The engagement of the activating isoforms of C-type lectin inhibitory receptor (CLIR) or killer Ig-like receptor (KIR) by their natural ligands, represented by soluble HLA-I (sHLA-I) molecules, induced programmed cell death of natural killer (NK) cells. Indeed, NK cell apoptosis elicited by either putative HLA-E and HLA-F (sHLA-I non-A, -B, -C, and -G) or sHLA-I–Cw4 or –Cw3 from untransfected or –Cw4 or –Cw3 alleles transfected HLA-A−, B−, C−, G−, E+, F+ 721.221 lymphoblastoid cell line, respectively, was blocked by covering the corresponding activating receptor with either anti-CLIR– or anti-KIR–specific monoclonal antibodies (mAbs). After sHLA-I–activating receptor interaction, NK cells produced and released Fas ligand (FasL), which in turn led to NK cell apoptosis by interacting with Fas at the NK cell surface. Blocking anti-Fas mAb, or anti-FasL mAb, inhibited sHLA-I–mediated apoptosis via activating receptor in NK cell clones. This apoptosis was inhibited by NK cell treatment with cyclosporin A, whereas this drug had no effect on activating receptor–mediated activation of cytolysis. Conversely, concanamycin A, an inhibitor of vacuolar type H+–adenosine triphosphatase (H+-ATPase) of granules, inhibited activating receptor–induced NK cell cytolysis, suggesting that activating receptor–mediated apoptosis and cytolysis can use different intracellular pathways. Furthermore, a large amount of interferon-γ (IFN-γ) was detectable in culture supernatant of activating receptor+ NK cells incubated with the appropriate sHLA-I ligand. Again, cyclosporin A, but not concanamycin A, strongly reduced activating receptor–mediated IFN-γ production. This suggests that activating receptor–induced apoptosis of NK cells could play a role in eliminating potentially harmful NK cell clones and, at the same time, it leads to production of IFN-γ, an antiviral cytokine able to amplify immune responses.
Introduction
It is generally accepted that the major histocompatibility complex class I–specific inhibitory receptors on natural killer (NK) cells prevent the lysis of healthy autologous cells.1-3 This inhibitory receptor superfamily (IRS) can be subdivided into 2 structural types of molecules. The first one consists of immunoglobulin (Ig) superfamily receptors (ISIRs), the other one of C-type lectin inhibitory receptors (CLIRs). IRS engagement with the appropriate HLA–class I (HLA-I) allele delivers an inhibitory signal that down-regulates several NK and T-cell functions such as proinflammatory cytokine production, proliferation, and cytolytic activity.1-3 Recently, we have demonstrated that IRS members can function as survival receptors in NK cells. Indeed, the apoptosis of NK cells induced by soluble HLA-I (sHLA-I), via the engagement of CD8, is strongly down-regulated by ligation of sHLA-I with IRS.4
Some members of the IRS include receptors with activating rather than inhibitory function.1-3 These activating receptors are represented by the killer cell Ig-like receptor (KIR) family with 2 Ig domains (KIR2D) with a short cytoplasmic tail (KIR2DS or p50) or by CLIR isoforms, such as CD94/NKG2C complex, which lack the immunoreceptor tyrosine-based inhibition motif (ITIM).1-3KIR2DS and NKG2C associate with a short disulfide-linked homodimer of the protein called DAP12 that carries a cytoplasmic immunoreceptor tyrosine-based activation motif (ITAM).1-3 It has been shown that these receptors—besides other triggering molecules expressed on NK cells including CD16, CD2, CD69, NKp30, NKp44, NKp46, and NKG2D4,5—engaged by specific monoclonal antibodies (mAbs), deliver an activating signal in NK cells leading to interferon-γ (IFN-γ) production, cytotoxicity, and proliferation.1-3,7-13 The physiological significance of these activating receptors for, in some instances, unique subgroups of HLA-I alleles1-3 is still to be determined. Indeed, NK cells bearing activating receptor for HLA-I can damage autologous cells by interacting with self–HLA-I antigens, leading to autoimmune reaction.1-3 This effect should be switched off by the inhibitory signal delivered via KIR or CLIR.1-3 However, it is evident that NK and T-cell clones bearing activating receptor could be isolated from healthy donors and, more importantly, some T-cell clones expressing these activating receptors are present in autoimmune diseases.14 In this context, it has been reported that sHLA-I is present in serum of healthy donors, whereas increased levels of sHLA-I antigens are found in serum of patients affected by immune diseases such as rheumatoid arthritis, multiple sclerosis, and HIV-1 infection.15-19The functional role of sHLA-I in these pathophysiological conditions is not clear, but it is possible that sHLA-I delivers an activating signal through its natural ligand CD8 at the T and NK cell surface, leading to their apoptosis. This could represent a mechanism of down-regulation of NK- and cytolytic T lymphocyte–mediated functions, which ultimately limits self-reaction.6,17,18 Indeed, it has been shown that ligation of CLIR CD94 with specific mAbs could induce apoptosis of a subpopulation of interleukin-2 (IL-2)–stimulated NK cells.20 It remains unclear whether or not sHLA-I alleles can deliver an apoptotic signal by interacting with their specific activating receptor at the NK cell surface. Indeed, activating receptor may represent additional surface structures, besides CD8, through which NK cell response is down-regulated.6 Here, we show that activating receptor for HLA-I engaged by specific sHLA-I alleles can deliver an apoptotic signal through the Fas ligand (FasL)/Fas pathway. This phenomenon is accompanied by the production of IFN-γ. Finally, activating receptor–induced NK cell death is sensitive—different from activating receptor–mediated cytolytic activity—to NK cell treatment with the immunosopressive drug cyclosporin A (CsA).
Materials and methods
Monoclonal antibodies and reagents
The anti-CD16 (NK54, IgG1) mAb, the anti-CD56 (TA181H12, IgG2a) mAb, the anti-CD54 (14D12D2, IgG1) mAb, the anti-CD8α (astra102, IgG1) mAb, the NKVFS1 mAb, recognizing a common epitope of CD158a and CD158b and p50.3, and the anti-CD69 mAb (31C4, IgG2a) were produced as described.1,21,22 The anti-CD3 (Leu4, IgG1), the anti-CD4 (Leu3a, IgG1), and the anti-CD8 (Leu2a, IgG1) mAbs were from Becton Dickinson (Palo Alto, CA). The anti-FasL (NOK-1, IgG1) was from Pharmingen International (San Diego, CA). The anti-CD94 (HP-3B1, IgG2a), the anti-CD158a (EB6, IgG1), and the anti-CD158b (GL183, IgG1) mAbs were from Serotec (Kidlington, United Kingdom). The blocking anti-Fas mAb ZB4 (IgG1) and the apoptosis-inducing anti-Fas mAb CH11 (IgM) were from MBL (Naka-ku Nagoya, Japan); the anti-FasL mAb Alf-2.1a was from Ancell (Bayport, MN). The anti-CD8α-chain OKT8 mAb was purchased from Ortho (Milan, Italy). The mAbs W6/32 to HLA-I heavy chain α3 and α2 domain was a kind gift from S. Ferrone (Roswell Park Memorial, Buffalo, NY). Fluorescein isothiocyanate (FITC)–annexin V and propidium iodide (PI) were from Sigma (Milan, Italy). The affinity-purified goat antimouse (GAM) anti-isotype–specific antiserum was from Southern Biotechnology (Birmingham, AL). Purified GAM anti-Ig(H+L) was purchased from Sigma, the immunomagnetic beads coated with GAM were from Oxoid (Dynal, Oslo, Norway), and recombinant IL-2 from Chiron (Proleukin, Chiron Italia, Siena, Italy). Cells were cultured in RPMI 1640 medium with glutamine and penicillin-streptomycin (Biochrom, Berlin, Germany) supplemented with 10% fetal calf serum (FCS) (Sigma). The immunosuppressive agent cyclosporin A23 and concanamycin A (CMA), a specific inhibitor of vacuolar type H+–adenosine triphosphatase (H+-ATPase),24 25 were from Sigma.
Indirect immunofluorescence
Single fluorescence staining was performed as described.26 Briefly, aliquots of 105 cells were stained with the corresponding mAb followed by phycoerythrin (PE)–conjugated anti-isotype specific GAM serum or with an unrelated mAb followed by the fluorescent second reagent. Samples were analyzed on a flow cytometer (FACSort, Becton Dickinson), and results are expressed as log red mean fluorescence intensity (MFI) in arbitrary units (au) (x-axis) versus number of cells (y-axis).
Isolation and culture of polyclonal and clonal NK cell populations
Peripheral blood mononuclear cells (PBMCs) from healthy volunteers were isolated by Ficoll-Hypaque gradient. CD3−CD4− cells were isolated after negative immunodepletion as described.26 The resulting cell population was 50% to 70% CD16+ (range of 8 different experiments) but 99% CD3−CD4−. Highly purified CD3−CD4− cells were stimulated with 10 μg/mL phytohemagglutinin (PHA) and cultured in 96-well U-bottomed microplates (Becton Dickinson) with complete medium in the presence of 100 U/mL recombinant IL-2 (rIL-2) in a final volume of 200 μL per well in the presence of 105-per-well irradiated allogeneic PBMCs and 5 × 103-per-well 721.221 lymphoblastoid cell line transfected with HLA-G.26 CD3−CD16+clones were obtained by culturing highly purified CD3−CD4− NK cells under limiting dilution conditions as previously reported.13,26 Cloning efficiency was of 5% to 10% calculated as described.27 All NK cell clones were analyzed for the expression of CD158a (p50.1), CD158b (p50.2), p50.3 (CD158a− and CD158b−but NKVFS1+), CD94/NKG2A (mAb Z199, Serotec), CD16, and CD56. Each clone was analyzed in a killing assay using the FcγR+ P815 murine mastocytoma cell line in the presence of mAb recognizing either KIRs or C-type lectin inhibitory receptors at the effector-target ratio (E/T) of 20:1 or 2:1 to identify clones with inhibiting or activating forms of these HLA-I receptors, respectively.1-3,10-13 22
Soluble HLA-I antigen preparations
Soluble HLA-A2, -Cw3, and -Cw4 were prepared from culture supernatant of HLA-I–A−, –B−, –C−, and –G− 721.221 cells transfected with the corresponding HLA-I alleles 6,28,29 while soluble HLA non-A, -B, -C, and -G was prepared from culture supernatant of untransfected 721.221 cells by precipitation with ammonium sulfate, low-medium pressure chromatography, strong anionic and strong cationic ion exchange, and gel filtration as described 28,29 and purified by affinity chromatography on anti-HLA class I mAb W6/32 (10 mg/mL) coupled to cyanogen bromide–activated Sepharose 4B (Pharmacia). The purity of sHLA-I molecule preparations was analyzed by 1-dimensional polyacrylamide gel electrophoresis (PAGE) under nonreducing/nondenaturing (Figure 2A) or reducing/denaturing conditions (not shown) followed by silver staining.28,29 721.221 untransfected cells appeared to be faintly stained with W6/32 mAb followed by PE-conjugated GAM antiserum; thus, in accordance with previous reports,30-36 it is conceivable that the sHLA-I derived from these cells was represented, at least in part, by HLA-E and HLA-F molecules.37
Determination of sFasL and IFN-γ in culture supernatants
Soluble Fas ligand present in culture supernatant derived from NK cell clones after different incubation times (6, 12, 24, 36, 48 hours) with medium alone, or under the various culture conditions indicated in “Results” and in the figure legends, was evaluated by enzyme-linked immunosorbent assay (ELISA).6,28 29 Standard curve was obtained using progressive dilutions of recombinant FasL from Alexis (Leufelfingen, Switzerland). Results were expressed as mean ± SD of triplicate wells. IFN-γ present in supernatant from NK cell clones incubated for 48 hours with medium alone, or under different culture conditions as indicated in “Results,” was evaluated by ELISA (Bender MedSystems Diagnostics, Vienna).
Induction and detection of apoptosis
Bulk NK cell populations or NK cell clones (105 per milliliter) were cultured in 24-well flat-bottomed plates with culture medium either alone or with different amounts of sHLA-I molecules (0.5 to 4 μg/mL) for different periods (6, 12, 24, 36, 48, 60, 72 hours) at 37°C in a 5% CO2 atmosphere. In some experiments cells were incubated with anti-CD158a or anti-CD158b or anti-CD94 or anti-CD54 mAb for 30 minutes at 4°C, washed, and either used in apoptotic assays (to analyze the effect of the covering of a given activating receptor) or further incubated for different times with 4-per-cell GAM-coated magnetic beads 6,28,29 (to obtain the optimal cross-linking of a given activating receptor). Early apoptotic events were evaluated by annexin V labeling method, and viable apoptotic cells were differentiated from necrotic cells by flow cytometry after PI staining of nonpermeabilized cells.6Apoptotic cells were identified as annexin V+PI− cells6,28,29; 104 cells per sample were analyzed and results plotted as the percentage of annexin V+ cells and PI− cells. Apoptosis was also detected by PI staining after permeabilization (DNA content < 2n) and by DNA laddering after DNA extraction and agarose gel electrophoresis.6,28 29
Isolation of RNA, reverse transcription, and PCR amplification
Total RNA was isolated from cell pellets by using the RNAzol B (Biotecx Laboratories, Houston, TX) method.28Complementary DNA (corresponding to 2 μg RNA) was synthesized from oligo(dT)-primed RNA as described.28 The polymerase chain reaction (PCR) mixture was amplified using the following primer sequences: β-actin 5′-ACTCCATCATGAAGTGTGACG, β-actin 3′-CCTAGTCGTTCGTCCTCATAC (228-bp fragment); FasL 5′-CAAGTCCAACTCAAGGTCCATGCC, FasL 3′-CAGAGAGAGCTCAGATACGTTGAC (350-bp fragment).6 28 PCR products were size-fractionated by agarose gel electrophoresis and normalized according to the amount of β-actin detected in the same mRNA sample.
Cytolytic assays
Cytolytic activity of CD3−CD16+ NK cell clones was tested in a 4-hour 51Cr-release assay as previously described.13,22 Polyclonal or clonal CD3−CD16+ NK cell populations, selected for the expression of activating forms of either KIR and/or CLIR, were used as effector cells with the FcγR+ murine mastocytoma cell line P815 in the presence of mAb directed against activating receptor for HLA, at an effector-target ratio (E/T) of 2:1, in a final volume of 200 μL RPMI 1640 medium in V-bottomed microwells.13 22NK cell–mediated cytolysis was also analyzed against a panel of target cells including K562 (erythroleukemia), Jurkat (T-cell lymphoma), and 721.221 lymphoblastoid cell line. The effect of cyclosporin A (5, 50, 500 ng/mL) or concanamycin A (0.3-3 μM) on NK cell–mediated cytolytic activity was analyzed after pretreatment of NK cells for 15 minutes. The optimal concentration for each compound is indicated in “Results.” Cell viability was analyzed after incubation with the different drugs or the appropriate dilution buffer and was always more than 98%.
Results
Soluble HLA-I induces NK cell apoptosis upon engagement of the activating isoforms of KIR or C-type lectin inhibitory receptor
Besides inhibiting receptors for HLA-I, NK cells express at the cell surface the activating isoforms of these receptors.1-3 It appears that these activating receptors recognize the same HLA-I allele, which can interact with their inhibiting counterparts.1-3,7-13,30 NK cells bearing activating receptor can kill cells expressing the appropriate HLA-I allele,1-3,7-13,30 because their engagement delivers an activating signal that ultimately leads to NK cell degranulation of perforins and granzymes.1-3 Thus, these findings would suggest that the activating receptor for HLA-I is potentially harmful and, thus, control mechanisms that down-regulate activating receptor–mediated function should exist. It is conceivable that apoptosis can represent a useful tool to switch off effector cell–mediated activities.4 Thus, to define these mechanism(s), we first analyzed whether the engagement of activating receptor by its natural ligand, HLA-I, can induce NK cell apoptosis. To this aim, to demonstrate that HLA-I interacting with activating receptor can directly deliver an apoptotic signal, we incubated NK cell clones with the soluble form of HLA-I derived from the lymphoblastoid 721.221 HLA-I− cell line either untransfected or stably transfected with appropriate HLA-I allele35 36 (Figure1A).
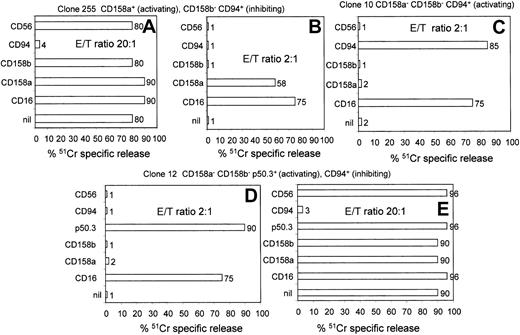
Selection of NK cell clones bearing activating and/or inhibiting receptors for HLA-I antigens.
NK cell clones, selected for the homogeneous expression of CD158a, CD158b, p50.3, and CD94, were analyzed in a 4-hour killing assay using the FcγR+ P815 target cells in the presence of mAb recognizing the indicated surface molecules. An HLA-I receptor was defined as inhibiting or activating when P815 killing at the E/T ratio of 20:1 or 2:1 was either inhibited or increased, respectively, as described.1-3 10-13 Surface phenotype of each clone and functional behavior of HLA-I receptor are indicated above each panel. nil indicates lysis of P815 cells in the absence of any mAb. Results obtained with anti-CD56 mAb, as isotype-matched negative control antibody, are shown in each panel. In some experiments, results obtained with an anti-CD16 mAb, used for comparison as antibody to an NK-triggering molecule, are shown. Results are expressed as51Cr-specific release, and E/T ratios used are indicated in each panel.
Selection of NK cell clones bearing activating and/or inhibiting receptors for HLA-I antigens.
NK cell clones, selected for the homogeneous expression of CD158a, CD158b, p50.3, and CD94, were analyzed in a 4-hour killing assay using the FcγR+ P815 target cells in the presence of mAb recognizing the indicated surface molecules. An HLA-I receptor was defined as inhibiting or activating when P815 killing at the E/T ratio of 20:1 or 2:1 was either inhibited or increased, respectively, as described.1-3 10-13 Surface phenotype of each clone and functional behavior of HLA-I receptor are indicated above each panel. nil indicates lysis of P815 cells in the absence of any mAb. Results obtained with anti-CD56 mAb, as isotype-matched negative control antibody, are shown in each panel. In some experiments, results obtained with an anti-CD16 mAb, used for comparison as antibody to an NK-triggering molecule, are shown. Results are expressed as51Cr-specific release, and E/T ratios used are indicated in each panel.
To select NK cells bearing activating receptor, NK cell clones were first chosen on the basis of the homogeneous expression of receptors for HLA-I. Secondly, each clone was tested in a killing assay against the FcγR+ murine mastocytoma cell line P815, in the presence of anti-KIR– or anti-CLIR–specific mAb, at the ratio of 20:1 or 2:1 to identify NK cell clones bearing inhibiting or activating receptors for HLA-I, respectively. Indeed, it has been suggested that either KIR or CLIR, engaged by specific mAbs, may deliver an inhibiting or activating signal if these mAbs are cross-linked by FcγR or goat antimouse antisera.1-3 10-13 In Figure 1, the functional assays of some representative NK cell clones are shown. Indeed, for example, clone 255 was found to bear the inhibiting form of CLIR CD94, because P815 lysis was strongly inhibited at a 20:1 E/T ratio (80% of specific lysis in medium alone vs 4% in the presence of anti-CD94 mAb) (Figure 1A). Conversely, this clone expressed the activating form of KIR CD158a, as P815 lysis was strongly increased at the E/T ratio of 2:1 (Figure 1B) (58% specific lysis with anti-CD158a mAb vs 1% lysis in medium alone).
Among the clones analyzed, about 10% expressed activating receptor isoforms of either KIR or CLIR (35 of 350 tested) (Table1). In addition, as we have demonstrated that CD8 ligation with sHLA-I can induce NK cell apoptosis by interacting with the α3 domain of sHLA-I, all selected NK cell clones were analyzed for CD8 expression (Table 1).
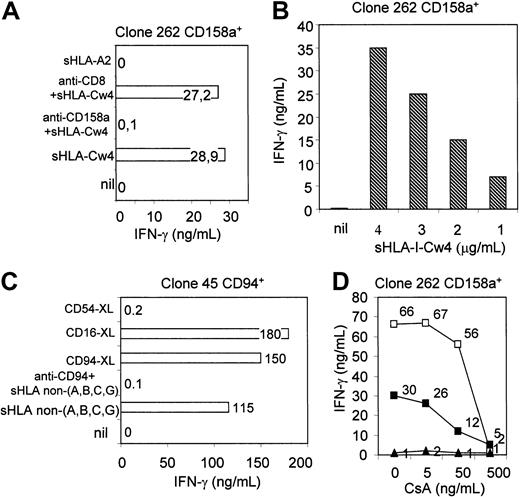
To determine whether the activating receptor may deliver an apoptotic signal in NK cells, only NK cell clones with a dull expression of CD8 were further analyzed. As shown in Figure2B, sHLA-I–Cw4 allele, the natural ligand of CD158a, induced apoptosis of the clone 262, which expressed the activating receptor form of CD158a. Preincubation of NK cells with anti-CD158a mAb strongly reduced this apoptosis, indicating that the interaction of sHLA-I–Cw4 with CD158a was necessary to induce cell death. The finding that the optimal cross-linking of CD158a, achieved by using anti-CD158a mAb followed by GAM-coated beads, led to NK cell apoptosis further indicates that the engagement of activating receptor may induce NK cell death. Noteworthy, the incubation of the NK cell clone 262 with the sHLA-I–A2 allele, an HLA-I allele that does not bind CD158a, did not induce apoptosis (Figure 2B), suggesting that the apoptotic signal can be delivered only when the activating receptor is engaged by its specific sHLA-I allele. Similar results were obtained using the NK cell clone A1.25 bearing the activating receptor form of CD158b and sHLA-I–Cw3 as specific ligand (Figure 2C). Because we have previously demonstrated that the engagement of CD8 with sHLA-I at the NK cell surface led to NK cell apoptosis, we further analyzed whether apoptosis induced by ligation of activating receptor was independent on CD8-mediated signaling. As shown in Figure 2D, we found that the covering of CD8 with specific mAb did not affect activating receptor–induced apoptosis.
Soluble HLA-I–specific alleles induce NK cell apoptosis upon interaction with activating forms of either killer Ig-like receptor (KIR) or C-type lectin inhibitory receptor (CLIR).
(A) One-dimensional PAGE under nonreducing/nondenaturing conditions and silver staining of sHLA-I molecules used to induce apoptosis of NK cells. These molecules were isolated from culture supernatant of either untransfected (lane 1: sHLA non-A, -B, -C, and -G [that is, putative sHLA-E and -F]) or HLA-I allele–transfected (lane 2, Cw3; lane 3, Cw4; lane 4, A2; lane 5, sHLA-G) 721.221 cells 6,28 29 by precipitation with ammonium sulfate, low-medium pressure chromatography, strong anionic and strong cationic ion exchange, and gel filtration and purified by affinity chromatography on anti–HLA class I mAb W6/32 coupled to cyanogen bromide. Molecular weights are indicated on the left. (B-E) NK cell clones selected for the expression of activating isoforms of CD158a ([B] clone 262; [D] clone 255) or CD158b ([C] clone A1.25) or CD94/NKG2 ([E] clone 45) were incubated for 48 hours with their specific sHLA-I ligand (sHLA-Cw4 for CD158a; sHLA-Cw3 for CD158b; sHLA non-A, -B, -C, and -G [that is, putative HLA-E and -F] for CD94/NKG2 complex, respectively), and percentage of apoptotic cells was determined by labeling cells with FITC-conjugated annexin V. In some experiments, covering of activating receptor (CD158a, CD158b, CD94/NKG2) was obtained by preincubating NK cells with specific anti-CD158a or CD158b or CD94 mAb. NK cell apoptosis induced by activating receptor was also achieved upon cross-linking of these receptors with specific mAb (1 μg/mL) followed by GAM-coated beads (4 per cell) ([A,C] CD158a-XL; [B] CD158b-XL; [D] CD94-XL). nil and CD54-XL:NK cells incubated with medium alone or upon cross-linking of CD54. In panel C, either covering of CD8 or its optimal cross-linking was obtained as for activating receptor to determine the role of CD8 in NK cell clones bearing activating receptor. Results are expressed as the percentage of apoptotic cells (FITC–annexin V+ but PI−) and are representative of 3 independent experiments using 3 different NK cell clones for each activating receptor.
Soluble HLA-I–specific alleles induce NK cell apoptosis upon interaction with activating forms of either killer Ig-like receptor (KIR) or C-type lectin inhibitory receptor (CLIR).
(A) One-dimensional PAGE under nonreducing/nondenaturing conditions and silver staining of sHLA-I molecules used to induce apoptosis of NK cells. These molecules were isolated from culture supernatant of either untransfected (lane 1: sHLA non-A, -B, -C, and -G [that is, putative sHLA-E and -F]) or HLA-I allele–transfected (lane 2, Cw3; lane 3, Cw4; lane 4, A2; lane 5, sHLA-G) 721.221 cells 6,28 29 by precipitation with ammonium sulfate, low-medium pressure chromatography, strong anionic and strong cationic ion exchange, and gel filtration and purified by affinity chromatography on anti–HLA class I mAb W6/32 coupled to cyanogen bromide. Molecular weights are indicated on the left. (B-E) NK cell clones selected for the expression of activating isoforms of CD158a ([B] clone 262; [D] clone 255) or CD158b ([C] clone A1.25) or CD94/NKG2 ([E] clone 45) were incubated for 48 hours with their specific sHLA-I ligand (sHLA-Cw4 for CD158a; sHLA-Cw3 for CD158b; sHLA non-A, -B, -C, and -G [that is, putative HLA-E and -F] for CD94/NKG2 complex, respectively), and percentage of apoptotic cells was determined by labeling cells with FITC-conjugated annexin V. In some experiments, covering of activating receptor (CD158a, CD158b, CD94/NKG2) was obtained by preincubating NK cells with specific anti-CD158a or CD158b or CD94 mAb. NK cell apoptosis induced by activating receptor was also achieved upon cross-linking of these receptors with specific mAb (1 μg/mL) followed by GAM-coated beads (4 per cell) ([A,C] CD158a-XL; [B] CD158b-XL; [D] CD94-XL). nil and CD54-XL:NK cells incubated with medium alone or upon cross-linking of CD54. In panel C, either covering of CD8 or its optimal cross-linking was obtained as for activating receptor to determine the role of CD8 in NK cell clones bearing activating receptor. Results are expressed as the percentage of apoptotic cells (FITC–annexin V+ but PI−) and are representative of 3 independent experiments using 3 different NK cell clones for each activating receptor.
Analogous results were obtained using NK cells bearing the activating receptor forms of CLIR represented by CD94/NKG2 complex (Figure 2E). However, in this case, we have used as specific sHLA-I the sHLA-I isolated from HLA-A−, -B− ,-C−, and -G− untransfected 721.221 cells. In fact, CLIR can recognize HLA-I–B7 allele as well as HLA-G.1-3Later, it was reported that CD94/NKG2 interacts with HLA-E.3,35-38,40 These discrepancies were due to the simultaneous expression of HLA-E and either HLA-B7 or HLA-G on 721.221 cell line transfected with HLA-B7 or HLA-G.3,33-40 In our hands, 721.221 cells reacted very faintly with the anti–HLA-I mAb W6/32, but this W6/32-reacted product can be purified from culture supernatant by affinity chromatography (Figure 2A, lane 1). As it has been reported that the 721.221 cell line can express very low amounts of HLA-E and HLA-F although it does not bear HLA-A, -B, and -C alleles and -G isoform,33-40 it is conceivable that the W6/32-reacting molecules isolated from 721.221 cell–derived supernatant (SN) was, at least in part, HLA-E and HLA-F.33-40 As shown in Figure 2E, sHLA non-A, -B, -C, and -G induced apoptosis of the NK cell clone 45 expressing activating receptor form of CLIR. This apoptosis was almost abolished by covering CD94/NKG2 receptor with anti-CD94–specific mAb (Figure 2E).
As depicted in Figure 3, that activating receptor actually induced apoptosis in NK cells after interaction with sHLA-I–specific allele was further supported by DNA analysis with PI (Figure 3B) and DNA laddering (Figure 3C), besides staining of NK cells with FITC–annexin V (Figure 3A). Indeed, the number of NK cells with a DNA content less than 2n (apoptotic cells) after covering of activating receptor form of CLIR (Figure 3Biii) with anti-CLIR mAb was strongly reduced compared with that found upon incubation of NK cells with sHLA non-A, -B, -C, and -G (Figure 3Bii; 85% vs 12%). Comparable results were obtained by DNA laddering (Figure 3C, compare lanes 2 and 3). The percentage of apoptotic cells was maximal at 4 μg/mL although it was detectable at 1 μg/mL (Figure 3D). Finally, kinetics of apoptosis revealed that the optimal incubation time of NK cells with the specific sHLA-I allele was 48 hours (Figure 3E).
Annexin V stainings and DNA analysis of NK cells bearing activating receptor incubated with the corresponding specific sHLA ligand.
Dose-response and kinetics of activating receptor–mediated apoptosis. (A) The NK cell clone 45 bearing the activating form of C-type lectin inhibiting receptor CD94 was incubated for 48 hours with medium alone (top left dot plot) or sHLA non-A, -B, -C, and -G (that is, putative sHLA-E and F) alone (top right dot plot) or after preincubation with anti-CD94 mAb (bottom left dot plot) or with anti-CD94 mAb (1 μg/mL) followed by GAM-coated beads to obtained optimal cross-linking (bottom right dot plot). Cells were then stained with FITC-conjugated annexin V and PI and analyzed on a FACSort. Results are expressed as log green fluorescence intensity versus log red fluorescence intensity (au), and shown in the lower right portion of each dot plot is the percentage of annexin V+PI− cells; that is, apoptotic cells. (B-C) DNA analysis with PI labeling or laddering of the NK cell clone 45 upon incubation for 72 hours with medium alone (Bi; C, lane 1) or sHLA non-A, -B, -C, and -G alone (Bii; C, lane 2) or after covering of CD94/NKG2 complex with anti-CD94–specific mAb (Biii; C, lane 3). In panel A, NK cells were labeled with PI and analyzed on a FACSort. Results are expressed as log red fluorescence intensity (arbitrary units [au]) (x-axis) versus cell number (y-axis). Numbers in each subpanel indicate the percentage of DNA content less than 2n; that is, apoptotic DNA. In panel C, DNA isolated from NK cells was subjected to agarose gel electrophoresis. DNA markers are shown on the left in base pairs (bp). (D) Titration of sHLA-I–induced apoptosis. The NK cell clone 255 bearing the activating receptor of CD158a was incubated with increasing amounts of sHLA-Cw4 for 48 hours, and apoptosis was evaluated after labeling with FITC–annexin V. Results are expressed as the percentage maximal apoptosis. Maximal apoptosis corresponds to 45% of apoptotic cells. (E) Kinetics of activating receptor–mediated apoptosis. The NK cell clone 255 activating receptor+CD158a+ (▪) or the NK cell clone 10 (activating receptor+ CD94+) (▴) was incubated with either sHLA-Cw4 or sHLA non-A, -B, -C, and -G (that is, putative HLA-E) for the indicated periods of time, and apoptosis was evaluated by staining cells with FITC–annexin V. ● indicates apoptosis of the NK cell clone 255 incubated with medium alone. Results are expressed as the percentage of apoptotic cells that are annexin V+ but PI−.
Annexin V stainings and DNA analysis of NK cells bearing activating receptor incubated with the corresponding specific sHLA ligand.
Dose-response and kinetics of activating receptor–mediated apoptosis. (A) The NK cell clone 45 bearing the activating form of C-type lectin inhibiting receptor CD94 was incubated for 48 hours with medium alone (top left dot plot) or sHLA non-A, -B, -C, and -G (that is, putative sHLA-E and F) alone (top right dot plot) or after preincubation with anti-CD94 mAb (bottom left dot plot) or with anti-CD94 mAb (1 μg/mL) followed by GAM-coated beads to obtained optimal cross-linking (bottom right dot plot). Cells were then stained with FITC-conjugated annexin V and PI and analyzed on a FACSort. Results are expressed as log green fluorescence intensity versus log red fluorescence intensity (au), and shown in the lower right portion of each dot plot is the percentage of annexin V+PI− cells; that is, apoptotic cells. (B-C) DNA analysis with PI labeling or laddering of the NK cell clone 45 upon incubation for 72 hours with medium alone (Bi; C, lane 1) or sHLA non-A, -B, -C, and -G alone (Bii; C, lane 2) or after covering of CD94/NKG2 complex with anti-CD94–specific mAb (Biii; C, lane 3). In panel A, NK cells were labeled with PI and analyzed on a FACSort. Results are expressed as log red fluorescence intensity (arbitrary units [au]) (x-axis) versus cell number (y-axis). Numbers in each subpanel indicate the percentage of DNA content less than 2n; that is, apoptotic DNA. In panel C, DNA isolated from NK cells was subjected to agarose gel electrophoresis. DNA markers are shown on the left in base pairs (bp). (D) Titration of sHLA-I–induced apoptosis. The NK cell clone 255 bearing the activating receptor of CD158a was incubated with increasing amounts of sHLA-Cw4 for 48 hours, and apoptosis was evaluated after labeling with FITC–annexin V. Results are expressed as the percentage maximal apoptosis. Maximal apoptosis corresponds to 45% of apoptotic cells. (E) Kinetics of activating receptor–mediated apoptosis. The NK cell clone 255 activating receptor+CD158a+ (▪) or the NK cell clone 10 (activating receptor+ CD94+) (▴) was incubated with either sHLA-Cw4 or sHLA non-A, -B, -C, and -G (that is, putative HLA-E) for the indicated periods of time, and apoptosis was evaluated by staining cells with FITC–annexin V. ● indicates apoptosis of the NK cell clone 255 incubated with medium alone. Results are expressed as the percentage of apoptotic cells that are annexin V+ but PI−.
NK cell apoptosis induced by activating receptor of KIR or CLIR is mediated by FasL-Fas interaction
It has been shown that FasL-Fas interaction is responsible for inducing apoptosis through the ligation of CD8 by sHLA-I both in T and NK cells.6,15,16 29 Thus, to define which surface receptor is involved in delivering an apoptotic signal upon engagement of activating receptor isoforms of KIR or CLIR, we incubated NK cells with sHLA-I alleles in the presence of either anti-Fas or anti-FasL mAb alone or in combination. As shown in Figure4A, covering of Fas antigen at the NK cell surface and/or the incubation with anti-FasL mAb strongly reduced the activating receptor–mediated NK cell apoptosis. That FasL was implicated in this phenomenon was further supported by the finding that FasL was present in culture supernatants of NK cells incubated with the appropriate sHLA-I allele (Figure 4B-D). In addition, covering of CD8 with specific mAb did not reduce the amount of sFasL recovered from culture supernatant induced by incubation of the CD8dull NK cell clone A1.25 bearing activating receptor isoform of CD158b with its specific ligand sHLA-Cw3 (Figure 4C). This finding suggests that CD8 is not directly involved in activating receptor–mediated NK cell apoptosis. Furthermore, as shown in Figure 4E, the engagement of the activating receptor CD158a with sHLA-Cw4 (or upon cross-linking with anti-CD158a mAb plus GAM-coated beads) induced a strong increase of mRNA coding for FasL that was abolished by the covering of CD158a with specific mAb. Similar results were obtained by incubating NK cells bearing activating receptor of CD94 with sHLA non-A, -B, -C, and -G ligand (not shown). Altogether, these findings suggest that the engagement of activating receptor by sHLA-I–specific alleles delivers an apoptotic signal through the production of FasL that in turn is secreted in the extracellular milieu and eventually interacts with Fas at the NK cell surface, leading to NK cell apoptosis.
FasL-Fas interaction is responsible for activating receptor–mediated NK cell apoptosis.
(A) The NK cell clone 10 (CD94+ activating) was incubated with sHLA non-A, -B, -C, and -G (that is, putative HLA-E and HLA-F) for 48 hours alone or after preincubation with anti-Fas mAb (5 μg/mL) or in the presence of anti-FasL mAb (5 μg/mL) or anti-Fas plus anti-FasL mAbs (5 μg/mL plus 5 μg/mL) in combination. Apoptosis was evaluated by labeling NK cells with FITC–annexin V. The effect of the covering of CD94 (achieved by preincubating NK cells with anti-CD94–specific mAb) is shown for comparison. Results are expressed as the percentage of apoptotic cells (annexin V+ but PI−). “nil” indicates apoptosis in medium alone. (B-D) ELISA for the presence of FasL in supernatant recovered from the NK cell clone 262 (CD158a+ activating) (B) or A1.25 (CD158b+ activating) (C) or 1 (CD94+activating) (D) incubated for 48 hours with the corresponding sHLA-I allele ([B] sHLA-Cw4; [C] sHLA-Cw3; [D] sHLA non-A, -B, -C, and -G) alone or after covering of activating receptor with specific mAb ([B] anti-CD158a plus sHLA-Cw4; [C] anti-CD158b plus sHLA-Cw3; [D] anti-CD94 plus sHLA non-A, -B, -C, and -G). Some experiments were performed upon optimal cross-linking of the indicated activating receptor with specific mAb (1 μg/mL) followed by GAM-coated beads (4 per cell) ([B] CD158a-XL; [C] CD158b-XL; [D] CD94-XL) or with unrelated sHLA-A2 allele (C) or sHLA-Cw3 after covering of CD8 (C) with anti-CD8 mAb (OKT8, 1 μg/mL). (E) Analysis of mRNA coding for FasL in the NK cell clone 262 bearing activating form of CD158a upon incubation for 6 hours with sHLA-Cw4 alone or after covering of CD158a+ with specific anti-CD158a mAb. “nil” represents mRNA coding for FasL in NK cells incubated with medium alone, and results obtained after optimal cross-linking of CD158a achieved using anti-CD158a mAb followed by GAM-coated beads (CD158a-XL) are shown for comparison.
FasL-Fas interaction is responsible for activating receptor–mediated NK cell apoptosis.
(A) The NK cell clone 10 (CD94+ activating) was incubated with sHLA non-A, -B, -C, and -G (that is, putative HLA-E and HLA-F) for 48 hours alone or after preincubation with anti-Fas mAb (5 μg/mL) or in the presence of anti-FasL mAb (5 μg/mL) or anti-Fas plus anti-FasL mAbs (5 μg/mL plus 5 μg/mL) in combination. Apoptosis was evaluated by labeling NK cells with FITC–annexin V. The effect of the covering of CD94 (achieved by preincubating NK cells with anti-CD94–specific mAb) is shown for comparison. Results are expressed as the percentage of apoptotic cells (annexin V+ but PI−). “nil” indicates apoptosis in medium alone. (B-D) ELISA for the presence of FasL in supernatant recovered from the NK cell clone 262 (CD158a+ activating) (B) or A1.25 (CD158b+ activating) (C) or 1 (CD94+activating) (D) incubated for 48 hours with the corresponding sHLA-I allele ([B] sHLA-Cw4; [C] sHLA-Cw3; [D] sHLA non-A, -B, -C, and -G) alone or after covering of activating receptor with specific mAb ([B] anti-CD158a plus sHLA-Cw4; [C] anti-CD158b plus sHLA-Cw3; [D] anti-CD94 plus sHLA non-A, -B, -C, and -G). Some experiments were performed upon optimal cross-linking of the indicated activating receptor with specific mAb (1 μg/mL) followed by GAM-coated beads (4 per cell) ([B] CD158a-XL; [C] CD158b-XL; [D] CD94-XL) or with unrelated sHLA-A2 allele (C) or sHLA-Cw3 after covering of CD8 (C) with anti-CD8 mAb (OKT8, 1 μg/mL). (E) Analysis of mRNA coding for FasL in the NK cell clone 262 bearing activating form of CD158a upon incubation for 6 hours with sHLA-Cw4 alone or after covering of CD158a+ with specific anti-CD158a mAb. “nil” represents mRNA coding for FasL in NK cells incubated with medium alone, and results obtained after optimal cross-linking of CD158a achieved using anti-CD158a mAb followed by GAM-coated beads (CD158a-XL) are shown for comparison.
NK cell–mediated apoptosis via activating receptor isoforms of KIR or CLIR is CsA dependent
Apoptosis induced by the activating isoforms of KIR and/or CLIR can represent a mechanism that limits cell tissue damage due to activation of NK cells against autologous cells. On the other hand, activating isoforms of KIR and/or CLIR can recognize at the cell surface of autologous tumor cells the corresponding HLA-I allele and, thus, this recognition can trigger target cell lysis. Thus, we analyzed whether the activating receptor–mediated NK cell apoptosis was sensitive to blocking drugs, different from activating receptor–mediated target cell lysis. In this context, it has been shown that NK cells lyse tumor target cells by means of secretion of perforins and granzymes present in intracellular discrete granules and that this process is sensitive to the specific inhibitor of vacuolar type H+-ATPase concanamycin (CMA).24,25 On the other hand, the FasL induction and its consequent secretion, in human NK cells, is strictly dependent on the activation of calcineurin and nuclear factors of activated T cell (NFAT), which in turn are highly cyclosporin A sensitive.23 Thus, we analyzed whether apoptosis and/or cytolysis induced through activating receptor were alternatively sensitive to CsA or CMA.
NK cell treatment with CsA, in a dose-dependent fashion, led to a strong reduction of sHLA-I–mediated apoptosis through ligation of activating receptor (Figure 5A). Indeed, the apoptosis induced by ligation of p50.3 on the NK cell clone 12, with either sHLA-Cw3 or with anti-p50 mAb followed by optimal cross-linking with GAM-coated beads, was reduced by 99% and 92%, respectively, in the presence of 500 ng/mL CsA (Figure 5A). Importantly, CsA treatment of NK cells was accompanied by a strong reduction of the amount of sFasL present in culture supernatant after ligation of p50.3 with sHLACw3 or with anti-p50 mAb followed by optimal cross-linking with GAM-coated beads (Figure 5B). Although not shown, similar results were obtained with NK cell clones bearing the activating receptor CD158a or CD158b or CD94. By contrast, CMA only marginally influenced NK cell apoptosis induced by p50.3 (Figure 5C) or via activating receptor isoforms of CD158a or CD158b or CD94 (not shown).
Activating receptor–mediated NK cell apoptosis is cyclosporin A sensitive.
(A) The NK cell clone 45 (CD94+ activating; ▴) or the NK cell clone 12 (p50.3+ activating; ▪) was incubated with their specific sHLA ligands (sHLA non-A, -B, -C, and -G [that is, HLA-E and HLA-F] for CD94/NKG2 complex or sHLA-Cw3 for p50.3) either alone (0 ng/mL CsA; left symbols in panel A) or with increasing doses of cyclosporin A (5, 50, 500 ng/mL) for 48 hours, and apoptosis was evaluated by FITC–annexin V labeling. Results are expressed as the percentage of maximal apoptosis. Maximal apoptosis was 85% for NK cell clone 45 and 78% for NK cell clone 12, respectively. ♦ indicates percentage of maximal apoptosis of the NK cell clone 45 incubated for 48 hours in medium alone. (B) Amount of sFasL present in culture supernatant recovered after 24 hours of incubation under the experimental conditions described in panel A with the same NK cell clones. Results are expressed as nanograms per milliliter as determined by ELISA. (C) Concanamycin A does not affect activating receptor–mediated apoptosis. The NK cell clone 12 (p50.3+activating) was incubated with sHLA-Cw3 or GAM-coated beads (4 per cell) after staining with anti-p50 mAb (1 μg/mL) (p50.3-XL) for 48 hours in the presence or absence of 3 μg/mL concanamycin A or with 500 ng/mL CsA and stained with FITC–annexin V. “nil” indicates apoptosis in medium alone. Results are expressed as the percentage of apoptotic cells (FITC–annexin V+ but PI−) and are representative of 3 independent experiments.
Activating receptor–mediated NK cell apoptosis is cyclosporin A sensitive.
(A) The NK cell clone 45 (CD94+ activating; ▴) or the NK cell clone 12 (p50.3+ activating; ▪) was incubated with their specific sHLA ligands (sHLA non-A, -B, -C, and -G [that is, HLA-E and HLA-F] for CD94/NKG2 complex or sHLA-Cw3 for p50.3) either alone (0 ng/mL CsA; left symbols in panel A) or with increasing doses of cyclosporin A (5, 50, 500 ng/mL) for 48 hours, and apoptosis was evaluated by FITC–annexin V labeling. Results are expressed as the percentage of maximal apoptosis. Maximal apoptosis was 85% for NK cell clone 45 and 78% for NK cell clone 12, respectively. ♦ indicates percentage of maximal apoptosis of the NK cell clone 45 incubated for 48 hours in medium alone. (B) Amount of sFasL present in culture supernatant recovered after 24 hours of incubation under the experimental conditions described in panel A with the same NK cell clones. Results are expressed as nanograms per milliliter as determined by ELISA. (C) Concanamycin A does not affect activating receptor–mediated apoptosis. The NK cell clone 12 (p50.3+activating) was incubated with sHLA-Cw3 or GAM-coated beads (4 per cell) after staining with anti-p50 mAb (1 μg/mL) (p50.3-XL) for 48 hours in the presence or absence of 3 μg/mL concanamycin A or with 500 ng/mL CsA and stained with FITC–annexin V. “nil” indicates apoptosis in medium alone. Results are expressed as the percentage of apoptotic cells (FITC–annexin V+ but PI−) and are representative of 3 independent experiments.
As shown in Figure 6A-B, CMA strongly reduced (by 50%), in a killing assay, lysis of P815 target cells induced by the engagement of activating isoforms of KIR or CLIR, whereas CsA had no effect. Furthermore, CsA did not inhibit NK cell–mediated lysis of different tumor target cells including K562 (Figure 6C), 721.221 (Figure 6D) and Jurkat (Figure 6E) cell lines, whereas CMA reduced lysis of these target cells by 50% to 80% (Figure 5C-E). Altogether, these findings indicate that apoptosis induced via the engagement of activating receptor is selectively CsA sensitive but this drug does not affect the activating receptor–mediated activation of NK cell cytolysis.
Activating receptor–mediated triggering of NK cell cytolysis is not cyclosporin A sensitive.
(A-B) Killing assay using the murine FcγR+ P815 cell line with the NK cell clone 262 ([A] CD158a+activating) or the NK cell clone 10 ([B] CD94+activating) at the effector-target ratio (E/T) of 2:1 in the presence of anti-CD158a mAb (A) or anti-CD94 mAb (B) with 3 μg/mL CMA or 500 ng/mL CsA. Results obtained with anti-CD16 mAb are shown for comparison. “nil” indicates NK cell cytolysis in the absence of mAb. Cytolytic activity of the NK cell clone 45 against K562 (C) or 721.221 (D) or Jurkat (E) target cells in medium alone (○) or in the presence of either 3 μg/mL CMA (▪) or 500 ng/mL CsA (▴) was evaluated in a 4-hour 51Cr-release cytotolytic assay at the indicated E/T ratio. Results are expressed as the percentage of specific 51Cr release and are representative of 3 independent experiments using different NK cell clones.
Activating receptor–mediated triggering of NK cell cytolysis is not cyclosporin A sensitive.
(A-B) Killing assay using the murine FcγR+ P815 cell line with the NK cell clone 262 ([A] CD158a+activating) or the NK cell clone 10 ([B] CD94+activating) at the effector-target ratio (E/T) of 2:1 in the presence of anti-CD158a mAb (A) or anti-CD94 mAb (B) with 3 μg/mL CMA or 500 ng/mL CsA. Results obtained with anti-CD16 mAb are shown for comparison. “nil” indicates NK cell cytolysis in the absence of mAb. Cytolytic activity of the NK cell clone 45 against K562 (C) or 721.221 (D) or Jurkat (E) target cells in medium alone (○) or in the presence of either 3 μg/mL CMA (▪) or 500 ng/mL CsA (▴) was evaluated in a 4-hour 51Cr-release cytotolytic assay at the indicated E/T ratio. Results are expressed as the percentage of specific 51Cr release and are representative of 3 independent experiments using different NK cell clones.
Soluble HLA-I induces IFN-γ production by the engagement of activating isoforms of KIR or CLIR
To determine whether the engagement of activating receptor with specific sHLA-I alleles can stimulate NK cells to produce inflammatory cytokines able to regulate immune cell responses, supernatant derived from NK cells incubated with sHLA-I were analyzed for the presence of IFN-γ by ELISA. As shown in Figure7A, a detectable amount of IFN-γ was found in culture supernatant of the representative CD158a+NK cell clone 262 incubated with sHLA-Cw4. Covering of CD158a with a specific mAb almost completely inhibited sHLA-Cw4–induced IFN-γ production (Figure 7A), indicating that ligation of CD158a with corresponding sHLA-I allele was necessary to deliver the activating signal leading to IFN-γ production. As clone 262 expressed CD8 at low levels, we further analyzed whether IFN-γ found in culture supernatant was due to the engagement of CD8 by sHLA-Cw4. As shown in Figure 7A, anti-CD8 mAb had no effect on sHLA-Cw4–induced IFN-γ production, indicating that CD8 was not involved in activating receptor–mediated IFN-γ production.
Soluble HLA-I alleles trigger NK cells to produce IFN-γ upon ligation of activating receptor.
(A) The NK cell clone 262 (CD158a+ activating) was incubated for 24 hours with either sHLA-Cw4 or unrelated sHLA-A2 allele alone or with sHLA-Cw4 after covering of CD158a or CD8 with either anti-CD158a– or anti-CD8–specific mAb (1 μg/mL), respectively. (B) Production of IFN-γ upon incubation of the NK cell clone 262 with the indicated increasing amounts of the specific ligand sHLA-Cw4. (C) IFN-γ production by the NK cell clone 45 (CD94+activating) in the presence of sHLA non-A, -B, -C, and -G alone (that is, putative HLA-E) or after covering of CD94 with anti-CD94 mAb (1 μg/mL). The amount of IFN-γ produced upon incubation of the NK cell clone 45 (CD94+activating) with anti-CD94 or anti-CD16 or anti-CD54 mAb followed by GAM-coated beads (4 per cell) (to induce optimal cross-linking of the indicated molecules, CD94-XL, CD16-XL, CD54-XL) is shown for comparison. (D) The NK cell clone 262 (CD158a+activating) was incubated with sHLA-Cw4 (▪) or with anti-CD158a mAb followed by optimal cross-linking with GAM-coated beads (4 per cell) (■) for 24 hours alone or in the presence of increasing doses of CsA (5, 50, 500 ng/mL). ▴ indicates IFN-γ produced by the same NK cell clone in medium alone. Culture supernatant was recovered and analyzed for the presence of IFN-γ by ELISA. Results are expressed as nanograms per milliliter and are representative of 3 independent experiments.
Soluble HLA-I alleles trigger NK cells to produce IFN-γ upon ligation of activating receptor.
(A) The NK cell clone 262 (CD158a+ activating) was incubated for 24 hours with either sHLA-Cw4 or unrelated sHLA-A2 allele alone or with sHLA-Cw4 after covering of CD158a or CD8 with either anti-CD158a– or anti-CD8–specific mAb (1 μg/mL), respectively. (B) Production of IFN-γ upon incubation of the NK cell clone 262 with the indicated increasing amounts of the specific ligand sHLA-Cw4. (C) IFN-γ production by the NK cell clone 45 (CD94+activating) in the presence of sHLA non-A, -B, -C, and -G alone (that is, putative HLA-E) or after covering of CD94 with anti-CD94 mAb (1 μg/mL). The amount of IFN-γ produced upon incubation of the NK cell clone 45 (CD94+activating) with anti-CD94 or anti-CD16 or anti-CD54 mAb followed by GAM-coated beads (4 per cell) (to induce optimal cross-linking of the indicated molecules, CD94-XL, CD16-XL, CD54-XL) is shown for comparison. (D) The NK cell clone 262 (CD158a+activating) was incubated with sHLA-Cw4 (▪) or with anti-CD158a mAb followed by optimal cross-linking with GAM-coated beads (4 per cell) (■) for 24 hours alone or in the presence of increasing doses of CsA (5, 50, 500 ng/mL). ▴ indicates IFN-γ produced by the same NK cell clone in medium alone. Culture supernatant was recovered and analyzed for the presence of IFN-γ by ELISA. Results are expressed as nanograms per milliliter and are representative of 3 independent experiments.
The amount of IFN-γ recovered in culture SN was proportional to the amount of the specific sHLA-I allele employed to stimulate NK cells (Figure 7B). Similar results were obtained when sHLA non-A, -B, -C, and -G was incubated with NK cells bearing the activating receptor CD94/NKG2 complex (Figure 7C). In this case, the amount of IFN-γ produced was similar to that obtained upon ligation of CD16 (Figure7C). Finally, the incubation of NK cells with increasing doses of CsA led to a progressively increased inhibition of IFN-γ production induced by sHLA-I–specific alleles (Figure 7D).
Discussion
Activating forms of KIR or CLIR can deliver an apoptotic signal in human NK cells after their engagement with the appropriate ligand represented by sHLA. This interaction leads to production and secretion of FasL, which in turn induces apoptosis upon ligation with Fas at the NK cell surface. This phenomenon is accompanied by the production of the proinflammatory cytokine IFN-γ. Interestingly, apoptosis induced via activating receptor for HLA-I, at variance with the activation of NK cell–mediated cytolytic activity, is strictly dependent on the immunosupressive drug CsA.
The physiological significance of the presence of activating receptor at the NK cell surface specific for discrete HLA-I allele is still debated.1-3 Conceivably, these activating receptors react with the same HLA-I allele reacting with their inhibiting counterpart, but their engagement can induce the activation of NK cell–mediated functional activities such as cytolysis and cytokine production.1-3 Several authors have claimed that, to avoid autologous tissue cell damage induced by activating receptor–triggered cytolysis, the inhibiting receptors for the HLA-I can down-regulate this effect because the inhibitory signal via KIR and/or CLIR should overcome the triggering signal via their activating counterpart.1-3 If this is the case, because the HLA-I NK cell receptors seem to recognize specific HLA-I allele,1-3 NK cells should bear at the same time inhibiting and activating receptors specific for the same HLA-I allele; otherwise, the interaction of different HLA-I alleles with different counter-receptors not necessarily should take place in close proximity at the NK cell surface in order to shut down cytolysis initiated upon the engagement of activating receptor. In fact, it has been stated that inhibiting signal can switch off the activating one only if these occur closely in NK cells.1-3 Among the NK cell clones analyzed in this and previous studies8-13 we found that it is not exceptional to find NK cell clones that express an activating receptor for one HLA-I allele and inhibiting receptors for other HLA-I alleles. Indeed, we found NK cell clones with activating receptor form of KIR but CLIR+, and vice versa, without any detectable expression (both in immunofluorescence and functional assays) of known inhibiting form of KIR (not shown). In addition, although to a very low frequency (in our culture conditions) some NK cell clones with only activating forms of KIR and CLIR can be found. In these instances, it would be very hard to block NK cell–mediated cytolysis of HLA-I+ autologous cells bearing the appropriate counter-receptor. Our present results can explain, at the same time, because NK cell clones with activating receptor do not usually exist at higher frequencies and because they do not kill all autologous cells. Indeed, a certain amount ranging from 0.5 to 2 μg/mL sHLA-I (composed of a mixture of HLA-A, -B, and -C alleles) is present in serum of healthy donors,17-19,27,41 this amount would be enough to induce apoptosis of activated NK cells with activating receptor without any NK cell interaction with autologous cells. In addition, during the interaction with self–HLA-I+ tissue cells, activating receptor on NK cells may deliver the apoptotic signal, thus leading to autolimitation of any self-reaction. If this were true, what is the physiological significance of activating receptor on NK cells? One can hypothesize that activating receptor plays a role in the elimination of tumor cells. Indeed, this study and others show that when tumor cells express the appropriate self–HLA-I antigens, it is possible that the engagement of activating receptor for HLA-I can efficiently trigger cytolysis.8-13 Thus, NK cells kill self-tumor target cells by means such as perforins and granzymes, and the consequent up-regulation of production and secretion of FasL leading to NK cell apoptosis can switch off innate immune response.
Recently, several receptors responsible for NK cell–mediated cytolysis have been identified.4,5,42 These receptors are represented by natural cytotoxicity receptors (NCR), including NKp30, NKp44, and NKp46 molecules,4 which are specifically expressed only by NK cells, and NKG2D5,42 that is present on NK cells and on cytolytic lymphocytes bearing α/β or γ/δ T-cell receptor (TCR).5,42Apparently, NCR and NKG2D can recognize target cells independently on the HLA-I expression.4,5,42 This finding supports the hypothesis that NK cells can use activating receptors for HLA-I when HLA-I is expressed on target cells while they are triggered by NCR and/or NKG2D upon interaction with HLA-I– tumor target cells. Indeed, it has been demonstrated that tumor cells do not express HLA-I in several instances.43 The interrelationship between activating receptors for HLA-I− and NCR and/or NKG2D remains to be analyzed. In fact, a cross-talk between activating receptors for HLA-I and NCR/NKG2D could take place during interaction with HLA-I+ target cells bearing at the same time counter-receptors for NCR and/or NKG2D. If NCR or NKG2D, and likewise CD16,44 can induce apoptosis of NK cells, they could play—besides activating receptors for HLA-I—a key role in down-regulating NK cell–mediated cytolysis.
Notably, we found that FasL-induced apoptosis of NK cells is strongly reduced by the immunosuppressive drug CsA. Indeed, CsA can block FasL expression induced via T-cell antigen receptor in cytolytic CD8+ T-cell clones.45 Thus, CsA treatment of NK cell clones with activating receptor may be a tool to maintain killing of autologous tumor target cells without the elimination of these really potent cytolytic effector cells. In fact, CsA did not inhibit activating receptor–mediated cytolysis as well as triggering of NK cells via CD16 (this study) or other activating NK cell receptors as CD69 (not shown), suggesting that CsA did not affect NK cell–mediated killing.
However, it has been reported that cytolytic effector cells can lyse tumor target cells by 2 independent mechanisms: the first is represented by degranulation of perforin/granzyme, whereas the second one is FasL mediated. In fact, several tumor target cells express at the cell surface Fas antigen and can die upon Fas engagement by FasL.25 46-48 CsA administration could then block the second cytolytic pathway of inducing tumor target cell death. Thus, to plan a CsA treatment of patients affected by neoplasia, one should consider that a certain tumor can show different sensitivity to cytolysis mediated via the 2 above-mentioned mechanisms. In fact, one should first define whether a correlation exists between tumor histotypes and their sensitivity to CsA-treated NK cells in order to select which tumor is much more sensitive to cytolysis via perforin/granzymes. Secondly, CsA treatment can strongly augment the frequency of NK cells bearing activating receptor for self–HLA-I antigens and, thus, aside from the potential and wished elimination of tumor cells, it can evoke an undesired powerful autoimmune reaction.
Our findings also indicate a possible mechanism of tumor escape. In fact, tumor cells can shed sHLA-I molecules, which upon interaction with activating receptor for HLA-I on the NK cell surface may induce and secrete FasL, which in turn evokes NK cell apoptosis. This effect could take place anywhere—either far from tumor cell localization or in close proximity of tumor cells. Thus, inactivation of NK cells with activating receptor is obtained by their own apoptosis far from tumor cells without any evident effect on tumor cells. In other circumstances, the interaction of sHLA-I with activating receptor not only leads to NK cell death, but it can induce production and secretion of a sufficient amount of IFN-γ, which in turn may up-regulate the expression of HLA-I on target cells, possibly leading to a stronger inhibitory effect on NK cells via KIR and/or CLIR. Otherwise, IFN-γ can increase the shedding of HLA-I from tumor cells and thus increase the degree of NK cell apoptosis.
In addition, it is well known that NK cells express CD8, another receptor for a monomorphic portion of HLA-I. Recently, we have demonstrated that the engagement of CD8 induces NK cell apoptosis via FasL-Fas interaction.6 Indeed, we found a direct correlation between the level of CD8 expression on a given clone with the degree of sHLA-I–induced NK cell apoptosis. On this basis, we defined that only NK cell clones with intermediate or bright expression of CD8 were susceptible to sHLA-I–mediated apoptosis.6Furthermore, we observed that the same NK cell clone could increase the expression of CD8 during the culture period, thus increasing its susceptibility to sHLA-I–mediated apoptosis. Herein, we could not find a correlation between the level of expression of activating receptor and the degree of their induced apoptosis (not shown). In fact, the amount of a given activating receptor for HLA-I remained constant along the culture period analyzed (4 to 8 weeks; not shown). Altogether, these findings would suggest that down-regulation of NK cell–mediated functional activities consequent to apoptosis may be determined by 2 distinct types of HLA-I receptors with different regulation of cell surface expression and be able to recognize peculiar portions of HLA-I. In fact, it has been demonstrated that CD8 can recognize the α3 domain of HLA-I, common to different alleles,49-53 whereas activating and/or inhibiting forms of KIR and CLIR would bind to a specific HLA-I allele and site-directed mutagenesis of critical amino acidic residues contained into the α2 domain is sufficient to avoid this interaction.1-3 Herein, we have chosen NK cell clones that do not express CD8 in order to analyze only the contribution of activating receptor for HLA-I to NK cell apoptosis. These findings and our previous report6suggest that CD8 and activating receptor function independently, and thus the final outcome of their engagement can be different due to the presence in the extracellular milieu of either a given or a mixture of several HLA-I alleles.
Prepublished online as Blood First Edition Paper, July 18, 2002; DOI 10.1182/blood-2002-04-1284.
Supported in part by grants from Ministero della Sanità(2000-2002) and from Associazione Italiana per la Ricerca sul Cancro (AIRC2002) (A.P.); Istituto Superiore di Sanità (ISS) AIDS project (M.R.Z.), and MURST National Program 2000 MN06118858-001 (F.P.). G.M.S. is a fellow of Federazione Italiana per la Ricerca sul Cancro (FIRC).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Alessandro Poggi, Laboratory of Immunology, National Institute for Cancer Research (IST), c/o CBA, Torre A1, Largo R. Benzi, 10, 16132 Genoa, Italy; e-mail:poggi@vega.cba.unige.it.

![Fig. 2. Soluble HLA-I–specific alleles induce NK cell apoptosis upon interaction with activating forms of either killer Ig-like receptor (KIR) or C-type lectin inhibitory receptor (CLIR). / (A) One-dimensional PAGE under nonreducing/nondenaturing conditions and silver staining of sHLA-I molecules used to induce apoptosis of NK cells. These molecules were isolated from culture supernatant of either untransfected (lane 1: sHLA non-A, -B, -C, and -G [that is, putative sHLA-E and -F]) or HLA-I allele–transfected (lane 2, Cw3; lane 3, Cw4; lane 4, A2; lane 5, sHLA-G) 721.221 cells 62829 by precipitation with ammonium sulfate, low-medium pressure chromatography, strong anionic and strong cationic ion exchange, and gel filtration and purified by affinity chromatography on anti–HLA class I mAb W6/32 coupled to cyanogen bromide. Molecular weights are indicated on the left. (B-E) NK cell clones selected for the expression of activating isoforms of CD158a ([B] clone 262; [D] clone 255) or CD158b ([C] clone A1.25) or CD94/NKG2 ([E] clone 45) were incubated for 48 hours with their specific sHLA-I ligand (sHLA-Cw4 for CD158a; sHLA-Cw3 for CD158b; sHLA non-A, -B, -C, and -G [that is, putative HLA-E and -F] for CD94/NKG2 complex, respectively), and percentage of apoptotic cells was determined by labeling cells with FITC-conjugated annexin V. In some experiments, covering of activating receptor (CD158a, CD158b, CD94/NKG2) was obtained by preincubating NK cells with specific anti-CD158a or CD158b or CD94 mAb. NK cell apoptosis induced by activating receptor was also achieved upon cross-linking of these receptors with specific mAb (1 μg/mL) followed by GAM-coated beads (4 per cell) ([A,C] CD158a-XL; [B] CD158b-XL; [D] CD94-XL). nil and CD54-XL:NK cells incubated with medium alone or upon cross-linking of CD54. In panel C, either covering of CD8 or its optimal cross-linking was obtained as for activating receptor to determine the role of CD8 in NK cell clones bearing activating receptor. Results are expressed as the percentage of apoptotic cells (FITC–annexin V+ but PI−) and are representative of 3 independent experiments using 3 different NK cell clones for each activating receptor.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/100/12/10.1182_blood-2002-04-1284/5/m_h82323474002.jpeg?Expires=1766279320&Signature=gVBYXsI6KZjl-sTdPJ7MhNsOsAfT4o1N3kiL30DFCeRUWJlZH6fW0WI~GgqnZn4KL6ovWnGrQL1GprOJFImIl0FAVN3HOSOjSaeN149OtukohciFy1sOjh4FjRpkTnYIpaLR78vzGEOdrvxQVpMj0ZaP3cMvYWtbmQfbhpxfvIWUQyPV0u7EwuqENHQuUPNpsd6PqhmDQJQQIQ~BjaBPIbjeT0t1Y8U5uPu~gsWenvHadxClOo~uZxdaRuIjvT73wvZj2U5V8QgtcPOAcuJKG7RLDVUeJ2elxC1PeHgwzLjVHBgVmtKgkQxUWUowmW8EJ~surhZ8w5phsp6sVkd~ZQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 3. Annexin V stainings and DNA analysis of NK cells bearing activating receptor incubated with the corresponding specific sHLA ligand. / Dose-response and kinetics of activating receptor–mediated apoptosis. (A) The NK cell clone 45 bearing the activating form of C-type lectin inhibiting receptor CD94 was incubated for 48 hours with medium alone (top left dot plot) or sHLA non-A, -B, -C, and -G (that is, putative sHLA-E and F) alone (top right dot plot) or after preincubation with anti-CD94 mAb (bottom left dot plot) or with anti-CD94 mAb (1 μg/mL) followed by GAM-coated beads to obtained optimal cross-linking (bottom right dot plot). Cells were then stained with FITC-conjugated annexin V and PI and analyzed on a FACSort. Results are expressed as log green fluorescence intensity versus log red fluorescence intensity (au), and shown in the lower right portion of each dot plot is the percentage of annexin V+PI− cells; that is, apoptotic cells. (B-C) DNA analysis with PI labeling or laddering of the NK cell clone 45 upon incubation for 72 hours with medium alone (Bi; C, lane 1) or sHLA non-A, -B, -C, and -G alone (Bii; C, lane 2) or after covering of CD94/NKG2 complex with anti-CD94–specific mAb (Biii; C, lane 3). In panel A, NK cells were labeled with PI and analyzed on a FACSort. Results are expressed as log red fluorescence intensity (arbitrary units [au]) (x-axis) versus cell number (y-axis). Numbers in each subpanel indicate the percentage of DNA content less than 2n; that is, apoptotic DNA. In panel C, DNA isolated from NK cells was subjected to agarose gel electrophoresis. DNA markers are shown on the left in base pairs (bp). (D) Titration of sHLA-I–induced apoptosis. The NK cell clone 255 bearing the activating receptor of CD158a was incubated with increasing amounts of sHLA-Cw4 for 48 hours, and apoptosis was evaluated after labeling with FITC–annexin V. Results are expressed as the percentage maximal apoptosis. Maximal apoptosis corresponds to 45% of apoptotic cells. (E) Kinetics of activating receptor–mediated apoptosis. The NK cell clone 255 activating receptor+CD158a+ (▪) or the NK cell clone 10 (activating receptor+ CD94+) (▴) was incubated with either sHLA-Cw4 or sHLA non-A, -B, -C, and -G (that is, putative HLA-E) for the indicated periods of time, and apoptosis was evaluated by staining cells with FITC–annexin V. ● indicates apoptosis of the NK cell clone 255 incubated with medium alone. Results are expressed as the percentage of apoptotic cells that are annexin V+ but PI−.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/100/12/10.1182_blood-2002-04-1284/5/m_h82323474003.jpeg?Expires=1766279320&Signature=su~AvTduIQ2Tb8oljRbZ37O1ZIrto4IaIImI8p2SILAKp7pi-QY~aMNLogzQn-6lKb4I~TduhOV2f93vZMBOiR11BAdZYdCI8c0PON3wP7Q3MhjNizV4Yyu4isPILIsgNv3LuCUNEgGBbiCVKpvbUVNGxYjpxPkWde2I8iGkbZk8Sx6mGAvnhvWE5wWjiyzZ4zm1Uzq-~zisZLo5Iam~p8xuP0X6HESk1p8ScBPhQLFpI8zy7aWUK4Umivr-1-aJ9NcKpb4NMnru5--TYMd4VBnWhvoFPWNdqLV3DtrVaY~828PVRLerDcbrZSm2rMxDo0vKSGdYFvD0kfcD-rvP7g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 4. FasL-Fas interaction is responsible for activating receptor–mediated NK cell apoptosis. / (A) The NK cell clone 10 (CD94+ activating) was incubated with sHLA non-A, -B, -C, and -G (that is, putative HLA-E and HLA-F) for 48 hours alone or after preincubation with anti-Fas mAb (5 μg/mL) or in the presence of anti-FasL mAb (5 μg/mL) or anti-Fas plus anti-FasL mAbs (5 μg/mL plus 5 μg/mL) in combination. Apoptosis was evaluated by labeling NK cells with FITC–annexin V. The effect of the covering of CD94 (achieved by preincubating NK cells with anti-CD94–specific mAb) is shown for comparison. Results are expressed as the percentage of apoptotic cells (annexin V+ but PI−). “nil” indicates apoptosis in medium alone. (B-D) ELISA for the presence of FasL in supernatant recovered from the NK cell clone 262 (CD158a+ activating) (B) or A1.25 (CD158b+ activating) (C) or 1 (CD94+activating) (D) incubated for 48 hours with the corresponding sHLA-I allele ([B] sHLA-Cw4; [C] sHLA-Cw3; [D] sHLA non-A, -B, -C, and -G) alone or after covering of activating receptor with specific mAb ([B] anti-CD158a plus sHLA-Cw4; [C] anti-CD158b plus sHLA-Cw3; [D] anti-CD94 plus sHLA non-A, -B, -C, and -G). Some experiments were performed upon optimal cross-linking of the indicated activating receptor with specific mAb (1 μg/mL) followed by GAM-coated beads (4 per cell) ([B] CD158a-XL; [C] CD158b-XL; [D] CD94-XL) or with unrelated sHLA-A2 allele (C) or sHLA-Cw3 after covering of CD8 (C) with anti-CD8 mAb (OKT8, 1 μg/mL). (E) Analysis of mRNA coding for FasL in the NK cell clone 262 bearing activating form of CD158a upon incubation for 6 hours with sHLA-Cw4 alone or after covering of CD158a+ with specific anti-CD158a mAb. “nil” represents mRNA coding for FasL in NK cells incubated with medium alone, and results obtained after optimal cross-linking of CD158a achieved using anti-CD158a mAb followed by GAM-coated beads (CD158a-XL) are shown for comparison.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/100/12/10.1182_blood-2002-04-1284/5/m_h82323474004.jpeg?Expires=1766279320&Signature=gw3H47XDNOJEMLrISCurdGpCKYbA9z8YFIl4TOk5DHvaMExpChnH6TdmwRmvu6yK1AsfZkwpa2mr-0-87Cq-zhHBSY9B0u7nvCAppgjooQqUw8oC0ujtxguKDPkqcvjjDMzLXOClh63uTCk~KWHcYeQwOTZ~gq2DRL9Xt42BXusM4XEP2IRJMKd9UHrdp57e4WraQET1S0H5dGymP1rZubApxdoAEGGNptvcDR1Egz2JjTycImgbLs~cgmX~2d~FmcoA9FciASVlcFkV2qRgAjYHmoMpy43hOYPnEy5AWIaNj4luJaT~RP2lUmvNPEarpUmLezJ9Cis69pwxhntXxw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 5. Activating receptor–mediated NK cell apoptosis is cyclosporin A sensitive. / (A) The NK cell clone 45 (CD94+ activating; ▴) or the NK cell clone 12 (p50.3+ activating; ▪) was incubated with their specific sHLA ligands (sHLA non-A, -B, -C, and -G [that is, HLA-E and HLA-F] for CD94/NKG2 complex or sHLA-Cw3 for p50.3) either alone (0 ng/mL CsA; left symbols in panel A) or with increasing doses of cyclosporin A (5, 50, 500 ng/mL) for 48 hours, and apoptosis was evaluated by FITC–annexin V labeling. Results are expressed as the percentage of maximal apoptosis. Maximal apoptosis was 85% for NK cell clone 45 and 78% for NK cell clone 12, respectively. ♦ indicates percentage of maximal apoptosis of the NK cell clone 45 incubated for 48 hours in medium alone. (B) Amount of sFasL present in culture supernatant recovered after 24 hours of incubation under the experimental conditions described in panel A with the same NK cell clones. Results are expressed as nanograms per milliliter as determined by ELISA. (C) Concanamycin A does not affect activating receptor–mediated apoptosis. The NK cell clone 12 (p50.3+activating) was incubated with sHLA-Cw3 or GAM-coated beads (4 per cell) after staining with anti-p50 mAb (1 μg/mL) (p50.3-XL) for 48 hours in the presence or absence of 3 μg/mL concanamycin A or with 500 ng/mL CsA and stained with FITC–annexin V. “nil” indicates apoptosis in medium alone. Results are expressed as the percentage of apoptotic cells (FITC–annexin V+ but PI−) and are representative of 3 independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/100/12/10.1182_blood-2002-04-1284/5/m_h82323474005.jpeg?Expires=1766279320&Signature=aL--VDolixekRi~xp4MI2b9Mj2~yaCHuzOnSMTzD-NlNyeeemCOFNbpL222Dexfl6A7YNbYPxwNmB319ubMi2j5Uo3vDBJRJc1Ca~zDsTbvENuQ6PAJK7KrNGEXp~vrHlYn4QmnOXLHZSPAhaCp93mkSBL0~Ymfdm4W-w1iffTnbxNqJdb837SZ6pKUl~82OAt7HYlw8Z9aA~1~vxB0SC3hiy7bfN86Mot~2ZIERP~PY8iiy61YhX3TiTL4eVX1uUucYdsI0nT~GmVXhKCELApRMfy0baT3jJD9qQcQw3m-rP8oeCCTfuMZpLxZRvEvXlR~Jx-qiBbuDPA3PANjauw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 6. Activating receptor–mediated triggering of NK cell cytolysis is not cyclosporin A sensitive. / (A-B) Killing assay using the murine FcγR+ P815 cell line with the NK cell clone 262 ([A] CD158a+activating) or the NK cell clone 10 ([B] CD94+activating) at the effector-target ratio (E/T) of 2:1 in the presence of anti-CD158a mAb (A) or anti-CD94 mAb (B) with 3 μg/mL CMA or 500 ng/mL CsA. Results obtained with anti-CD16 mAb are shown for comparison. “nil” indicates NK cell cytolysis in the absence of mAb. Cytolytic activity of the NK cell clone 45 against K562 (C) or 721.221 (D) or Jurkat (E) target cells in medium alone (○) or in the presence of either 3 μg/mL CMA (▪) or 500 ng/mL CsA (▴) was evaluated in a 4-hour 51Cr-release cytotolytic assay at the indicated E/T ratio. Results are expressed as the percentage of specific 51Cr release and are representative of 3 independent experiments using different NK cell clones.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/100/12/10.1182_blood-2002-04-1284/5/m_h82323474006.jpeg?Expires=1766279320&Signature=tsYEzi1~V0R33FmEBhBMy-uMJP7cMLhQ53DBJCkkosw3MA1PmzJfKEnItxsE5bA6RUsmto7DsjKXSyGgoBmkN0o2rKVipDvB0cawD2Sf6O-HlSPvP1gQYh70SUbAcy4PNr2eBEYztsiPlFoG~NXX-o6FOBZth2gAX-UmqCvdYC2fbLmnOCDplRIjyUIGOWy86HmI6Odvxgf90GYi12vzBZQmV6Dt5FUjn7dupcnuFNYHQnDJ-YPqRhRSKQ96G9Y9wEUvtudn6dIQmQOkf-r~63JhbBshBFOtkL2DNGaLL692Rs0TFTvXsJvcx0scgGs~9QLmGQZnugxsiZTzjJiC3Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)


![Fig. 2. Soluble HLA-I–specific alleles induce NK cell apoptosis upon interaction with activating forms of either killer Ig-like receptor (KIR) or C-type lectin inhibitory receptor (CLIR). / (A) One-dimensional PAGE under nonreducing/nondenaturing conditions and silver staining of sHLA-I molecules used to induce apoptosis of NK cells. These molecules were isolated from culture supernatant of either untransfected (lane 1: sHLA non-A, -B, -C, and -G [that is, putative sHLA-E and -F]) or HLA-I allele–transfected (lane 2, Cw3; lane 3, Cw4; lane 4, A2; lane 5, sHLA-G) 721.221 cells 62829 by precipitation with ammonium sulfate, low-medium pressure chromatography, strong anionic and strong cationic ion exchange, and gel filtration and purified by affinity chromatography on anti–HLA class I mAb W6/32 coupled to cyanogen bromide. Molecular weights are indicated on the left. (B-E) NK cell clones selected for the expression of activating isoforms of CD158a ([B] clone 262; [D] clone 255) or CD158b ([C] clone A1.25) or CD94/NKG2 ([E] clone 45) were incubated for 48 hours with their specific sHLA-I ligand (sHLA-Cw4 for CD158a; sHLA-Cw3 for CD158b; sHLA non-A, -B, -C, and -G [that is, putative HLA-E and -F] for CD94/NKG2 complex, respectively), and percentage of apoptotic cells was determined by labeling cells with FITC-conjugated annexin V. In some experiments, covering of activating receptor (CD158a, CD158b, CD94/NKG2) was obtained by preincubating NK cells with specific anti-CD158a or CD158b or CD94 mAb. NK cell apoptosis induced by activating receptor was also achieved upon cross-linking of these receptors with specific mAb (1 μg/mL) followed by GAM-coated beads (4 per cell) ([A,C] CD158a-XL; [B] CD158b-XL; [D] CD94-XL). nil and CD54-XL:NK cells incubated with medium alone or upon cross-linking of CD54. In panel C, either covering of CD8 or its optimal cross-linking was obtained as for activating receptor to determine the role of CD8 in NK cell clones bearing activating receptor. Results are expressed as the percentage of apoptotic cells (FITC–annexin V+ but PI−) and are representative of 3 independent experiments using 3 different NK cell clones for each activating receptor.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/100/12/10.1182_blood-2002-04-1284/5/m_h82323474002.jpeg?Expires=1766447394&Signature=i24IMU8dRBVBqOXz87ossN~44VHdMD-UomAwhgeHFaMm4f~PLbgHrJcx-3XN2evge7tWii6czqDHzLmBvSktQjtZMgnnjmEWYXPj~gFaxNhrkztl~KoVZayf2v9Oxevh~7tMI2fo-gdujB-TQdL6Vde1tTczpNGyMGudbVJPcM45W4JUNQFKaZBHRzZT52IUYaQ2Ae~QevYYigMXlX604lvsOK0TRoZOerBYt~nhgoiM8R1O4Pr3VanU9Asp74Hp3Q-Qk1uGX1-cU9xgRlJ4g3BBMnsEe4nFSTwPn4fSBd26Wl0A4QdBpjpRvGsWaApaX9xjgy7KZ9d~QsotvJoVMA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 3. Annexin V stainings and DNA analysis of NK cells bearing activating receptor incubated with the corresponding specific sHLA ligand. / Dose-response and kinetics of activating receptor–mediated apoptosis. (A) The NK cell clone 45 bearing the activating form of C-type lectin inhibiting receptor CD94 was incubated for 48 hours with medium alone (top left dot plot) or sHLA non-A, -B, -C, and -G (that is, putative sHLA-E and F) alone (top right dot plot) or after preincubation with anti-CD94 mAb (bottom left dot plot) or with anti-CD94 mAb (1 μg/mL) followed by GAM-coated beads to obtained optimal cross-linking (bottom right dot plot). Cells were then stained with FITC-conjugated annexin V and PI and analyzed on a FACSort. Results are expressed as log green fluorescence intensity versus log red fluorescence intensity (au), and shown in the lower right portion of each dot plot is the percentage of annexin V+PI− cells; that is, apoptotic cells. (B-C) DNA analysis with PI labeling or laddering of the NK cell clone 45 upon incubation for 72 hours with medium alone (Bi; C, lane 1) or sHLA non-A, -B, -C, and -G alone (Bii; C, lane 2) or after covering of CD94/NKG2 complex with anti-CD94–specific mAb (Biii; C, lane 3). In panel A, NK cells were labeled with PI and analyzed on a FACSort. Results are expressed as log red fluorescence intensity (arbitrary units [au]) (x-axis) versus cell number (y-axis). Numbers in each subpanel indicate the percentage of DNA content less than 2n; that is, apoptotic DNA. In panel C, DNA isolated from NK cells was subjected to agarose gel electrophoresis. DNA markers are shown on the left in base pairs (bp). (D) Titration of sHLA-I–induced apoptosis. The NK cell clone 255 bearing the activating receptor of CD158a was incubated with increasing amounts of sHLA-Cw4 for 48 hours, and apoptosis was evaluated after labeling with FITC–annexin V. Results are expressed as the percentage maximal apoptosis. Maximal apoptosis corresponds to 45% of apoptotic cells. (E) Kinetics of activating receptor–mediated apoptosis. The NK cell clone 255 activating receptor+CD158a+ (▪) or the NK cell clone 10 (activating receptor+ CD94+) (▴) was incubated with either sHLA-Cw4 or sHLA non-A, -B, -C, and -G (that is, putative HLA-E) for the indicated periods of time, and apoptosis was evaluated by staining cells with FITC–annexin V. ● indicates apoptosis of the NK cell clone 255 incubated with medium alone. Results are expressed as the percentage of apoptotic cells that are annexin V+ but PI−.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/100/12/10.1182_blood-2002-04-1284/5/m_h82323474003.jpeg?Expires=1766447394&Signature=0ENMZmEtMCrHyzg7k0mXrNzuKDZahlQCN~-RYFWWFl6AjyIMNylq1nCmIUvXzPHld0O1xfe-lB-q9mFAJwEwgfbWFn58fHhHvn0Up0~Zf-v-jY1yOsHCubjCOD7V6~~LcdqYOwWerEXxk~etcboRIap4e0eLE-Px7WVw5uHuLv0lKPNy7UopghToxrYz-KkzrsLHeDIx6Ft82vraFzedzpJqVK4TUy9vY-WeXKd6vQQGjJjs6vtnz4IciXHFRDMgrdzynajO3UZM0LlCLNh1SNNGqir6ig51gArYtq0YiqCXLtrTaLZjWVF~j5rgrKjpDuzt4X2v6w7LMyxg2ET3Bw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 4. FasL-Fas interaction is responsible for activating receptor–mediated NK cell apoptosis. / (A) The NK cell clone 10 (CD94+ activating) was incubated with sHLA non-A, -B, -C, and -G (that is, putative HLA-E and HLA-F) for 48 hours alone or after preincubation with anti-Fas mAb (5 μg/mL) or in the presence of anti-FasL mAb (5 μg/mL) or anti-Fas plus anti-FasL mAbs (5 μg/mL plus 5 μg/mL) in combination. Apoptosis was evaluated by labeling NK cells with FITC–annexin V. The effect of the covering of CD94 (achieved by preincubating NK cells with anti-CD94–specific mAb) is shown for comparison. Results are expressed as the percentage of apoptotic cells (annexin V+ but PI−). “nil” indicates apoptosis in medium alone. (B-D) ELISA for the presence of FasL in supernatant recovered from the NK cell clone 262 (CD158a+ activating) (B) or A1.25 (CD158b+ activating) (C) or 1 (CD94+activating) (D) incubated for 48 hours with the corresponding sHLA-I allele ([B] sHLA-Cw4; [C] sHLA-Cw3; [D] sHLA non-A, -B, -C, and -G) alone or after covering of activating receptor with specific mAb ([B] anti-CD158a plus sHLA-Cw4; [C] anti-CD158b plus sHLA-Cw3; [D] anti-CD94 plus sHLA non-A, -B, -C, and -G). Some experiments were performed upon optimal cross-linking of the indicated activating receptor with specific mAb (1 μg/mL) followed by GAM-coated beads (4 per cell) ([B] CD158a-XL; [C] CD158b-XL; [D] CD94-XL) or with unrelated sHLA-A2 allele (C) or sHLA-Cw3 after covering of CD8 (C) with anti-CD8 mAb (OKT8, 1 μg/mL). (E) Analysis of mRNA coding for FasL in the NK cell clone 262 bearing activating form of CD158a upon incubation for 6 hours with sHLA-Cw4 alone or after covering of CD158a+ with specific anti-CD158a mAb. “nil” represents mRNA coding for FasL in NK cells incubated with medium alone, and results obtained after optimal cross-linking of CD158a achieved using anti-CD158a mAb followed by GAM-coated beads (CD158a-XL) are shown for comparison.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/100/12/10.1182_blood-2002-04-1284/5/m_h82323474004.jpeg?Expires=1766447394&Signature=gVXJ6GCkL-Fzz2-L1W-KTQf3QScJonT5iheE8tHt3q3C5kZ-CKQ5Fnb-FVDNZpGVag-XVj8MzUYogz9PtG~RyM6Cegd0ZEW8L7fysv1c~Ca5Vf4Yaon4wuGaiqfoV32JjDXzaeWfHXGKDf-r6F8blCk1TLctTabeUK57ZPlGIF7yULGuEUz6AqjnbtSPZiqzem3mK0J2J4HD-P4hXaOf8e26MifX9KYH-tspGY1NQU0DD-HiS04dEGE0abb9Qy1xK29-II7EMdjJ7bvzzxySLk5vdSJ-PYxZ9Y3P7xNLXIgx3TDmuv9mwVbGMJjFXgSB6Cl~6fONyXcG4Mw~nCIEkA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 5. Activating receptor–mediated NK cell apoptosis is cyclosporin A sensitive. / (A) The NK cell clone 45 (CD94+ activating; ▴) or the NK cell clone 12 (p50.3+ activating; ▪) was incubated with their specific sHLA ligands (sHLA non-A, -B, -C, and -G [that is, HLA-E and HLA-F] for CD94/NKG2 complex or sHLA-Cw3 for p50.3) either alone (0 ng/mL CsA; left symbols in panel A) or with increasing doses of cyclosporin A (5, 50, 500 ng/mL) for 48 hours, and apoptosis was evaluated by FITC–annexin V labeling. Results are expressed as the percentage of maximal apoptosis. Maximal apoptosis was 85% for NK cell clone 45 and 78% for NK cell clone 12, respectively. ♦ indicates percentage of maximal apoptosis of the NK cell clone 45 incubated for 48 hours in medium alone. (B) Amount of sFasL present in culture supernatant recovered after 24 hours of incubation under the experimental conditions described in panel A with the same NK cell clones. Results are expressed as nanograms per milliliter as determined by ELISA. (C) Concanamycin A does not affect activating receptor–mediated apoptosis. The NK cell clone 12 (p50.3+activating) was incubated with sHLA-Cw3 or GAM-coated beads (4 per cell) after staining with anti-p50 mAb (1 μg/mL) (p50.3-XL) for 48 hours in the presence or absence of 3 μg/mL concanamycin A or with 500 ng/mL CsA and stained with FITC–annexin V. “nil” indicates apoptosis in medium alone. Results are expressed as the percentage of apoptotic cells (FITC–annexin V+ but PI−) and are representative of 3 independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/100/12/10.1182_blood-2002-04-1284/5/m_h82323474005.jpeg?Expires=1766447394&Signature=O9tFz0QG8gV92Te39vUKEV~DL5dlYb5ARpoXCcHjF~bLxVBA9Nns7d7YY8xfspcrT5P73mnieyUxTzQkjA1iKY4QWIRE5KacmZmazh85Mrmilmj7drwiE~fftXO2VR7c6TFSClM~E7CCv9KEkJaG0RdPdur98Gh3ZBubXv8gGZ6OPXTSkytpqoBSqdvBY0cBId5amWlwRIHsdOwaTZV1suGwH2uqp4xflNiOqnqgVT0gXbmoCbofodXEuNSw~NHDDlVHmfo-wRomGjxQ8SxyI12BnexMCBFGPHYTZ71YbvUYKl6g9U6HCK4a758gjNLj464xOzxlwq50bGMD95RM9g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 6. Activating receptor–mediated triggering of NK cell cytolysis is not cyclosporin A sensitive. / (A-B) Killing assay using the murine FcγR+ P815 cell line with the NK cell clone 262 ([A] CD158a+activating) or the NK cell clone 10 ([B] CD94+activating) at the effector-target ratio (E/T) of 2:1 in the presence of anti-CD158a mAb (A) or anti-CD94 mAb (B) with 3 μg/mL CMA or 500 ng/mL CsA. Results obtained with anti-CD16 mAb are shown for comparison. “nil” indicates NK cell cytolysis in the absence of mAb. Cytolytic activity of the NK cell clone 45 against K562 (C) or 721.221 (D) or Jurkat (E) target cells in medium alone (○) or in the presence of either 3 μg/mL CMA (▪) or 500 ng/mL CsA (▴) was evaluated in a 4-hour 51Cr-release cytotolytic assay at the indicated E/T ratio. Results are expressed as the percentage of specific 51Cr release and are representative of 3 independent experiments using different NK cell clones.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/100/12/10.1182_blood-2002-04-1284/5/m_h82323474006.jpeg?Expires=1766447394&Signature=XaN0qhoSZ5dNo~8wiCUv4BYKCk23M6zL0onucuKFLB-WKqh0kVOX5l-uGeLnfYYVIbNxVBj9KHum8ip-4dQIhKaEgUoSPHJT3Uuvl5U1LPx4J1mm40PHkuo3scVYJw8n173R90CiIsd3vY9MWVaRURevChxKTbD0WHP5IbfhLWKJj0USM4hrXIGPhzmMxN0lEQ6Ac3DafdroPODRlOurRZ2Tj6f-vuKEcqe6hNRcZYvFaIMzZ0yJHqJz2IKpw5HPgvdKE2MgzHGpzlfXgv5Ns-FV7B8eKyKMMmV~AgY9QrQ3mEybP-LAuKKI1Ww8fR5LL5N~eM0uYedfC9dP5pZU2w__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
