05;n = 8). These results are suggestive that PECAM-1 is required for T-cell–independent but not T-cell–dependent humoral immune responses.
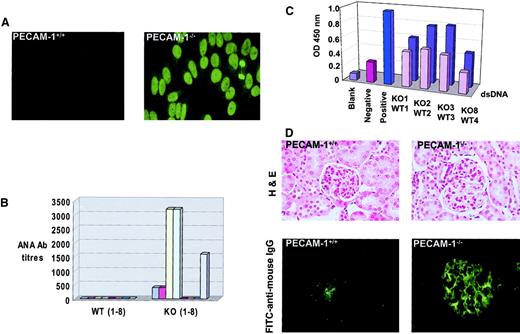
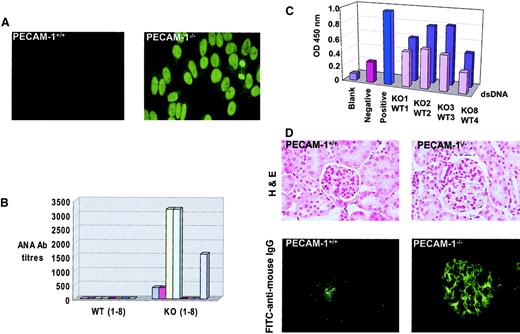
Autoantibody production in PECAM-1−/− mice with age
Because deficiency of inhibitory coreceptors such as FcγRIIB1 22 and CD22 23 have rendered mice susceptible to induction of autoimmune disease, we monitored sequential serum samples from aging wild-type control and PECAM-1−/−mice for the presence of ANAs. As shown in Figure8A, sera from 9-month-old PECAM-1−/− mice showed positive homogenous nuclear staining of HEp-2 epithelial cells, while sera from age-matched wild-type control mice showed no reactivity. Fifty percent of PECAM-1−/− mice showed evidence of ANA positivity, with titers reaching levels of 3200 at 9 months of age. By 17 months of age, 63% of PECAM-1−/− mice showed evidence of ANAs with titers up to 3200 (Figure 8B). Because the homogenous nuclear staining pattern of HEp-2 cells is consistent with autoantibody specificity against dsDNA and dsDNA complexed to histone 2A/2B or histone 1, we next determined IgG titers against dsDNA by ELISA. As shown in Figure8C, the sera from 9-month-old PECAM-1−/− mice that stained positive for ANAs (Figure 8A) were confirmed to have specificity of antibody recognition for dsDNA with higher readings than age- and sex-matched wild-type control sera.
PECAM-1−/− mice develop detectable ANAs and autoimmune disease with age.
PECAM-1−/− (n = 8) and wild-type (PECAM-1+/+) (n = 8) mice were maintained in a barrier-protected facility and bled on a monthly basis over a 17-month period. Serum samples were analyzed from an initial 1:50 dilution on HEp-2000 cell substrates and ANAs detected with antimouse FITC. (A) Photomicrograph of a negative wild-type and positive PECAM-1−/− sera obtained from a PECAM-1−/−mouse at 9 months of age. The PECAM-1−/− sera produced a homogeneous staining pattern of the cell nuclei (magnification × 132). (B) End-point serum dilutions of all serum samples from PECAM-1+/+ and PECAM-1−/− mice at 17 months of age. (C) ELISA readings of IgG antibodies to dsDNA for sera obtained from PECAM-1+/+ (front row) (n = 4) and PECAM-1−/− (back row) (n = 4) mice at 9 months of age. Values are representative of triplicate experiments. (D) Frozen kidney sections of PECAM-1+/+ (n = 8) and PECAM-1−/− (n = 8) were stained for hematoxylin and eosin (magnification × 132) and immune complex deposition by immunofluorescence with FITC–antimouse IgG (Fab′2) (magnification × 132). This figure shows representative hematoxylin and eosin and immunofluorescence stains of frozen kidney sections from 17-month-old PECAM-1+/+ and PECAM-1−/− mice. All slides were photographed and reproduced with identical times, microscope settings, and exposure times, thereby ensuring that the background was equivalent across all photographs shown.
PECAM-1−/− mice develop detectable ANAs and autoimmune disease with age.
PECAM-1−/− (n = 8) and wild-type (PECAM-1+/+) (n = 8) mice were maintained in a barrier-protected facility and bled on a monthly basis over a 17-month period. Serum samples were analyzed from an initial 1:50 dilution on HEp-2000 cell substrates and ANAs detected with antimouse FITC. (A) Photomicrograph of a negative wild-type and positive PECAM-1−/− sera obtained from a PECAM-1−/−mouse at 9 months of age. The PECAM-1−/− sera produced a homogeneous staining pattern of the cell nuclei (magnification × 132). (B) End-point serum dilutions of all serum samples from PECAM-1+/+ and PECAM-1−/− mice at 17 months of age. (C) ELISA readings of IgG antibodies to dsDNA for sera obtained from PECAM-1+/+ (front row) (n = 4) and PECAM-1−/− (back row) (n = 4) mice at 9 months of age. Values are representative of triplicate experiments. (D) Frozen kidney sections of PECAM-1+/+ (n = 8) and PECAM-1−/− (n = 8) were stained for hematoxylin and eosin (magnification × 132) and immune complex deposition by immunofluorescence with FITC–antimouse IgG (Fab′2) (magnification × 132). This figure shows representative hematoxylin and eosin and immunofluorescence stains of frozen kidney sections from 17-month-old PECAM-1+/+ and PECAM-1−/− mice. All slides were photographed and reproduced with identical times, microscope settings, and exposure times, thereby ensuring that the background was equivalent across all photographs shown.
Immune complex–mediated glomerulonephritis has been strongly associated with the deposition of specific isotypes of autoantibodies and/or cationicity to produce pathogenicity in animal models of autoimmune disease and in human systemic lupus erythematosus.24,25 Previous studies on the CD22-deficient mice revealed that these mice developed high-titer anti-dsDNA antibodies of IgG2a isotype but showed no evidence of immune complex–mediated glomerulonephritis with age.23 Seminal studies of FcγRIIB−/− mice did not show evidence of autoantibody development or autoimmune disease when bred on a Sv129/C57BL/6 hybrid background.11 However, subsequent backcrossing onto pure B6 background revealed that these mice develop autoantibodies and spontaneous autoimmune disease.22Because the PECAM-1–deficient mice had been backcrossed onto pure B6 background, we wanted to test for evidence of underlying pathology with particular emphasis on detection of spontaneous immune complex–mediated glomerulonephritis compared with PECAM-1+/+ mice. At age 17 months, 5 of 8 C57/BL6 PECAM-1−/− mice exhibited typical features of spontaneous lupuslike glomerulonephritis, including proteinuria (> 100 mg/dL protein in urine, data not shown), histologic evidence of endocapillary proliferative glomerulonephritis, and immune complex deposition in the glomeruli of the kidneys as indicated by PAS staining. Histologic analysis of the kidneys revealed that the PECAM-1–deficient mice showed systemic vasculitis with inflammatory cell infiltration. The glomeruli showed evidence of extensive glomerular sclerosis, hypercellularity, hyperlobularity, and mesangial thickening (Figure 8D). These features are consistent with a chronic inflammatory disease of glomerulonephritis that is reminiscent of human systemic lupus erythematosus. Examination of stained kidney sections from long-term PECAM-1−/− mice revealed deposition of PAS-positive material in thickened glomerular capillary and capsular basement membranes, sometimes forming discrete globules. PAS positivity was also observed in tubular luminal aspect with occasional discrete globules in lumina (data not shown). Apart from deposition, we also observed occasional interstitial aggregates of mononuclear cells, particularly lymphocytes within the kidney architecture (data not shown). Immunofluorescence analysis of the kidney revealed predominant immune complex deposition in the glomeruli of 5 of 8 aged PECAM-1−/− mice, while there was little evidence of immune complex deposition in 8 aged PECAM-1+/+ mice (Figure8D). Apart from underlying pathology observed in the kidney of PECAM-1–deficient mice, we did not observe arthritic changes in foot joints or vasculitis in other organs, including lungs, heart, liver, and gut (data not shown). These results are consistent with our hypothesis that the presence of PECAM-1 gene provides a protective factor in suppressing the emergence of autoimmune disease by negatively regulating the threshold of activation of autoreactive B cells.
Discussion
Our studies highlight that PECAM-1 represents a new B-cell coreceptor that serves to negatively regulate BCR signaling and B-cell tolerance in vivo. Using a PECAM-1 knock-out mouse model we demonstrate that absence of PECAM-1 leads to dysregulated B-cell responses consistent with a phenotype of an inhibitory B-cell coreceptor knock-out. The main features of PECAM-1−/− mice identified include a developmental defect in transition from immature to mature B cells associated with reduced B-2 cells in the periphery, elevated B-1 cells in peritoneum, hyperresponsive B cells in response to BCR cross-linking with anti-IgM and polyclonal stimulation with LPS, elevated antibody responses to T-cell independent antigens, spontaneous autoantibody development, and immune complex glomerulonephritis with age.
The reduced numbers of mature IgM+IgDhiPECAM-1−/− B cells in the periphery may reflect a block in transition from immature to mature B cell during development or changes in the kinetics of the peripheral conventional B-cell pool. Based upon in vitro apoptosis assays, we excluded the possibility that the reduction in mature B cells was due to increased apoptosis caused by excessive signaling through the BCR complex on PECAM-1−/− B cells following anti-IgM stimulation (Figure4A). However, the reduced numbers of mature PECAM-1−/− B cells may reflect an increase in in vivo B-cell turnover. In support of this hypothesis, our in vivo CSFE-labeled splenic lymphocyte transfer studies revealed that PECAM-1–deficient B cells showed an increase in B-cell turnover but not T-cell turnover in the spleen compared with wild-type B cells (Figure 4B-D). This finding is consistent with previous reports based on other inhibitory receptor knock-outs such as CD22 and CD72, where bromodeoxyuridine incorporation studies revealed an increase in B-cell turnover in bone marrow and spleen.18,19 Apart from a reduction in conventional B-2 cells, an expansion in the number of B-1 cells was observed in the peritoneum of PECAM-1−/− mice compared with wild-type mice (Figure 3C). This elevation in B-1 cells is consistent with an inhibitory receptor knock-out phenotype and has been observed in other B-cell coreceptor and signaling molecule knock-outs, CD22−/−, CD72−/−, PD-1−/−, SHP-1 mev/mev, and Lyn−/−mice.13,14,18,19,20,26 This is distinct from activatory receptor knock-outs, which typically show reduced B-1 cells, including BCR, CD19, C3d complement receptor, CD21, and nonreceptor protein-tyrosine kinase, Btk.27-29
We observed that PECAM-1−/− B cells were hyperproliferative to stimulation following IgM cross-linking and polyclonal stimulation by LPS, suggesting that PECAM-1 may regulate early activation of B cells through either the BCR complex or by polyclonal mitogens. In contrast, treatment of PECAM-1−/−B cells with anti-CD40 antibodies in the presence and absence of IL-4 did not lead to enhancement of B-cell proliferative responses. Our studies also demonstrate that PECAM-1 expression is not required for FcγRIIB1-mediated inhibition of B-cell proliferation. In addition, PECAM-1−/− B cells showed enhanced kinetics of calcium mobilization compared with wild-type B cells following anti-IgM F(ab′)2 stimulation. This difference in calcium responsiveness was particularly noticeable at subthreshold concentrations. These findings are consistent with PECAM-1 serving to negatively regulate the signaling threshold of early B-cell activation initiated through the BCR complex, which directs downstream effector functions including B-cell proliferation.
Further evidence of aberrant signaling in PECAM-1−/− mice was also observed in the enhanced ability to mount an immune response to classical soluble type II T-cell–independent antigen, DNP-Ficoll, but not the primary and secondary IgG and IgM immune responses induced by a classical T-cell–dependent antigen, DNP-KLH, in the presence of adjuvant. PECAM-1−/− mice showed elevated basal IgM levels and a modest elevation to antigen-specific IgG2a, IgG2b, IgG3, and IgA anti-DNP antibody response after challenge with T-cell–independent antigen, DNP-Ficoll, primarily at day 7 and to a lesser extent at day 14. This enhancement in type II T-cell–independent antigen responses can be attributed to the elevated B-1 cell population observed in PECAM-1−/− mice. The B-1 cell population is a subset of conventional B-2 cells that is thought to be responsible for basal immunoglobin levels and antibody responses to T-cell–independent antigens.30 31
Our recent in vitro studies in DT40 B cells demonstrated that coligation of the BCR complex with a FcγRIIB1 chimeric receptor containing the cytoplasmic domain of PECAM-1 could deliver inhibitory signals to negatively regulate B-cell activation. This inhibitory signaling event required intact ITIMs of PECAM-1 and recruitment and activation of distinct PTPs, primarily involving SHP-2 and to a lesser extent SHP-1. Based upon studies with other inhibitory coreceptors, we would predict that PECAM-1 would block signaling events by recruitment of SHP-1 and SHP-2, which leads to dephosphorylation of substrates such as protein-tyrosine kinases, ITAM motifs of the BCR complex, SLP-76, PLC-γ1, and/or linker activator of T cells (LAT) recruited by activated receptors. Of the 4 B-cell coinhibitory receptor knock-outs reported to date, CD22, CD72, and PD-1 preferentially recruit and functionally utilize SHP-1 to delivery inhibitory signals, while FcγRIIB1 recruits and functionally utilizes SHIP to negatively regulate signaling events in B cells.32-36 Because PECAM-1 preferentially utilizes SHP-2, the phenotype of PECAM-1−/− B cells would suggest that SHP-2 may be acting as a negative regulator in B cells. This is an interesting observation because SHP-2 has been implicated in both positive and negative signaling pathways depending upon the cell type and signaling complexes formed.37 Consistent with a potential negative regulator role of SHP-2 in B cells, SHP-2 has been demonstrated to be involved in negative signaling events in the context of T cells.10While our studies indicate that absence of PECAM-1 in B cells leads to loss of inhibitory coreceptor function responsible for controlling the signaling threshold of B-cell activation events, further studies will be required to define that this inhibitory function of PECAM-1 is ITIM-dependent in vivo.
Our studies reveal that the PECAM-1 gene may contribute to the maintenance of peripheral tolerance and protection from autoimmunity. Aged B6 PECAM-1−/− mice produced autoantibodies characterized by ANA positivity and anti-dsDNA antibodies that correlated with immune complex–mediated glomerulonephritis (Figure 8A-D). Deficiencies in several inhibitory coreceptor, complement component, and signaling molecule knock-outs, including FcγRIIB, PD-1, C1q, transgenic expression of Bcl2, Lyn, and SHP-1, have shown development of autoimmune disease.22,26,38-41Specifically, FcγRIIB−/− and PD-1−/− mice develop spontaneous autoimmune disease when modified onto a specific genetic B6 and not BALB/C background, indicating the importance of epistatic genetic modifiers.22 Another contributing factor in the development of autoimmune disease is the chromosomal location of specific loci, which are functionally linked with autoimmune diseases. Regions on chromosomes 1 (FcγRIIB), 7, and 17 (major histocompatibility complex locus) have been identified as having certain loci, which contributes to susceptibility to human autoimmune diseases. Interestingly, the human PECAM-1 gene maps to chromosome 17q23.42 Further studies will be required to define the role of PECAM-1 gene in the emergence of autoimmunity.
An important aspect of our study is that we have defined that PECAM-1 is a new B-cell coreceptor that appears to function in a similar manner to CD22 and CD72. PECAM-1−/− mice resemble mice deficient in CD22−/−, CD72−/−, PD-1−/−, and Lyn−/− and mice expressing a catalytically inactive form of SHP-1 (mev).13,14,18,19,21,26,38,41 However, in terms of the severity of the phenotype, the PECAM-1−/−mice closely resemble the more modest phenotypes of CD22−/−, CD72−/−, and PD-1−/−mice and not the more severe phenotypes observed in the tyrosine kinase Lyn−/− and moth-eaten SHP-1 mev mice. Absence of either Lyn or SHP-1 would be expected to give a more severe phenotype because they function as negative regulators utilized by multiple inhibitory and cytokine receptors in a direct feedback pathway. Consistent with B-cell coreceptor knock-out mouse models, loss of CD72 rendered the B cells sensitive to BCR-mediated triggering events, led to a reduction in mature conventional B-2 cells and overrepresentation of B-1 cells, but did not lead to the development of autoantibodies or spontaneous lupuslike autoimmune disease.19 Similarly, loss of CD22 in B cells led to hyperresponsive B cells, alteration in B-1a and B-2 cell compartments, development of high-affinity autoantibodies, but no evidence of spontaneous autoimmune disease by 5 months of age.13,18,21 23 These results suggest that the phenotype observed with the PECAM-1−/− mice resembles many of the features observed more closely in the CD22−/− mice rather than CD72−/− mice. However, neither the CD72−/− nor CD22−/− mice developed autoimmune disease. The potential importance of this report is to define that PECAM-1 has a functional role in regulating immunomodulation, thereby adding a new B-cell coreceptor to the list of known negative regulators of BCR-mediated signaling. Because CD22, PD-1, and CD72 preferentially functionally utilize SHP-1 and PECAM-1 preferentially utilizes SHP-2 to modulate BCR-mediated signaling, it is likely that these PTPs may regulate different signaling pathways and regulate B-cell responsiveness in different contexts. Further studies will be required to examine whether different B-cell coreceptors utilize similar or different pathways to down-modulate BCR-mediated signaling. These studies will provide an insight into the unique or overlapping roles of B-cell coreceptors in regulating B-cell responsiveness and B-cell tolerance.
The authors thank Dr Tak Mak and Dr Gordon Duncan (Amgen, Canada) for providing the PECAM-1−/− mice. We thank Dr Margaret Hibbs (Ludwig Institute, Melbourne) and Dr David Tarlinton (WEHI Institute, Melbourne) for advice with ELISA assays. We thank Dr John Hayball for immunological advice. We thank Dr John Finney and Mr John Cassidy (IMVS, Adelaide) for performing autopsies and arranging stained paraffin and frozen tissue sections on organs derived from the long-term mice. We thank Mr Alan Bishop for advice with flow cytometry studies.
Prepublished online as Blood First Edition Paper, April 17, 2002; DOI 10.1182/blood-2002-01-0027.
Supported by the National Health and Medical Research Council, Australia (D.E.J.). D.E.J. is a recipient of an NHMRC R. Douglas Wright fellowship.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Denise E. Jackson, Division of Haematology, Hanson Institute, IMVS, Frome Rd, Adelaide, South Australia; 5000; e-mail: denise.jackson@imvs.sa.gov.au.