Abstract
Hydrops fetalis is rarely caused by congenital dyserythropoietic anemia (CDA). We report a patient with hydrops fetalis as a result of severe anemia. This patient needed intrauterine transfusions from 21 weeks of gestation until birth. The hematologic study showed an atypical CDA (hydrops fetalis–associated CDA) characterized by features resembling CDA type II, but negative acidified serum lysis test (HEMPAS negative). The patient was regularly transfused for a year, after which an allogeneic bone marrow transplantation (BMT) from an HLA-identical sibling was successfully carried out. His actual hemoglobin is 127 g/L, and he has not received transfusions for more than a year. In conclusion, intrauterine transfusions and BMT could cure an otherwise lethal atypical CDA.
Introduction
Congenital dyserythropoietic anemias (CDAs) are an uncommon inherited cause of anemia characterized by ineffective erythropoiesis with dysplastic morphologic features. The anemia is usually mild to moderate in patients with CDA; however, it may be more serious and require transfusions. Anemia is rarely very severe, resulting in fatal hydrops fetalis.
Apart from the 3 classic types of CDA (CDA type I, II, and III) defined by Heimpel and Wendt,1 a number of CDAs are heterogenous and cannot be included in these types. At least 3 or 4 new categories have been proposed, designated as IV (CDA with nonspecific erythroid dysplasia), V (congenital ineffective erythropoiesis without significant dysplasia), VI (vitamin B12– and folate-independent megaloblastic and dysplastic erythropoiesis), and CDA with intraerythroblastic precipitation of a nonglobin protein, although many cases remain to be classified in a subgroup of CDA.2
Bone marrow transplantation (BMT) has been used to treat severe congenital erythroid disorders such as major thalassemias or hemoglobinopathies, and BMT is now considered as a potential curative option for many patients with transfusion-dependent disorders.
We report on a patient with hydrops fetalis as a result of a severe transfusion-dependent CDA who was successfully treated with a BMT.
Study design
Hematologic parameters were determined by standard procedures, including blood cell counts, reticulocytes, iron, vitamin B12, folate, haptoglobin, and so forth. Other causes of dyserythropoiesis were ruled out after studying the propositus and his parents. Accordingly, enzyme, membrane disorders, thalassemia-hemoglobin disorders, and sideroblastic anemia were excluded with the use of conventional methods and genetic molecular studies (α and β thalassemia) in the propositus and his family. Acidified serum lysis test (Ham test), sucrose lysis test, CD55 and CD59 glycoprotein analyses, and laboratory tests for excluding fetal isoimmunization were performed following routine methods.
For transmission electron microscopy, bone marrow cells were fixed with glutaraldehyde in cacodylate buffer, postfixed with osmium tetroxide, dehydrated in ethanol, and embedded in Epon 812. The ultrathin sections were stained with uranyl acetate and lead citrate and were examined at 80 Kv.
Results and discussion
The propositus was a Gypsy boy diagnosed with hydrops fetalis as a result of severe anemia in the prenatal diagnosis unit during an echographic control. His parents, who were cousins, showed apparently normal hematologic values. He was the fourth pregnancy (2 live brothers, one dead hydropic fetus, and the patient). During the pregnancy, the patient needed 5 intrauterine transfusions from diagnosis at 21 weeks of gestation (intrauterine hemoglobin, 16 g/L with marked erythroblastosis) until birth at week 34 by cesarean section (hemoglobin, 69 g/L). Intrauterine cytogenetic study showed 46 XY chromosomes without abnormalities. Intrauterine infections were also excluded.
At birth his weight was 1915 g, and he showed pallor, jaundice (maximal total bilirubin, 190 μmol/L at 4 days of life), and generalized edema. The patient required phototherapy, which was initiated at 24 hours (total bilirubin, 120 μmol/L), and mechanical ventilation during the newborn period.
In this period, an extensive study ruled out red cell enzyme, membrane, hemoglobin, and thalassemic diseases. Tests for maternofetal isoimmunization were normal, and a bone marrow biopsy showed a normal osteogenesis with erythroid hyperplasia, excluding an aplastic red cell anemia. His red cells showed a normal agglutinability with anti-i antibody, and an acidified serum lysis test was negative (HEMPAS negative) in 3 samples and against 25 sera. At 3 months of life when the immunohematologic study was carried out, his hemoglobin was 67 g/L, mean corpuscular volume was 80 fL, platelets were 568 × 109/L, leucocytes were 10.2 × 109/L, including 10% of nucleated red cells, and reticulocytes were 0.5% (0.013 × 1012/L). Red cell morphology in peripheral blood showed scant binuclear erythroblasts (2%) (Figure 1).
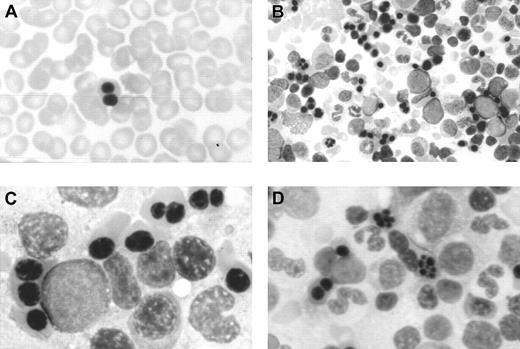
Optical microscopy morphology of peripheral and bone marrow hematopoiesis.
Binucleate erythroblast in peripheral blood (A). Erythroid hyperplasia with marked abnormalities (binucleate, trinucleate, and multinucleate late erythroblasts) (B,C,D). Abnormal erythroblasts showing anomalous distribution of chromatin (C,D), binucleate erythroblasts, and isolated trinuclear, tetranuclear (B), and aberrant multinuclear erythroblasts (B,D). May-Grünwald-Giemsa stain. Original magnifications: A and D, × 400; B, × 200; C, × 1000.
Optical microscopy morphology of peripheral and bone marrow hematopoiesis.
Binucleate erythroblast in peripheral blood (A). Erythroid hyperplasia with marked abnormalities (binucleate, trinucleate, and multinucleate late erythroblasts) (B,C,D). Abnormal erythroblasts showing anomalous distribution of chromatin (C,D), binucleate erythroblasts, and isolated trinuclear, tetranuclear (B), and aberrant multinuclear erythroblasts (B,D). May-Grünwald-Giemsa stain. Original magnifications: A and D, × 400; B, × 200; C, × 1000.
At the age of 2 months a bone marrow examination showed erythroid hyperplasia and markedly abnormal erythropoiesis (Figure 1), including a substantial proportion of binuclear erythroblasts (20%) and a much smaller proportion of trinuclear, tetranuclear, and multinuclear erythroblasts (2%). Sideroblastic or megaloblastic anemias were ruled out. Granulocytopoiesis and megakariocytopoiesis were normal.
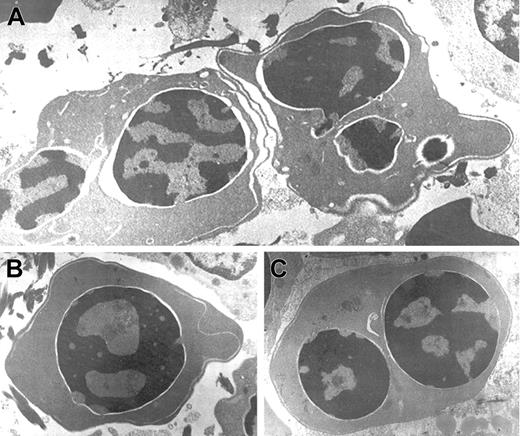
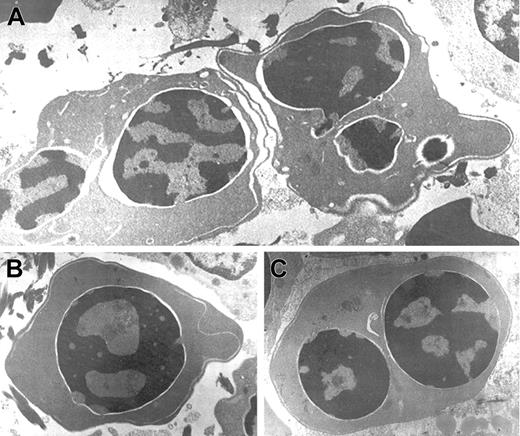
Electron microscopic aberrations were more prominent in late erythroblasts (Figure 2). The nuclear outline was often undulated or lobulated, and the nuclear membrane–associated heterochromatin was frequently absent over large areas of the nuclear membrane. In some cells, the heterochromatin was spongy and showed numerous “punched out” areas. The main cytoplasmic anomaly was the presence of abundant cytoplasmic membranes that are composed of excessive smooth endoplasmic reticulum. These characteristic cisternae usually ran parallel beneath the cell outer membrane producing the so-called “double membrane.” The dilatation of the space between the 2 layers of these cytoplasmic membranes was frequently observed, as was the dilatation of the space of the nuclear membrane.
Electron micrographs of several bone marrow late erythroblasts.
Multinucleate erythroblasts showing striking “double membranes” with partial dilatation of the intramembranous space (A). Erythroblasts revealing nondilated cisternae (B,C). Late erythroblast depicting spongy heterochromatin with numerous “punched out” areas (B). Binucleate erythroblasts showing large areas of nuclear membrane without attached heterochromatin (C). Uranyl acetate and lead citrate stain. Original magnification: A, × 10 600; B and C, × 18 000).
Electron micrographs of several bone marrow late erythroblasts.
Multinucleate erythroblasts showing striking “double membranes” with partial dilatation of the intramembranous space (A). Erythroblasts revealing nondilated cisternae (B,C). Late erythroblast depicting spongy heterochromatin with numerous “punched out” areas (B). Binucleate erythroblasts showing large areas of nuclear membrane without attached heterochromatin (C). Uranyl acetate and lead citrate stain. Original magnification: A, × 10 600; B and C, × 18 000).
The patient was regularly transfused to maintain hemoglobin exceeding 80 g/L every 3 to 4 weeks for 1 year. During this year an extensive examination did not show other dysmorphic features, and his development was normal.
The patient had the main features of the atypical CDA, causing severe transfusion-dependent anemia presenting as hydrops fetalis. Cantù-Rajnoldi et al3 reviewed the characteristics of 5 cases and suggested the name of “hydrops fetalis–associated congenital dyserythropoietic anaemia.” The hematologic findings are very similar in all cases, including a CDA II–like erythroblast morphology with a negative Ham test. Typically, the family history revealed repeated abortions and consanguinity. All patients who have survived are transfusion dependent.3
BMT is a potential curative treatment in patients with many hematologic severe diseases, including erythroid disorders such as major thalassemia, severe hemoglobinopathies, and so forth. The results are excellent when the procedure was carried out as early as possible and from an HLA-matched sibling. Thus, BMT is an alternative to regular transfusions and chelation treatment.4 In this regard, a BMT from his HLA-matched 12-year-old brother was carried out, although at that time the literature did not contain reports of the results of BMT in patients with CDA.
At the age of 13 months the BMT was carried out. The patient was O, Rh− (M−), and the serology for cytomegalovirus was positive. His 12-year-old brother was also O, Rh− (M+) and positive for cytomegalovirus. Both boys were HLA identical (A, B, and DRB1). Donor blood counts were normal (hemoglobin, 140 g/L; mean corpuscular volume, 86 fL; platelets, 220 × 109/L; and leucocytes, 5.9 × 109/L), as was his bone marrow including erythroid cells.
The conditioning treatment was oral busulfan (16 mg/kg over 4 days) and intravenous cyclophosphamide (200 mg/kg over 4 days). Cyclosporine A was used for graft-versus-host disease prophylaxis until day +30 in continuous infusion and after this date orally until 9 months after BMT. A total number of 7.7 × 108/kg mononuclear cells or 16.7 × 106/kg CD34+cells were infused on October 27, 2000. The hemopoietic reconstitution was observed on day +12 for neutrophils and on +29 for platelets.
The patient has not shown acute or chronic graft-versus-host disease complications. As for transplant-related complications during the BMT procedure, the propositus only showed transient arterial hypertension related to cyclosporine and a respiratory tract infection without a known microorganism.
A total chimerism was demonstrated by a DNA study from day +11 to day +236. Mixed chimerism persists now at day +391 in T lymphocytes (58% donor) and in granulocytes (52% donor). However, red blood cells belong to the donor (M+), and the patient did not require more transfusions since day +30. The patient is now 3 years old, his current hemoglobin is 127 g/L, and he has not received red cell transfusion for more than a year.
To our knowledge, this patient is the first in which a severe transfusion-dependent CDA that caused hydrops fetalis has been treated with intrauterine transfusions and BMT. This case raises the possibility of a curative treatment for patients with this rare disease. During the follow-up of our patient, a patient with a classical CDA type II in association with beta-thalassemia trait and severe iron overload was successfully treated with a BMT.5
Prepublished online as Blood First Edition Paper, April 17, 2002; DOI 10.1182/blood-2001-12-0351.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Angel F. Remacha, Department of Hematology, Hospital de Sant Pau, Avinguda Padre Claret 167, Barcelona 08025, Spain.