We have investigated the role of the Rho and Rac family small guanine triphosphate (GTP) exchange factors (RhoGEFs), Vav1 and Vav2, in the activation of platelets by the immunoreceptor tyrosine-based activation motif (ITAM)–coupled collagen receptor GPVI and by the G protein–coupled receptor agonist thrombin. The glycoprotein VI (GPVI)–specific agonist collagen-related peptide (CRP) and thrombin stimulated tyrosine phosphorylation of Vav1 but not Vav2 in human platelets. Surprisingly, however, CRP did not activate the low-molecular-weight G protein Rac and stimulated only a small increase in activity of p21-associated kinase 2 (PAK2), despite the fact that both proteins are regulated downstream of Vav1 in other cells. Further, activation of Rac and PAK2 by thrombin was maintained in platelets from mice deficient in Vav1. Activation of phospholipase C (PLC) by GPVI and thrombin was unaltered in Vav1-, Vav2-, and Vav1/Vav2-deficient platelets. A weak inhibition of late-stage aggregation to CRP and thrombin was observed in platelets deficient in Vav1 but not Vav2, whereas spreading on fibrinogen was not changed. The present results demonstrate that neither Vav1 nor Vav2 lie upstream of PLC or Rac in platelets, highlighting an important difference in their role in signaling by ITAM-coupled receptors in other cell types. The present study has provided evidence for a possible role of Vav1 but not Vav2 in the later stages of platelet aggregation.
Introduction
Vav proteins are GTP exchange factors for members of the Rho family of low-molecular-weight G proteins. In addition, they are involved in the regulation of phospholipase C (PLC) by a number of immunoreceptor tyrosine-based activation motif (ITAM)–coupled receptors. There are 3 members of the Vav family, all of which share a common structural arrangement. At the amino termini is a calponin homology domain, followed by an acidic region, a Dbl homology (DH) domain, a pleckstrin homology (PH) domain, a zinc finger (ZF) domain, a short proline-rich region, and a C-terminal SH3-SH2-SH3 region. Vav proteins are also tyrosine phosphorylated by Src and Syk family kinases and associate with many of the membrane proximal molecules in ITAM-dependent signaling cascades, including Syk and Zap70, SLP-76, Grb2, Nck, and the p85 subunit of phosphatidylinositol 3-kinase (PI 3-K) (reviewed in Bustelo1). Vav1 is specifically expressed in hematopoietic cells, whereas Vav22 3 and Vav34 show a broader profile of expression.
The DH, PH, and ZF domains form the guanine diphosphate (GDP)–GTP exchange factor region of Vav-family proteins with the DH domain containing the active site enabling activation of the Rho family of small G proteins. Rho proteins regulate critical changes in the cytoskeleton and participate in events such as lymphocyte development.5,6 Vav1 has been shown to selectively activate Rac1, Rac2, RhoG, and to a lesser extent RhoA. A majority of studies indicate that Cdc42 is not a good substrate for Vav1.1 3-Phosphoinositides and tyrosine phosphorylation of Vav1 have been shown to stimulate GTP-GDP exchange activity of Vav1.1,7 Vav2 and Vav3 also activate RhoA and RhoG but show less activity towards Rac1.4,8 The activity of these 2 members of the Vav family is also modulated by tyrosine phosphorylation.4 8
Insights into the role of Vav proteins have come from studying mice engineered to lack these proteins. T-cell development and proliferation are retarded in Vav1-deficient mice. Vav1−/− T cells are defective in their ability to activate PLCγ1 and mobilize Ca++ in response to T-cell receptor (TCR) crosslinking. This impairment in Ca++ mobilization appears to be at the heart of the developmental defects, as proliferation can be rescued by restoration of the Ca++ flux with ionophore.9Formation of cap structures, TCR clustering, and cytoskeletal remodeling, all of which are dependent on actin reorganization, are also blocked in Vav1−/− T cells.10,11 The loss of Vav1 from B cells causes mild defects in B-cell development and signaling via the B-cell receptor.12 However, Vav2 is required for these processes and a stronger phenotype is seen in Vav1/Vav2-deficient B cells, suggesting a degree of redundancy in this system. Vav1−/− Vav2−/− mice display a block in B-cell development and a severe impairment of B-cell receptor activation of PLCγ2 and elevation of Ca++ following antigen receptor triggering.13 14
Vav1 undergoes an increase in tyrosine phosphorylation in platelet suspensions challenged with thrombin and collagen but not in response to the thromboxane mimetic U46619 and adenosine diphosphate (ADP).15 In addition, platelet adhesion via the platelet integrin GPIIb-IIIa (αIIbβ3) to fibrinogen leads to a strong increase in Vav1 phosphorylation.15,16Evidence for a functional role of Vav1 downstream of GPIIb-IIIa is provided by studies in A5 Chinese hamster ovary (CHO) cells stably transfected with the integrin.17,18 Vav1 is strongly phosphorylated in the presence of Syk in the GPIIb-IIIa–transfected A5 CHO cells following adhesion to fibrinogen. Coexpression of Syk and Vav1 in the A5 cells is essential for Rac activation and formation of lamellapodia by fibrinogen.17,18 Dominant-negative Rac completely inhibits the increase in formation of lamellapodia placing the small G protein downstream of Syk and Vav in this cell line.17 Activation of Rac has been reported in platelets challenged by thrombin and collagen.19 20
Collagen activates platelets through a tyrosine kinase–dependent pathway via the receptor complex GPVI and Fc receptor (FcR) γ–chain.21 The GPVI signaling pathway has a number of features in common with other ITAM-coupled signaling pathways such as those used by the T- and B-cell antigen receptors.21-24Central roles for the FcR γ-chain, the tyrosine kinase Syk25 and the adapter proteins LAT26 and SLP-7627-29 in the pathway leading to PLCγ2 activation and Ca++ mobilization have been identified. Activation of GPVI can be accomplished by utilizing the specific agonists collagen-related peptide (CRP)30 and the more powerful snake toxin convulxin.31 32
In view of the similarities between the signaling events downstream of the T-cell and B-cell antigen receptors and those downstream of GPVI, we have analyzed the roles of Vav1 and Vav2 in GPVI signaling in platelets. Experiments were performed alongside the G protein–coupled receptor agonist thrombin in order to examine whether defects were specific to GPVI. Activation of PLCγ2 by CRP was maintained in platelets deficient in Vav1 and/or Vav2. Interestingly, a weak inhibition of the late phase of aggregation to CRP and thrombin was observed.
Materials and methods
Reagents
Antiphosphotyrosine monoclonal antibody (mAb) 4G10, anti-Vav1 mAb, and anti-Rac mAb were purchased from Upstate Biotechnology (TCS Biologicals, Bucks, United Kingdom). Anti-PAK2 polyclonal antibodies (pAbs) were purchased from Santa Cruz (Autogen Bioclear, Caine, Wiltshire, United Kingdom). Fluorescein isothiocyanate (FITC)–phalloidin was purchased from Sigma-Aldrich (Poole, Dorset, United Kingdom). Rhodamine phalloidin was from Molecular Probes (Leiden, The Netherlands). The Vav2 pAbs were a kind gift from Dr Daniel Billadeau (Mayo Clinic, Rochester, MN)33 and the PLCγ2 pAb was kindly supplied by Dr Mike Tomlinson (DNAX, Palo Alto, CA). The anti–SLP-76 pAb was a kind gift from Dr Gary Koretzky (University of Pennsylvania, Philadelphia, PA). The cDNA for the GST-CRIB domain of PAK1 (prepared essentially as described in Benard et al34) and the active form of Rac (L61Rac)35were kind gifts from Dr Doreen Cantrell (Imperial Cancer Research Fund, London, United Kingdom). Other reagents were from previously described sources.25
The generation of mice disrupted in the Vav1 gene (Vav1−/−) is described in Turner et al.36The generation of mice disrupted in the Vav2 gene (Vav2−/−) and those deficient in both Vav1 and Vav2 (Vav1−/− Vav2−/−) is described in Doody et al.13 Syk-deficient radiation chimeric mice were obtained as described previously in Turner et al.37 Mutant and control mice were age and background matched. TheVav1−/−, Vav2−/−, andVav1−/−Vav2−/− mice are on a C57 BL/6 background, whereas the Syk-deficient mice are on a RAG-1 background.
Platelet preparation, stimulation, and aggregation
Human platelets were isolated from blood taken on the day of the experiment as described previously.38 Platelets were resuspended at a concentration of 5 × 108 cells/mL in modified Tyrodes buffer. Murine blood was taken by cardiac puncture following carbon dioxide asphyxiation and the platelets prepared as described previously.25 Murine platelets were resuspended at a concentration of 1 × 108 cells/mL to 2 × 108 cells/mL. Unless stated, ethyleneglycotetraacetic acid (EGTA) (1 mM), indomethacin (10 μM), and apyrase (2 U/mL) were included in the resuspension buffer. Wortmannin (100 nM) and Ly294002 (20 μM) were preincubated with platelets for 15 minutes at 37°C, whereas PP1 (10 μM) and PP2 (20 μM) preincubations were for 10 minutes at 37°C. Stimulation of platelets with CRP and thrombin was performed in an aggregometer (Chrono-Log, Havertown, PA) with continuous stirring at 1200 rpm at 37°C for the times shown in the figure legends. Aggregation of platelets was monitored by measuring changes in light transmission under the conditions described above, except EGTA, indomethacin, and apyrase were not included in the resuspension medium.
Immunoblotting and immunoprecipitation
Platelets were lysed with an equal volume of lysis buffer (2% NP-40, 300 mM NaCl, 20 mM Tris, 10 mM ethylenediaminetetraacetic acid [EDTA], 2 mM Na3VO4, 1 mM phenylmethylsulfonyl fluoride, 10 μg/mL leupeptin, 10 μg/mL aprotinin, and 1 μg/mL pepstatin A, pH 7.4). Insoluble cell debris were removed by centrifugation for 5 minutes at 13000 rpm, 4°C, and cell lysates were precleared using protein A-Sepharose. Platelet lysates were incubated with the following antibodies: Vav1, Vav2, PLCγ2, and PAK2. Resulting protein complexes and immunoprecipitates were resolved by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to polyvinylidene difluoride (PVDF) membranes. Immunoblotting was performed as described previously25 with detection by enhanced chemiluminescence (ECL; Amersham Biosciences, Bucks, United Kingdom).
Measurement of phosphatidic acid levels and pleckstrin phosphorylation
Platelets were resuspended in modified Tyrodes without phosphate and incubated with [32P]orthophosphate (0.5 mCi/mL [18.5 MBq/mL]) for 1 hour at 37°C, washed, and activated as described above. An aliquot of each reaction was suspended in Laemmli buffer for measurement of pleckstrin phosphorylation by SDS-PAGE and subsequent autoradiography of the dried gel. The remaining reaction was stopped by the addition of one volume chloroform-methanol-HCl (100/200/1 vol/vol/vol), phospholipids extracted, and [32P]phosphatidic acid separated by thin layer chromatography and analyzed by subsequent autoradiography.39
Analysis of platelets by scanning electron microscopy
The analysis of platelets by scanning electron microscopy was carried out as described previously.40 Briefly, platelets were resuspended in modified Tyrodes, stimulated as described above for 60 seconds, and then mixed with an equal volume of 4% gluteraldehyde/phosphate-buffered saline (PBS), pH 7.4. Platelets were collected with gentle suction onto 0.6-μm polycarbonate filters (Whatman, Maidstone, United Kingdom). Filters were then washed, sequentially dehydrated with ethanol, subjected to critical point drying and gold coating, and then analyzed on a Philips 515 scanner (FEI UK, Cambridge, United Kingdom).
Platelet adhesion studies
The ability of mouse platelets to spread on fibrinogen was assessed as follows. Glass coverslips were coated with fibrinogen (400 μL of 100 μg/mL solution) for 1 hour at 37°C, followed by washing with PBS. Mouse platelets in modified Tyrodes (400 μL of 1 × 108 platelets/mL) were transferred to the coverslips. The coverslips were incubated at 37°C for 1 hour in a humid atmosphere, then washed gently with PBS and the attached platelets fixed with 3.7% formaldehyde (10 minutes, RT). After washing with PBS, the cells were permeabilized with 0.2% Triton X-100 (10 minutes, RT), washed, and F-actin–labeled with rhodamine phalloidin for 1 hour at room temperature. The coverslips were washed in PBS, mounted using Immuno Fluore Mounting Medium (ICN Biomedicals, Aurora, CA), and viewed in an inverted microscope (Axiovert S100; Carl Zeiss, Herts, United Kingdom) under a 100 × oil immersion lens using Openlab software (Improvision, Warwick, United Kingdom).
Measurement of Rac and PAK2 activity
Rac activity was measured essentially as described in Benard et al34 using the CRIB domain of PAK1 (amino acids 67-150), which binds the GTP-bound form of Rac. Reactions were stopped with an equal volume of 2 × lysis buffer (2% [vol/vol] NP-40, 2% [wt/vol] N-octyl glucoside, 300 mM NaCl, 20 mM Tris/HCl, 2 mM EGTA, 20 mM MgCl2, 1 mM phenylmethylsulfonyl fluoride, 10 μg/mL leupeptin, 10 μg/mL aprotinin, and 1 μg/mL pepstatin A; pH 7.4). Insoluble material was removed by centrifugation (5 minutes, 13000 rpm, 4°C), freshly prepared GST-PAK1 previously incubated with glutathione agarose was added and the samples incubated for 1 hour at 4°C. The beads were then washed with 1 × Rac assay lysis buffer and the bound protein taken up into Laemmli buffer. The resulting samples were separated by 12% SDS-PAGE and transferred to PVDF membranes for immunoblotting as described above. PAK2 activity was measured using an in-gel kinase technique as described in Borsch-Haubold et al41 with myelin basic protein (MBP) as the substrate.
Measurement of F-actin levels
The level of F-actin was measured as previously described.42 Basal or activated platelets (2 × 108/mL) were fixed with an equal volume of 3.6% formaldehyde-PBS at 37°C for 45 minutes. Fixed cells were permeabilized and stained with 0.1 volume 1% Triton X-100 containing 10 μM FITC-phalloidin at 25°C for 60 minutes. Platelets were gated by forward and side scatter in a flow cytometer and mean fluorescence of 10 000 cells quantitated per sample.
Analysis of data
Experiments were carried out on at least 3 occasions and are shown as representative data from one experiment. Where applicable, results are shown as mean plus SEM.
Results
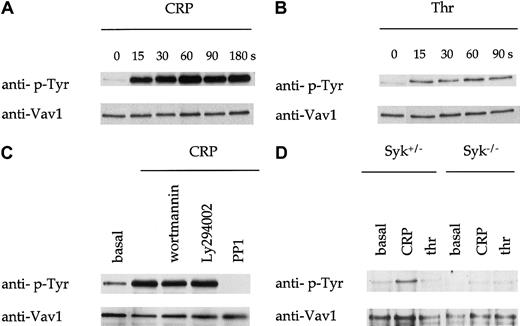
Vav1 is tyrosine-phosphorylated downstream of GPVI. Tyrosine phosphorylation of Vav1 has been shown to be critical for its GTP exchange function and to support its association with a number of signaling proteins. Vav1 undergoes rapid tyrosine phosphorylation in human platelets in response to the GPVI-specific agonist CRP that peaks by 30 seconds and is sustained for at least 180 seconds (Figure1A and not shown). In comparison, the G protein–coupled receptor agonist thrombin stimulated a weaker increase in tyrosine phosphorylation in human platelets but with a similar time course (Figure 1B). Importantly, these studies were performed under conditions that prevented the binding of fibrinogen to GPIIb-IIIa and the action of the secondary mediators, ADP and thromboxanes, demonstrating that the increase in phosphorylation was the direct consequence of receptor activation. Tyrosine phosphorylation of Vav1 by CRP was not significantly altered in the presence of the inhibitors wortmannin and Ly294002 but was abolished by the Src kinase inhibitor PP1 (Figure 1C). Similar results were observed for thrombin (not shown).
Tyrosine phosphorylation of Vav1.
(A-B) Human platelets were stimulated with CRP (1 μg/mL) as shown in (A) or with thrombin (thr; 1 U/mL) as shown in (B), and lysis buffer added at the indicated time point. Vav1 was immunoprecipitated from these samples and analyzed by immunoblotting with monoclonal antiphosphotyrosine antibody 4G10 (anti–p-Tyr). Immunoblots were then stripped and reprobed with an antibody to Vav1 (lower panel). (C) Human platelets were incubated with the PI 3-kinase inhibitors, wortmannin, or Ly294002, or the Src kinase inhibitor PP1, as described in “Materials and methods.” (D) CRP (1 μg/mL)–induced tyrosine phosphorylation of Vav1 in platelets from mice deficient in the tyrosine kinase Syk were analyzed at the indicated time points as described above.
Tyrosine phosphorylation of Vav1.
(A-B) Human platelets were stimulated with CRP (1 μg/mL) as shown in (A) or with thrombin (thr; 1 U/mL) as shown in (B), and lysis buffer added at the indicated time point. Vav1 was immunoprecipitated from these samples and analyzed by immunoblotting with monoclonal antiphosphotyrosine antibody 4G10 (anti–p-Tyr). Immunoblots were then stripped and reprobed with an antibody to Vav1 (lower panel). (C) Human platelets were incubated with the PI 3-kinase inhibitors, wortmannin, or Ly294002, or the Src kinase inhibitor PP1, as described in “Materials and methods.” (D) CRP (1 μg/mL)–induced tyrosine phosphorylation of Vav1 in platelets from mice deficient in the tyrosine kinase Syk were analyzed at the indicated time points as described above.
CRP also stimulated tyrosine phosphorylation of Vav1 in murine platelets (Figure 1D). In contrast, thrombin did not stimulate a significant increase in tyrosine phosphorylation of Vav1 in mouse platelets (Figure 1D). Phosphorylation of Vav1 by CRP was abolished in Syk-deficient platelets (Figure 1D).
Role of Vav1 in platelet functional responses induced by GPVI
To assess the role of Vav1 in platelets, we analyzed responses of platelets from mice carrying a mutation disrupting the Vav1gene resulting in no Vav1 production.36 The number of platelets, red cells, and leukocytes in whole blood from Vav1-deficient mice is no different than wild type (Table1). Bleeding problems, such as the intraperitoneal hemorrhage seen in Syk- and SLP-76–deficient mice, were not observed.
The role of Vav1 in the activation of PLC by CRP and thrombin was analyzed by measuring formation of [32P]phosphatidic acid and [32P]phosphorylation of pleckstrin, a substrate for protein kinase C. There was no significant difference in the level of [32P]phosphatidic acid or [32P]phosphorylation of pleckstrin between Vav1−/− and control platelets treated with either low or high concentrations of CRP and thrombin (Figure 2A-B). Consistent with this, there was no alteration in the level of tyrosine phosphorylation of PLCγ2 or the adapter SLP-76 in response to CRP in Vav1−/− platelets compared with control platelets (Figure 2C).
PLC activation is normal in Vav1-deficient mouse platelets.
(A) For analysis of phosphatidic acid (PA) levels, platelets from control and Vav1-deficient mice were labeled with [32P]orthophosphoric acid, and stimulated with the indicated concentrations of CRP or thrombin for 5 minutes at 37°C and PA extracted as described in “Materials and methods.” PA levels were detected by autoradiography of the TLC plate. (B) For analysis of pleckstrin phosphorylation, platelets from both control and Vav1−/− mice were labeled with [32P]orthophosphoric acid, and stimulated with the indicated concentrations of CRP or thrombin for 2 minutes at 37°C. Samples were separated by SDS-PAGE and the gel was dried and autoradiographed. Pleckstrin appears as a strongly phosphorylated band at approximately 45 kDa. (C) PLCγ2 was immunoprecipitated from control and Vav1−/− mouse platelets stimulated with CRP at the indicated concentrations for 60 seconds. The resulting immunoblots were probed for phosphotyrosine (anti–p-Tyr), stripped, and reprobed for PLCγ2. This procedure was repeated for SLP-76 (lower panels). One experiment representative of 3 is depicted.
PLC activation is normal in Vav1-deficient mouse platelets.
(A) For analysis of phosphatidic acid (PA) levels, platelets from control and Vav1-deficient mice were labeled with [32P]orthophosphoric acid, and stimulated with the indicated concentrations of CRP or thrombin for 5 minutes at 37°C and PA extracted as described in “Materials and methods.” PA levels were detected by autoradiography of the TLC plate. (B) For analysis of pleckstrin phosphorylation, platelets from both control and Vav1−/− mice were labeled with [32P]orthophosphoric acid, and stimulated with the indicated concentrations of CRP or thrombin for 2 minutes at 37°C. Samples were separated by SDS-PAGE and the gel was dried and autoradiographed. Pleckstrin appears as a strongly phosphorylated band at approximately 45 kDa. (C) PLCγ2 was immunoprecipitated from control and Vav1−/− mouse platelets stimulated with CRP at the indicated concentrations for 60 seconds. The resulting immunoblots were probed for phosphotyrosine (anti–p-Tyr), stripped, and reprobed for PLCγ2. This procedure was repeated for SLP-76 (lower panels). One experiment representative of 3 is depicted.
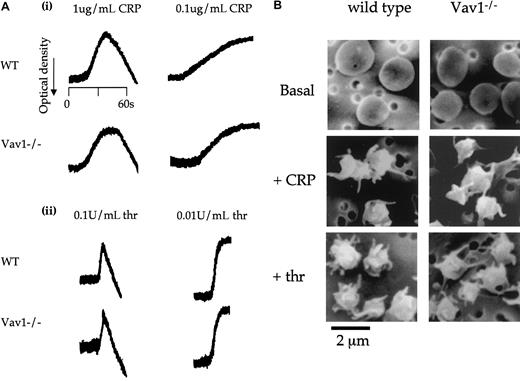
A possible role of Vav1 in response to CRP and thrombin was investigated by monitoring shape change, the earliest response that takes place upon platelet activation. Shape change induced by CRP is entirely dependent on the elevation of intracellular Ca++in the presence of inhibitors of the action of thromboxanes and ADP.43 In contrast, shape change induced by thrombin is mediated through a combination of Ca++- and rho-activated pathways.43 44 The magnitude and time course of shape change to low and high concentrations of thrombin and CRP was maintained in the Vav1-deficient platelets (Figure3A) as measured by an increase in optical density. To confirm these observations, we performed scanning electron microscopy of Vav1−/− platelets. Control and Vav1−/− platelets underwent the characteristic rounding and formation of filopodia that are observed during platelet shape change in response to both stimuli (Figure 3B).
Platelets from Vav1-deficient mice have a normal shape change response.
(A) Platelets from wild-type and Vav1−/− mice were stimulated with the indicated concentrations of CRP (i) or thrombin (ii) and the increase in optical density indicative of shape change monitored. One experiment representative of 3 is depicted. (B) Wild-type and Vav1−/− mouse platelets under resting and CRP (1 μg/mL, 90 seconds)– or thrombin (1 U/mL, 30 seconds)–activated conditions were studied by scanning electron microscopy. Original magnification, × 5200. Results are representative of 5 independently analyzed fields. Scanning electron micrographs were taken as described in “Materials and methods.”
Platelets from Vav1-deficient mice have a normal shape change response.
(A) Platelets from wild-type and Vav1−/− mice were stimulated with the indicated concentrations of CRP (i) or thrombin (ii) and the increase in optical density indicative of shape change monitored. One experiment representative of 3 is depicted. (B) Wild-type and Vav1−/− mouse platelets under resting and CRP (1 μg/mL, 90 seconds)– or thrombin (1 U/mL, 30 seconds)–activated conditions were studied by scanning electron microscopy. Original magnification, × 5200. Results are representative of 5 independently analyzed fields. Scanning electron micrographs were taken as described in “Materials and methods.”
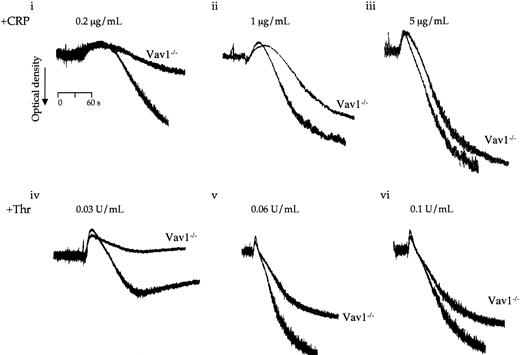
In contrast to the above, aggregation of Vav1−/−platelets to CRP and thrombin was reduced compared with control platelets throughout the length of the dose response curves (Figure4A). In both cases, the inhibitory effect could be most clearly seen as the aggregation response approached completion, with a noticeable tailing away in the curve. The reduction in late-stage aggregation is not due to an inhibition of secretion as it was maintained in the presence of inhibitors of thromboxane formation and ADP receptor activation (not shown). Further, there was only a very small reduction in α-granule secretion to CRP, as measured by exposure of P-selectin, whereas the response to thrombin was unaltered (not shown).
Platelets from Vav1-deficient mice have an impaired aggregation response.
Platelets from wild-type and Vav1−/− mice were stimulated with the indicated concentrations of CRP (i-iii) or thrombin (iv-vi) in the absence of inhibitors of secondary mediators or EGTA. The change in optical density indicative of aggregation was monitored. One experiment representative of 3 is depicted.
Platelets from Vav1-deficient mice have an impaired aggregation response.
Platelets from wild-type and Vav1−/− mice were stimulated with the indicated concentrations of CRP (i-iii) or thrombin (iv-vi) in the absence of inhibitors of secondary mediators or EGTA. The change in optical density indicative of aggregation was monitored. One experiment representative of 3 is depicted.
Spreading on fibrinogen is not altered in Vav1−/− mice
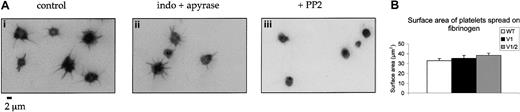
Studies in transfected COS cells have identified a role for Vav1 in supporting spreading to the integrin GPIIb-IIIa (discussed in “Introduction”). Spreading of Vav1-deficient platelets on fibrinogen-coated coverslips was therefore measured after labeling of polymerized actin with rhodamine phalloidin. Platelet spreading involves the extension of filopodia followed by a limited lamellapodia extension between the filopodia protrusions. Platelet spreading on fibrinogen was not significantly altered in the presence of inhibitors of the actions of ADP and thromboxanes but was completely blocked by the Src family kinase inhibitor PP2, demonstrating that it was mediated through a tyrosine kinase–dependent pathway (Figure5A). Surprisingly, no difference in the time course or extent of spreading of Vav1- or Vav1/Vav2-deficient platelets relative to wild-type cells was detected on the fibrinogen surface between 5 minutes and 30 minutes (not shown). A numerical analysis of the increase in platelet surface area of the Vav1- and Vav1/Vav2-deficient platelets revealed no significant difference from wild-type cells (Figure 5B).
Platelets form Vav1- and Vav1/Vav2-deficient mice spread normally on fibrinogen.
(A) Wild-type platelets were spread on a fibrinogen monolayer for 30 minutes in the presence of (i) DMSO, (ii) apyrase (2 U/mL) and indomethacin (10 μM), or (iii) the Src family kinase inhibitor PP2 (20 μM). Slides were stained with rhodamine phalloidin and imaged by fluorescence microscopy. (B) The ability of wild-type, Vav1−/−, and Vav1−/−Vav2−/−mouse platelets to spread on fibrinogen-coated coverslips was studied by labeling of F-actin with rhodamine phalloidin followed by fluorescence microscopy. The surface area of spread platelets was measured at 30 minutes. Data are shown as mean surface area plus SEM. One experiment representative of 3 is depicted.
Platelets form Vav1- and Vav1/Vav2-deficient mice spread normally on fibrinogen.
(A) Wild-type platelets were spread on a fibrinogen monolayer for 30 minutes in the presence of (i) DMSO, (ii) apyrase (2 U/mL) and indomethacin (10 μM), or (iii) the Src family kinase inhibitor PP2 (20 μM). Slides were stained with rhodamine phalloidin and imaged by fluorescence microscopy. (B) The ability of wild-type, Vav1−/−, and Vav1−/−Vav2−/−mouse platelets to spread on fibrinogen-coated coverslips was studied by labeling of F-actin with rhodamine phalloidin followed by fluorescence microscopy. The surface area of spread platelets was measured at 30 minutes. Data are shown as mean surface area plus SEM. One experiment representative of 3 is depicted.
Regulation of Rac and PAK2 activity in platelets
Vav1 has been shown to lie upstream of the low-molecular-weight G protein Rac and the low-molecular-weight G protein–activated serine-threonine kinase PAK2 in a number of cell types. Importantly, both proteins are implicated in regulating actin polymerization in platelets and other cell types,17,18 45an event that may contribute to the reduction in aggregation observed in Vav1-deficient platelets. To address this, we have investigated the role of Vav1 in the regulation of Rac and PAK2 and actin polymerization in platelets.
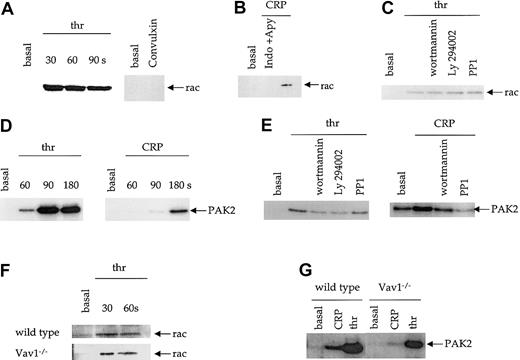
Rac activity was assessed using a method based on that of Benard et al34 utilizing a GST fusion protein incorporating the CRIB domain of PAK1 to bind GTP-Rac but not GDP-Rac. We confirmed the specificity of this assay by showing that the fusion protein bound to a constitutively active, GTP-bound form of Rac (L61 Rac) expressed in Hel cells but not to the endogenous, inactive Rac present within the cells (not shown). CRP and the stronger GPVI agonist, the snake venom toxin convulxin, did not cause a detectable activation of Rac (Figure 6A-B). By contrast, thrombin caused a rapid and sustained level of activation of Rac with maximal activity reached within 30 seconds (Figure 6A). Importantly, these experiments were carried out in the presence of apyrase and indomethacin to block the effects of the G protein–coupled secondary mediators ADP and thromboxanes. In the absence of these inhibitors, CRP stimulates Rac activation (Figure 6B). Thrombin-induced Rac activity was not blocked by the PI 3-K inhibitors, wortmannin and Ly294002, or the Src kinase inhibitor PP1 (Figure 6C).
Activation of Rac and PAK2 in platelets.
(A) Human platelets were stimulated with thrombin (1 U/mL) or the GPVI agonist convulxin (5 μg/mL), and Rac assay lysis buffer added at the indicated time points. Active Rac was pulled down with a GST-fusion protein containing the Rac binding domain of PAK1 and detected by immunoblotting for Rac. (B) Human platelets were stimulated with 3 μg/mL CRP in the presence and absence of apyrase (2 U/mL) and indomethacin (10 μM) as indicated. Active Rac was pulled down and blotted as above. (C) Human platelets, where indicated, were incubated with the PI 3-kinase inhibitors, wortmannin (100 nM), or Ly294002 (20 μM), or the Src kinase inhibitor PP1 (10 μM) followed by thrombin (1 U/mL, 30 seconds). Activation of Rac was measured as described above. (D) PAK2 activity in human platelets was measured using an in-gel kinase assay with MBP as the substrate. Platelets were activated with CRP (1 μg/mL) or thrombin (1 U/mL) and lysis buffer added at the indicated times. PAK2 immunoprecipitates were separated on SDS-PAGE gels containing MBP, the gels dried, and PAK2 activity detected by autoradiography. (E) The effect of wortmannin, Ly294002, and PP1 on thrombin- (1 U/mL, 90 seconds) and CRP-induced (1 μg/mL, 180 seconds) PAK2 activity was determined as described above. The autoradiograph for CRP activation of PAK2 is a longer exposure due to the weaker level of activity. (F-G) Rac (F) and PAK2 (G) activity was measured from control and Vav1-deficient mouse platelets as described above. Each panel depicts one experiment representative of at least 3 independent experiments.
Activation of Rac and PAK2 in platelets.
(A) Human platelets were stimulated with thrombin (1 U/mL) or the GPVI agonist convulxin (5 μg/mL), and Rac assay lysis buffer added at the indicated time points. Active Rac was pulled down with a GST-fusion protein containing the Rac binding domain of PAK1 and detected by immunoblotting for Rac. (B) Human platelets were stimulated with 3 μg/mL CRP in the presence and absence of apyrase (2 U/mL) and indomethacin (10 μM) as indicated. Active Rac was pulled down and blotted as above. (C) Human platelets, where indicated, were incubated with the PI 3-kinase inhibitors, wortmannin (100 nM), or Ly294002 (20 μM), or the Src kinase inhibitor PP1 (10 μM) followed by thrombin (1 U/mL, 30 seconds). Activation of Rac was measured as described above. (D) PAK2 activity in human platelets was measured using an in-gel kinase assay with MBP as the substrate. Platelets were activated with CRP (1 μg/mL) or thrombin (1 U/mL) and lysis buffer added at the indicated times. PAK2 immunoprecipitates were separated on SDS-PAGE gels containing MBP, the gels dried, and PAK2 activity detected by autoradiography. (E) The effect of wortmannin, Ly294002, and PP1 on thrombin- (1 U/mL, 90 seconds) and CRP-induced (1 μg/mL, 180 seconds) PAK2 activity was determined as described above. The autoradiograph for CRP activation of PAK2 is a longer exposure due to the weaker level of activity. (F-G) Rac (F) and PAK2 (G) activity was measured from control and Vav1-deficient mouse platelets as described above. Each panel depicts one experiment representative of at least 3 independent experiments.
We analyzed PAK2 activity by means of an in-gel kinase assay with MBP incorporated into the gel. Treatment of platelets with CRP resulted in weak activation of PAK2 at 90 seconds and stronger activation at 180 seconds (Figure 6D). Thrombin caused a more rapid and higher degree of PAK2 activation, which peaked at 90 seconds (Figure 6D). Activation of PAK2 by thrombin was weakly inhibited by PP1 and more strongly reduced by the PI 3-K inhibitors wortmannin and Ly294002 (Figure 6E). Activation of PAK2 by CRP was abolished by PP1 and wortmannin (Figure6E).
Vav1−/− platelets were used to investigate the role of Vav1 in the activation of Rac and PAK2. There was no difference between the level of thrombin-induced Rac1 activity detected in control and Vav1−/− mice (Figure 6F). On the other hand, PAK2 was slightly reduced in Vav1−/− platelets in response to thrombin, whereas the response to CRP was abolished (Figure 6G).
These results demonstrate that Vav1 does not lie upstream of sequential activation of Rac and PAK2 by thrombin and CRP. CRP does not stimulate activation of Rac, whereas robust activation of Rac and PAK2 by thrombin is largely maintained in Vav1−/− platelets. It remains to be determined whether PAK2 lies downstream of Rac in thrombin-stimulated platelets. Tiam1, which has been shown to activate Rac in a PI 3-K–independent manner,46 and the PAK interacting protein, PIX (Cool),47 are potential candidates for the exchange factor that promotes Rac activation in thrombin-stimulated platelets.
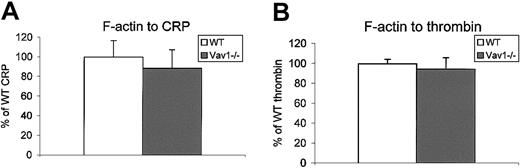
Actin polymerization was measured in platelets using a FACS-based assay in which FITC-conjugated phalloidin binds to F-actin in fixed, permeabilized platelets.42 CRP- and thrombin-induced formation of F-actin was not altered in Vav1−/− platelets relative to controls (Figure 7).
F-actin polymerization is normal in Vav1-deficient platelets.
The F-actin content of basal platelets or platelets stimulated with CRP (10 μg/mL, 45 seconds) or thrombin (1 U/mL, 30 seconds) from wild-type and Vav1−/− mice were measured by FACS as described in “Materials and methods.” Data are presented as the increase in mean fluorescence over basal as a percentage of wild type, plus the standard error of the mean. One experiment representative of 3 is depicted.
F-actin polymerization is normal in Vav1-deficient platelets.
The F-actin content of basal platelets or platelets stimulated with CRP (10 μg/mL, 45 seconds) or thrombin (1 U/mL, 30 seconds) from wild-type and Vav1−/− mice were measured by FACS as described in “Materials and methods.” Data are presented as the increase in mean fluorescence over basal as a percentage of wild type, plus the standard error of the mean. One experiment representative of 3 is depicted.
Vav2 is not activated in human platelets
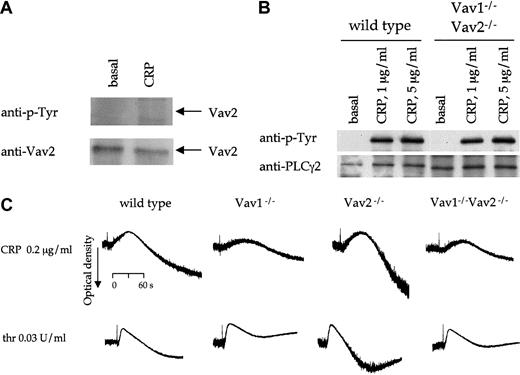
In consideration of recent reports showing that Vav1 and Vav2 play redundant roles in signaling by the B-cell receptor, we extended the present study to analyze Vav2 function in platelets. The presence of a low level of Vav2 in platelets was shown by immunoprecipitation from lysates and Western blotting (Figure 8A, lower panel, and not shown). K562 cells served as a positive control for these studies (not shown). Western blotting with an antiphosphotyrosine antibody demonstrated that Vav2 is not tyrosine phosphorylated in platelets in response to CRP or thrombin (Figure 8A, top panel). Because tyrosine phosphorylation of Vav2 has been shown to be critical for its RhoGEF activity,8 these results suggest that platelet activation does not lead to activation of Vav2.
Vav2 in platelets.
(A) Human platelets were stimulated with CRP (5 μg/mL, 60 seconds) and lysis buffer added. Vav2 was immunoprecipitated from these samples with a rabbit polyclonal antibody to Vav2 and analyzed by immunoblotting with a second antibody to Vav2 (bottom panel). Immunoblots were then stripped and reprobed with monoclonal antiphosphotyrosine antibody 4G10 (top panel). (B) PLCγ2 was immunoprecipitated from control and Vav1−/−Vav2−/− mouse platelets stimulated with CRP at the indicated concentrations for 60 seconds. The resulting immunoblots were probed for phosphotyrosine (anti–p-Tyr), stripped, and reprobed for PLCγ2. (C) Platelets from wild-type, Vav1−/−, Vav2−/−, and Vav1−/−Vav2−/− mice, without EGTA, indomethacin, or apyrase were stimulated with CRP (0.2 μg/mL) or thrombin (0.03 U/mL) and the change in optical density indicative of aggregation monitored. One experiment representative of 3 is depicted.
Vav2 in platelets.
(A) Human platelets were stimulated with CRP (5 μg/mL, 60 seconds) and lysis buffer added. Vav2 was immunoprecipitated from these samples with a rabbit polyclonal antibody to Vav2 and analyzed by immunoblotting with a second antibody to Vav2 (bottom panel). Immunoblots were then stripped and reprobed with monoclonal antiphosphotyrosine antibody 4G10 (top panel). (B) PLCγ2 was immunoprecipitated from control and Vav1−/−Vav2−/− mouse platelets stimulated with CRP at the indicated concentrations for 60 seconds. The resulting immunoblots were probed for phosphotyrosine (anti–p-Tyr), stripped, and reprobed for PLCγ2. (C) Platelets from wild-type, Vav1−/−, Vav2−/−, and Vav1−/−Vav2−/− mice, without EGTA, indomethacin, or apyrase were stimulated with CRP (0.2 μg/mL) or thrombin (0.03 U/mL) and the change in optical density indicative of aggregation monitored. One experiment representative of 3 is depicted.
In order to investigate the role of Vav2 in GPVI and thrombin receptor signaling, we used platelets from mice deficient in Vav2 as well as Vav1−/−Vav2−/− double-deficient platelets. Vav2−/− and Vav1−/−Vav2−/−mice have normal levels of platelets and red blood cells, although there is a significant increase in the level of leukocytes in the Vav2−/−-deficient mice, which is not seen in the double-deficient mice (Table 1). The mice do not show signs of intraperitoneal hemorrhaging.
The stimulation of shape change and aggregation by CRP and thrombin was not altered in Vav2−/− platelets (Figure8C). Moreover, a similar reduction in aggregation to both agonists was seen in Vav1−/−Vav2−/− compared with that in Vav1−/− platelets (Figure 8C). The stimulation of tyrosine phosphorylation of PLCγ2 by CRP was not altered in the Vav1−/−Vav2−/− platelets (Figure 8B).
Discussion
The primary aim of this study was to investigate the role of Vav1 and Vav2 in transducing signals from the platelet collagen receptor GPVI, with particular emphasis on the regulation of PLCγ2. Experiments were performed alongside thrombin as a control. GPVI stimulates robust tyrosine phosphorylation of Vav1 but not Vav2 in human and mouse platelets. Because tyrosine phosphorylation of Vav proteins is required for their RhoGEF activity and their localization into signaling complexes, these data indicate a possible functional role for Vav1 but not Vav2. Thrombin also stimulated tyrosine phosphorylation of Vav1 but not Vav2.
The phosphorylation of Vav1 by Syk and its association with SLP-76 further suggested a role for Vav1 in GPVI signaling.28However, neither tyrosine phosphorylation of PLCγ2 nor its activation, as measured by formation of phosphatidic acid and phosphorylation of pleckstrin, was altered in platelets from Vav1−/− mice. Further, the stimulation of shape change by CRP, which is mediated through a Ca++-dependent pathway,43 was not altered in Vav1−/−platelets. Moreover, there was no discernable alteration in tyrosine phosphorylation or functional responses to CRP in Vav2-deficient platelets, and Vav1/Vav2-deficient platelets have a similar phenotype to Vav1-deficient cells. Therefore, neither Vav1 nor Vav2 is involved in the regulation of PLCγ2 by GPVI.
The lack of a role for Vav1 and Vav2 in the regulation of PLCγ2 by GPVI contrasts with the situation for other ITAM-coupled receptors. For example, B cells deficient in both Vav1 and Vav2 show defective calcium mobilization.13 Vav1 and Vav2 are believed to form part of the PLCγ2 “signalosome” that lies downstream of the B-cell antigen receptor.13 Vav1 also forms part of the PLCγ signalosome that lies downstream of the T-cell receptor and its absence is associated with defective elevation of Ca++ and stimulation of PLC.9 One possible explanation for the difference in the role of Vav1 and Vav2 in GPVI signaling is the presence of Vav3 in platelets.16
The observation that GPVI stimulation does not lead to activation of Rac was unexpected, especially in light of 2 recent reports describing the activation of the low-molecular-weight G protein by collagen in platelets.20,48 The likely explanation for this apparent discrepancy is the role of secondary mediators ADP and thromboxanes in the activation of Rac downstream of GPVI. In the study by Soulet et al,20 inhibitors of thromboxanes and ADP were not used, and in the study by Suzuki-Inoue et al,48 an inhibitor of ADP was not used. It is therefore likely that the stimulation of Rac by collagen in these 2 studies was mediated downstream of thromboxanes and/or ADP. The demonstration of robust activation of Rac by thrombin in the present study confirms the ability of G protein–coupled receptors to activate Rac in platelets, as originally reported by Azim et al,19 and serves as a positive control for the absence of a response to CRP under the same conditions of secondary mediator blockade. Moreover, we also demonstrate that Rac is activated following CRP stimulation in the absence of blockers of ADP and thromboxanes. An alternative explanation, however, for the apparent differences between this study and those by Suzuki-Inoue et al and Soulet et al is the possible regulation of Rac by collagen downstream of α2β1. Direct evidence for the regulation of Rac by α2β1 was provided by Suzuki-Inoue et al48 who reported activation of the GTP-binding protein upon platelet adhesion to a monolayer of F(ab′)2 fragments of the anti-β1 antibody, TS2/16. The absence of a role of Rac in platelet activation by GPVI is in contrast to platelet activation induced by G protein–coupled receptor stimuli.
Interestingly, platelets from Vav1-deficient mice were found to have a weak impairment in aggregation to CRP and thrombin, which was delayed in onset. Vav1/Vav2-deficient platelets display the same defect, whereas aggregation of Vav2-deficient platelets to CRP and thrombin is identical to wild-type platelets. Potentially, this could be explained by a role of Vav1 in inside-out regulation of GPIIb-IIIa or a role in outside-in signaling by the integrin. There is substantial evidence for a role of Vav1 in outside-in signaling downstream of GPIIb-IIIa. Vav1 undergoes tyrosine phosphorylation upon platelet adhesion to fibrinogen.15,16 Studies in A5 CHO cells, which stably express GPIIb-IIIa, have shown that Vav1 is regulated downstream of Src and Syk tyrosine kinases and lies upstream of Rac activation and formation of lamellapodia.17,18 Surprisingly, there was no discernable difference in the spreading of wild-type, Vav1-, and Vav1/Vav2-deficient platelets on fibrinogen. It is conceivable that this might reflect a redundant role for Vav3 in this system which has recently been shown to undergo tyrosine phosphorylation following platelet adhesion to fibrinogen.16 On the other hand, the limited degree of lamellapodia formation may reflect limited activation of the Rho family proteins in platelets spread on fibrinogen in the presence of inhibitors of ADP and thromboxanes.
In conclusion, we have demonstrated that neither Vav1 nor Vav2 participates in the regulation of PLC downstream of the ITAM-coupled collagen receptor GPVI, thereby demonstrating a novel difference with respect to most other ITAM-coupled receptors. Further, we have provided evidence for a role of Vav1 but not Vav2 in the late stages of platelet aggregation, although the mechanism of this event is unclear. The surprisingly mild nature of the phenotype of Vav1/Vav2-deficient platelets with regard to signaling through GPVI and GPIIb-IIIa might reflect an important role for Vav3. Experiments on mice deficient in this member of the Vav family are of considerable interest.
We would like to thank Peter Wonerow for critical evaluation of this manuscript and Jenny Corrigan (Department of Zoology, University of Oxford) for assistance with the scanning electron microscopy.
Supported by the Wellcome Trust and the British Heart Foundation (S.P.W.), the Biotechnology and Biological Sciences Research Council (M.T.), and the Medical Research Council (V.L.J.T.). A.C.P. is a Wellcome Trust Prize student.
A.C.P. and J.I.W. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Andrew C. Pearce, Department of Pharmacology, University of Oxford, Oxford, OX1 3QT, United Kingdom; e-mail: andrew.pearce@spc.ox.ac.uk.

![Fig. 2. PLC activation is normal in Vav1-deficient mouse platelets. / (A) For analysis of phosphatidic acid (PA) levels, platelets from control and Vav1-deficient mice were labeled with [32P]orthophosphoric acid, and stimulated with the indicated concentrations of CRP or thrombin for 5 minutes at 37°C and PA extracted as described in “Materials and methods.” PA levels were detected by autoradiography of the TLC plate. (B) For analysis of pleckstrin phosphorylation, platelets from both control and Vav1−/− mice were labeled with [32P]orthophosphoric acid, and stimulated with the indicated concentrations of CRP or thrombin for 2 minutes at 37°C. Samples were separated by SDS-PAGE and the gel was dried and autoradiographed. Pleckstrin appears as a strongly phosphorylated band at approximately 45 kDa. (C) PLCγ2 was immunoprecipitated from control and Vav1−/− mouse platelets stimulated with CRP at the indicated concentrations for 60 seconds. The resulting immunoblots were probed for phosphotyrosine (anti–p-Tyr), stripped, and reprobed for PLCγ2. This procedure was repeated for SLP-76 (lower panels). One experiment representative of 3 is depicted.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/100/10/10.1182_blood.v100.10.3561/4/m_h82223405002.jpeg?Expires=1765986830&Signature=J6vYDjw1thYulS8JlPp45sZWeZKZS92-2ihFJ5ISvznlq6A6I9LZRf5GY6GvK7AD8NULuOD1fobcCKwoqwjXoU9uBQ5cFJAha4HwypF5YOcu6TyuWmlooYR-K89sX29RwP-k7BpOpbc793hjxJVHV5~C1iteaNtGyxZMztU1BHG21OfbKuuohKMhEHjTJ8M8U~39mgawgmIRE~MXAWvxkswPJsnxm2nMOGMhNVPCBDh~xo1R5wBg3bAe3tqRJirbO2cBtO5rs-ogcmReHMVDfcSiJPKAf7CWgnxe1cZ-mOeUcOVXx0xMWYvRSwOCtpRXiLC5-27xoKgcuYA4NOQ7iA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)