We have previously reported that stressed apoptotic tumor cells are more immunogenic in vivo than nonstressed ones. Using confocal microscopy we have confirmed our previous observation that heat-stressed apoptotic 12B1-D1 leukemia cells(BCR-ABL+) express HSP60 and HSP72 on their surface. To explore how the immune system distinguishes stressed from nonstressed apoptotic tumor cells, we analyzed the responses of dendritic cells to these 2 types of apoptotic cells. We found that nonstressed and heat-stressed apoptotic 12B1-D1 cells were taken up by dendritic cells in a comparable fashion. However, when stressed apoptotic 12B1-D1 cells were coincubated with immature dendritic cells for 24 hours, this resulted in greater up-regulation of costimulatory molecules (CD40, CD80, and CD86) on the surface of dendritic cells. Moreover, stressed apoptotic 12B1-D1 cells were more effective in stimulating dendritic cells to secrete interleukin-12 (IL-12) and in enhancing their immunostimulatory functions in mixed leukocyte reactions. Furthermore, we demonstrated that immunization of mice with stressed apoptotic 12B1-D1 cells induced the secretion of T helper-1 (TH1) profile of cytokines by spleen cells. Splenocytes from mice immunized with stressed apoptotic cells, but not nonstressed ones, were capable of lysing 12B1-D1 and the parental 12B1 line, but not a B-cell leukemia line, A20. Our data indicate that stressed apoptotic tumor cells are capable of providing the necessary danger signals, likely through increased surface expression of heat shock proteins (HSPs), resulting in activation/maturation of dendritic cells and, ultimately, the generation of potent antitumor T-cell responses.
Introduction
The deficiency of self/nonself paradigm has led to new hypotheses proposed by Janeway,1 Medzhitov and Janeway,2 and Matzinger,3,4 in that the immune system is thought to respond to “danger” associated with infections or stress. According to this paradigm, the occurrence of pathologic or necrotic cell death, with cell products released during tissue damage, is a “danger signal” that may initiate protective immune responses. In contrast to necrotic cell death, apoptosis is a physiologic process critical to normal development, tissue remodeling, and cell turnover and is thought to be noninflammatory or bland to the immune system or even tolerigenic.5-7 The intact membrane and exposure of surface molecules to specific receptors on phagocytes seems to allow the safe disposition of undesirable cellular remains.8 This idea, however, has been challenged recently. Lopes and colleagues argued that phagocytosis of apoptotic cells played a previously unrecognized role in regulating the nature of immune responses against pathogens.9 Restifo proposed that apoptotic death associated with viral infection could trigger innate and adaptive immune responses though activation of caspase-1 (an activator of interleukin-1β [IL-1β] and IL-18).10Recent studies have documented that apoptosis induced by pathogens can elicit potent immune responses.11-13 These immune responses are associated with the induction of type 1 cytokines, such as interferon-γ (IFN-γ),11 and the generation of specific cytotoxic T lymphocytes(CTLs).13 Therefore, when apoptotic cell death is associated with “danger signals,” it can be sensed by the immune system and consequently induces active immune responses.
We have reported that vaccination of mice with heat-stressed autologous apoptotic tumor cells induces antitumor immunity that significantly retards tumor progression when compared with vaccination with nonstressed apoptotic cells.14 Moreover, we have found that stressed apoptotic tumor cells are effective immunogens when loaded onto syngeneic dendritic cells (DCs).14Using this artificial but useful model, we have further explored the mechanisms behind this and demonstrated that stressed apoptotic cells have striking effects on DCs, such as up-regulating costimulatory molecules on their surface and stimulating the production of the proinflammatory cytokine IL-12. Moreover, DCs stimulated by stressed apoptotic tumor cells (and not by nonstressed ones) have substantial enhancement of immunostimulatory function in a mixed leukocyte reaction (MLR). Furthermore, we have found that vaccination with stressed apoptotic tumor cells induces more prominent IL-2 and IFN-γ secretion by murine splenocytes and generation of tumor-specific CTLs with high lytic activities. In summary, we have documented that apoptotic tumor cells can be immunogenic when stressed and that DCs play a key role in determining whether a T-cell response will be generated.
Materials and methods
Mice
Six- to 10-week-old female BALB/c (H-2d) and C57BL/6 (H-2b) mice (Harlan Sprague Dawley, Indianapolis, IN) were used for the experiments. The mice were housed in a dedicated pathogen-free facility and cared for according to the University of Arizona Institutional Animal Care and Use Committee guidelines.
Cell lines
12B1 is a murine leukemia cell line derived by retroviral transformation of BALB/c bone marrow cells with the humanBCR-ABL (b3a2) fusion gene and expresses the p210 BCR-ABL protein.15 This is an aggressive leukemia, with the 100% lethal dose (LD100) being 100 cells following tail vein injection.16 12B1 cells are membrane Fas-negative and fail to undergo apoptosis with anti-Fas antibody treatment.14To induce pure apoptosis through Fas/Fas ligand (FasL) apoptotic pathway, we genetically generated an engineered cell line 12B1-D114 by stably transfecting 12B1 with cDNA encoding a recombinant protein that consists of 2 mutant FK506 binding protein (FKBP) domains and the Fas death domain (Fas DD).17-19Using this novel system we were able to trigger classical apoptotic death in stably transfected 12B1-D1 leukemia cells with the use of a mutant FKBP-specific dimerizing drug, AP20187. AP20187 has been tested and has no toxicity on nontransfected cells and has no effects in modifying DC function or phenotype.14 As a control target in CTL assays, we used the B-cell lymphoma/leukemia line, A20.
Generation of apoptosis or necrotic lysate of 12B1-D1
Nonstressed or heat-stressed (42°C, 1 hour) 12B1-D1 cells were treated with 40 nM AP20187 for 6 hours to generate nonstressed or stressed apoptotic tumor cells as described previously.14The lysate was generated by 5 cycles of freeze-thaw using liquid nitrogen and a 37°C water bath.
Confocal microscopy and flow cytometry to evaluate surface HSP expression
Nonstressed or heat-stressed 12B1-D1 cells were treated with AP20187 for 4 to 6 hours as previously described14 and then washed in phosphate-buffered saline (PBS) containing 2% heat-inactivated fetal bovine serum and 0.1% sodium azide (Sigma Chemical, St Louis, MO). A total of 2 × 105 cells were placed in each well of 96-well U-bottomed microtiter plates. Surface expression of specific antigens was determined by incubating the cells with saturating amounts of monoclonal antibodies (Mabs) for 30 minutes at 4°C. Antibodies used included anti-HSP72 (clone C92F3A-5, mouse immunoglobulin G1 [IgG1]; Stressgen, Victoria, BC), anti-HSP60 (clone LK-1, mouse IgG1; Stressgen), and anti-HSP90 (clone AC88, mouse IgG1; Stressgen). The cells were then washed 3 times in PBS containing 2% heat-inactivated fetal bovine serum and 0.1% sodium azide. Secondary antibody used was cyanine 3 (Cy3)–conjugated affiniPure F(ab′)2 fragment goat antimouse IgG (Jackson ImmunoResearch, West Grove, PA). After 30 minutes of incubation the cells were washed 3 times and further stained with annexin V–fluorescein isothiocyanate (FITC) using the Annexin-V-FLUOS staining kit (Roche, Basel, Switzerland). Cells were then washed and transferred onto microscope slides using Cytospin (Thermo Shandon, Pittsburgh, PA) centrifugation followed by examination using a Nikon TE300 microscope (Tokyo, Japan) and Bio-Rad 1024 MRC confocal imaging system (Bio-Rad, Hercules, CA). For flow cytometry, the secondary antibody was an F(ab′)2 fragment goat antimouse IgG conjugated with ALEXA FLUOR 488 (Molecular Probes, Eugene, OR). Following staining with the secondary antibody, the cells were further stained with the vital dye propidium iodide (PI) (Roche) and then analyzed by flow cytometry.
Generation of bone marrow–derived DCs
BALB/c mouse bone marrow DCs were generated as described previously.14 Briefly, bone marrow was harvested from femurs and tibiae and filtered through a Falcon 100-μm nylon cell strainer (Becton Dickinson Labware, Franklin Lakes, NJ). Red blood cells were lysed in a hypotonic buffer, and the marrow was cultured in complete RPMI medium (therapeutic grade; Gibco, Gaithersburg, MD), which contains 10% fetal calf serum, l-glutamine, human serum albumin, 50 μg/mL streptomycin sulfate, and 10 μg/mL gentamicin sulfate. Murine granulocyte-macrophage colony-stimulating factor (GM-CSF) (10 ng/mL) and IL-4 (10 ng/mL) (Peprotech, Rocky Hill, NJ) were added to the culture. After 6 days, the nonadherent and loosely adherent cells were harvested, washed, and used for in vivo and in vitro experiments. Less than 10% of these cells were contaminated by macrophages (CD14+ cells).16
Phagocytosis assay
DC and 12B1-D1 cells were stained with PKH 26 and PKH 67 (Sigma Chemical), respectively, following the manufacturer's instruction. PKH 67–stained nonstressed or heat-stressed (42°C water bath for 1 hour) 12B1-D1 cells were treated with AP20187 for 6 hours. DCs were cocultured with nonstressed or stressed apoptotic cells for 18 hours and then examined by flow cytometry or confocal laser microscopy (Bio-Rad).
Examination of DC surface marker expression by flow cytometry
DCs were washed in PBS containing 2% heat-inactivated fetal bovine serum and 0.1% sodium azide. A total of 2 × 105cells were placed in each well of 96-well U-bottom microtiter plates. Surface expression of specific antigens was determined by incubating the cells first with an Fc receptor–blocking antibody (BD Pharmingen, San Diego, CA) for 5 minutes and then with saturating amounts of monoclonal antibodies (all from Pharmingen) for 30 minutes at 4°C. Antibodies used included purified FITC-conjugated anti–I-Ad (clone AMS-32.1, mouse IgG2b), anti-CD80 (clone 16-10A1, hamster IgG), anti-CD86 (clone GL1, rat IgG2a), anti-CD40 (clone HM40-3, hamster IgM), and purified phycoerythrin (PE)–conjugated anti-CD11c (clone HL3, hamster IgG). After a 30-minute incubation, the cells were washed 3 times in PBS containing 2% heat-inactivated fetal bovine serum and 0.1% sodium azide and fixed with PBS containing 1% paraformaldehyde (Polysciences, Warrington, PA). A total of 10 000 cells were analyzed using a FACScan (Becton Dickinson Immunocytometry, San Jose, CA).
Mixed leukocyte reaction
BALB/c (H-2d) DCs were incubated with stressed or nonstressed apoptotic 12B1-D1 cells at a 1:1 ratio in the presence of 10 ng/mL murine GM-CSF and IL-4 for 24 hours. DCs were collected, treated with 50 μg/mL mitomycin C (Sigma Chemical) for 20 minutes, and then washed 3 times with PBS. Splenocytes (105 per well) from naive C57BL/6 (H-2b) mice were plated in 96-well plates. DCs were serially diluted and incubated with splenocytes at a ratio of splenocytes to DCs ranging from 10:1 to 40:1. After a 4-day coculture, 20 μL of 50 mCi/mL (1.85 GBq/mL) [3H]thymidine (ICN Pharmaceuticals, Costa Mesa, CA) was added to each well. The cells were harvested 18 hours later using a 96-well Packard cell harvester and the radioactivity measured on a Packard β counter (Packard Biosciences, Meriden, CT).
T-cell proliferation assay and IL-2 bioassay
BALB/c mice were immunized with 5 × 105 stressed or nonstressed apoptotic 12B1-D1 cells. Control mice were immunized with saline. Splenocytes from immunized mice were harvested 5 days later and cocultured with mitomycin C–treated 12B1-D1 cells. The ratio of splenocytes to tumor cells was 10 to 1. After a 72-hour culture, the supernatant from each group was collected and serially diluted in 96-well plates. IL-2–dependent CTLL-2 cells (2 × 103cells per well) were added to each well. Human recombinant IL-2 was used to generate standard curve. All assays were performed in triplicate wells. After 24 hours of culture, [3H]thymidine (1 μCi per well) was added. For T-cell proliferation assay, splenocytes were cocultured with apoptotic 12B1-D1 cells for 4 days before the addition of [3H]thymidine. The cells were harvested 18 hours later using a 96-well Packard cell harvester, and the radioactivity was measured on a Packard β counter.
ELISPOT assays
Enzyme-linked immunospot (ELISPOT) assays were performed to measure IFN-γ production by splenocytes and IL-12 production by DCs. To measure the IFN-γ secretion, heat-stressed or nonstressed 12B1-D1 cells were treated with 40 nM AP20187 for 6 hours and then injected into BALB/c mice subcutaneously. Splenocytes of the immunized mice were harvested 5 days later and washed. Between 105 and 106 splenocytes were then cultured in Millipore MultiScreen-HA 96-well plates (MAHA S45; Millipore, Bedford, MA). To measure IL-12 secretion by DCs, 105 to 106 DCs were cultured alone or with stressed or nonstressed apoptotic 12B1-D1 cells at a 1:1 ratio in the presence of 10 ng/mL GM-CSF and IL-4 for 24 hours on Millipore MultiScreen-HA 96-well plates. The plates had been previously coated overnight with anti–IFN-γ capture antibody (10 μg/mL, clone R4-6A2, rat Mab antimouse IFN-γ; BD PharMingen) or anti–IL-12 capture antibody (10 μg/mL, clone 9A5, rat Mab antimouse IL-12p70; BD PharMingen). Cells were then washed with copious amounts of PBST (PBS plus 0.05% Tween 20). Biotinylated anti–IFN-γ antibody (2 μg/mL, clone XMG1.2, rat Mab antimouse IFN-γ; BD PharMingen) or anti–IL-12 antibody (2 μg/mL, clone C17.8, rat Mab antimouse IL-12p40/p70; BD PharMingen) was added for 2 hours. Free antibody was washed out, and the plates were incubated with horseradish peroxidase (HRP)–linked avidin (ABC Elite reagent, 1 drop each of Reagent A and Reagent B per 10 mL PBS; Vector Laboratories, Burlingame, CA) for 1 hour, following extensive washing with PBST and then with PBS. Spots were visualized following the addition of the HRP substrate 3-amino-9-ethylcarbazole (AEC; Sigma Chemical) prepared in acetate buffer (pH 5.0) with 0.015% hydrogen peroxide. Spots were examined using a dissecting microscope. Wells of interest were photographed with a microscope-mounted Cool SNAP20187 CCD camera (RS Photometrics, Tucson, AZ) and images captured with RS Image, version 1.07 (Roper Scientific, Tucson, AZ).
Cytotoxicity assay
BALB/c mice were immunized with heat-stressed (42°C, 1 hour) or nonstressed 12B1-D1 cells that had been treated with 40 nM AP20187 for 6 hours. Splenocytes from immunized mice were harvested 5 days later and then cocultured with mitomycin-C–treated 12B1-D1 cells for 5 days. Stimulated effector cells were tested for cytolytic activity against 12B1-D1 cells, parental 12B1 cells, or A20 (B-cell leukemia) cells using a 4- to 6-hour cytotoxicity assay (Promega, Madison, WI) following the manufacturer's instruction.
Results
Heat-stressed apoptotic tumor cells express HSP60 and HSP72 on their surface
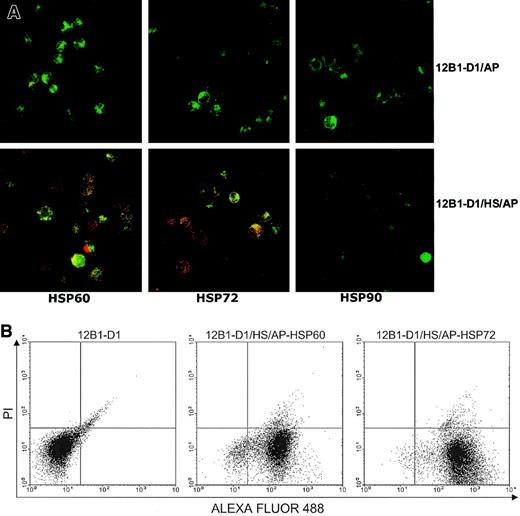
Using flow cytometry, we have previously shown that heat-stressed apoptotic 12B1-D1 cells express HSP60 and HSP72 on their surface.14 We further evaluated the presence of membrane heat shock proteins (HSPs) on apoptotic bodies by confocal microscopy. Figure 1 demonstrates that both nonstressed and stressed apoptotic cells stained positively with annexin V–FITC. However, only the stressed apoptotic cells displayed double staining of green and red dyes; in some areas, colocalization is evident as yellow or orange patches (Figure 1A). This confirmed that stressed apoptotic 12B1-D1 cells expressed HSP60 and HSP72 on their surface. Interestingly, the HSP staining displayed a distinct punctuate pattern rather than a more homogeneous membrane staining. The significance of this is not clear, but it suggests that the distribution of the membrane HSPs may be restricted. We also examined these cells for HSP90 expression by confocal microscopy and found that neither stressed nor nonstressed apoptotic 12B1-D1 cells expressed membrane HSP90 (Figure 1A). Using flow cytometry, most stressed apoptotic cells that were positively stained with HSP60 or HSP72 excluded the vital dye PI (Figure 1B), indicating that these cells were in early stages of apoptosis.
Stressed apoptotic tumor cells express membrane HSPs.
(A) Nonstressed or heat-stressed (42°C, 1 hour) 12B1-D1 cells were treated with AP20187 for 4 to 6 hours and then washed. Surface expression of specific antigens was determined by incubating with anti-HSP72, anti-HSP60, or anti-HSP90 monoclonal antibodies followed by staining with Cy3-conjugated affiniPure F(ab′)2 fragment goat antimouse IgG. Stained cells were washed and further stained with annexin V–FITC. Cells were then washed and transferred onto microscopic slides using cytospin centrifugation followed by examination under confocal laser microscopy. Original magnification × 63, oil objective. (B) For flow cytometry, the secondary antibody was F(ab′)2 fragment goat antimouse IgG conjugated with ALEXA FLUOR 488. Following staining with secondary antibody, the cells were further stained with the vital dye propidium iodide (PI) and analyzed by flow cytometry (12B1-D1/AP indicates 12B1-D1 cells treated with AP20187; 12B1-D1/HS/AP indicates heat-stressed 12B1-D1 cells treated with AP20187).
Stressed apoptotic tumor cells express membrane HSPs.
(A) Nonstressed or heat-stressed (42°C, 1 hour) 12B1-D1 cells were treated with AP20187 for 4 to 6 hours and then washed. Surface expression of specific antigens was determined by incubating with anti-HSP72, anti-HSP60, or anti-HSP90 monoclonal antibodies followed by staining with Cy3-conjugated affiniPure F(ab′)2 fragment goat antimouse IgG. Stained cells were washed and further stained with annexin V–FITC. Cells were then washed and transferred onto microscopic slides using cytospin centrifugation followed by examination under confocal laser microscopy. Original magnification × 63, oil objective. (B) For flow cytometry, the secondary antibody was F(ab′)2 fragment goat antimouse IgG conjugated with ALEXA FLUOR 488. Following staining with secondary antibody, the cells were further stained with the vital dye propidium iodide (PI) and analyzed by flow cytometry (12B1-D1/AP indicates 12B1-D1 cells treated with AP20187; 12B1-D1/HS/AP indicates heat-stressed 12B1-D1 cells treated with AP20187).
Immunostimulatory effects of stressed apoptotic 12B1-D1 cells on DCs
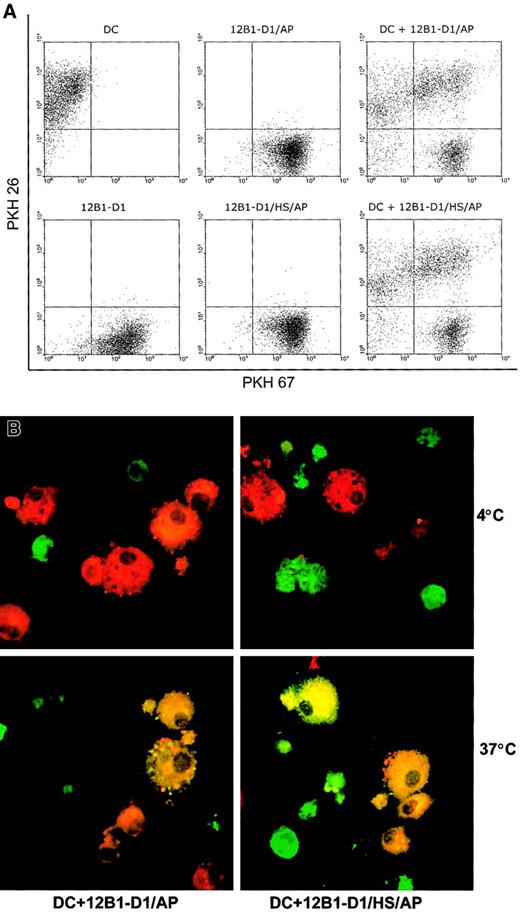
To explore whether DCs can respond differently to heat-stressed and nonstressed apoptotic cells, we first examined whether there was differential uptake of nonstressed versus stressed apoptotic bodies by DCs. 12B1-D1 cells were stained with PKH67 and then induced to undergo apoptosis by exposure to AP20187. The AP20187-treated cells were then incubated with DCs for 18 hours. Using flow cytometry, we found that DCs engulfed nonstressed and stressed apoptotic cells at the same rate as illustrated by the double-positive staining (Figure2A). Confocal microscopy confirmed that internalization was not affected by the application of heat stress to the apoptotic tumor cells (Figure 2B). As expected, phagocytosis was temperature sensitive, because it occurred at 37°C but not at 4°C (Figure 2B).
Nonstressed and stressed apoptotic 12B1-D1 cells are taken up by DCs.
DC and 12B1-D1 cells were stained with PKH 26 and PKH 67, respectively. PKH 67-stained nonstressed or heat-stressed 12B1-D1 cells were then treated with AP20187 for 6 hours to induce apoptosis. DCs were cocultured with nonstressed or stressed apoptotic cells for 18 hours and then examined by (A) flow cytometry or (B) confocal laser microscopy. Original magnification × 63, oil objective. Representative data from 1 of 3 experiments are shown.
Nonstressed and stressed apoptotic 12B1-D1 cells are taken up by DCs.
DC and 12B1-D1 cells were stained with PKH 26 and PKH 67, respectively. PKH 67-stained nonstressed or heat-stressed 12B1-D1 cells were then treated with AP20187 for 6 hours to induce apoptosis. DCs were cocultured with nonstressed or stressed apoptotic cells for 18 hours and then examined by (A) flow cytometry or (B) confocal laser microscopy. Original magnification × 63, oil objective. Representative data from 1 of 3 experiments are shown.
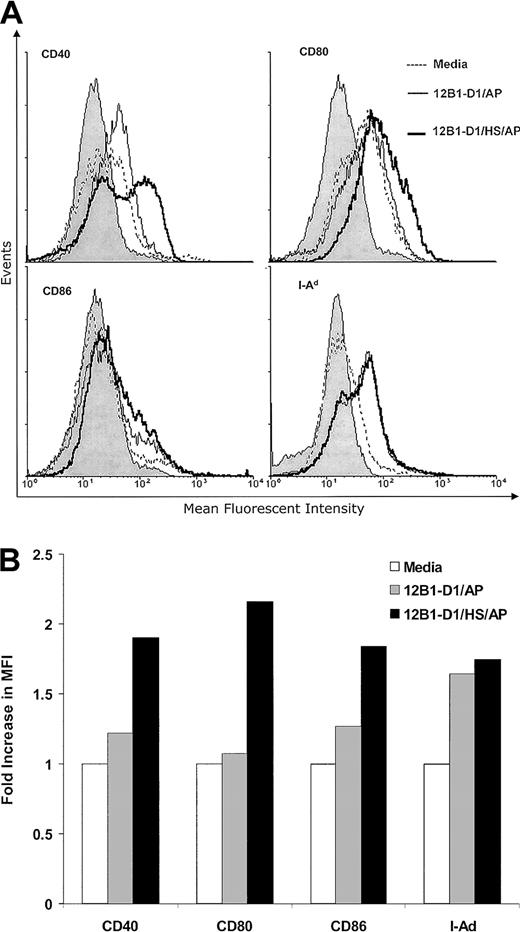
We next examined whether these groups of apoptotic tumor cells may differ in their abilities to stimulate DC maturation. DCs were cultured with nonstressed or stressed apoptotic 12B1-D1 cells at a 1:1 ratio for 24 hours. The CD11c+ gated cells that coexpressed CD40, CD80 (B7-1), CD86 (B7-2), and major histocompatibility complex (MHC) class II were determined. Both nonstressed and stressed apoptotic 12B1-D1 up-regulated the expression of CD40, CD80, CD86, and MHC class II on the surface of DCs (Figure3) as determined by increase of mean fluorescent intensity (MFI). However, stressed apoptotic 12B1-D1 cells reproducibly had a more pronounced effect on inducing CD40, CD80, and CD86 expression (Figure 3). Several groups have reported that necrotic lysate from tumors releases cellular HSPs and matures DCs.20,21 Although HSP release might be restricted in apoptotic cells even after 24 hours into apoptosis,20 we incorporated 2 additional control groups to explore the possibility that the up-regulation of costimulatory molecules on DCs was due to cellular components, such as HSPs released from apoptotic 12B1-D1 cells. A total of 106 heat-stressed or nonstressed 12B1-D1 cells were lysed by freeze-thaw and then incubated with 106DCs for 24 hours. The lysate derived from either heat-stressed or nonstressed 12B1-D1 cells failed to up-regulate the costimulatory molecules on DCs (data not shown). This is in agreement with our reported in vivo data that vaccination with tumor lysate generated by freeze-thaw fails to induce significant protective immunity against 12B1-D1 tumor challenge.14 In addition, we found that heat-stressed but viable 12B1-D1 cells (without induction of apoptosis by AP20187 treatments) failed to stimulate the up-regulation of costimulatory molecules on DCs (data not shown).
Stressed apoptotic 12B1-D1 cells up-regulate the expression of MHC class II and costimulatory molecules on DCs.
Bone marrow–derived DCs were cocultured with heat-stressed or nonstressed apoptotic 12B1-D1 cells at a 1:1 ratio for 24 hours. DCs were then harvested and analyzed by flow cytometry for expression of the cell surface markers indicated. The CD11c+ gated population was analyzed for expression of CD40, CD80, CD86, and MHC class II. (A) Overlay histograms and (B) fold increase in MFI. The gray portion of the histograms in panel A indicates isotype control. Representative data from 1 of 4 experiments are shown.
Stressed apoptotic 12B1-D1 cells up-regulate the expression of MHC class II and costimulatory molecules on DCs.
Bone marrow–derived DCs were cocultured with heat-stressed or nonstressed apoptotic 12B1-D1 cells at a 1:1 ratio for 24 hours. DCs were then harvested and analyzed by flow cytometry for expression of the cell surface markers indicated. The CD11c+ gated population was analyzed for expression of CD40, CD80, CD86, and MHC class II. (A) Overlay histograms and (B) fold increase in MFI. The gray portion of the histograms in panel A indicates isotype control. Representative data from 1 of 4 experiments are shown.
IL-12 is one of most important proinflammatory cytokines secreted by DCs that determines T helper-1/T helper-2 (TH1/TH2) polarization.22 23 Using ELISPOT assays, we next investigated the effect of stressed and nonstressed apoptotic cells on IL-12 production by DCs. Apoptotic cells were cocultured with DCs for 24 hours. Stressed apoptotic 12B1-D1 cells had a dramatic effect on stimulating IL-12 production by DCs, whereas nonstressed apoptotic tumor cells failed to induce measurable IL-12 (Figure 4A).
Effects of stressed apoptotic 12B1-D1 cells on DCs.
(A) Stressed apoptotic 12B1-D1 cells induce IL-12 production by DCs. ELISPOT assays were performed to measure the IL-12 secretion by DCs. A total of 3 × 105 DCs were cocultured with media with heat-stressed or with nonstressed apoptotic 12B1-D1 cells at a 1:1 ratio for 24 hours in the presence of 10 ng/mL GM-CSF and IL-4 for 24 hours. (B) Stressed apoptotic 12B1-D1 cells increase DC capacity to stimulate allogeneic splenocyte proliferation in MLR. DCs were cocultured with heat-stressed or nonstressed apoptotic 12B1-D1 cells at a 1:1 ratio for 24 hours in the presence of 10 ng/mL GM-CSF and IL-4 for 24 hours. DCs were then collected and treated with mitomycin C and washed as described in “Materials and methods.” A total of 105 splenocytes from C57BL6 mice were added per well and cultured with the indicated ratio of pretreated BALB/c DCs for 4 days. [3H]thymidine was added, and the cells were cultured for an additional 18 hours before the incorporated radioactivity was counted. Representative data from 1 of 3 experiments are shown.
Effects of stressed apoptotic 12B1-D1 cells on DCs.
(A) Stressed apoptotic 12B1-D1 cells induce IL-12 production by DCs. ELISPOT assays were performed to measure the IL-12 secretion by DCs. A total of 3 × 105 DCs were cocultured with media with heat-stressed or with nonstressed apoptotic 12B1-D1 cells at a 1:1 ratio for 24 hours in the presence of 10 ng/mL GM-CSF and IL-4 for 24 hours. (B) Stressed apoptotic 12B1-D1 cells increase DC capacity to stimulate allogeneic splenocyte proliferation in MLR. DCs were cocultured with heat-stressed or nonstressed apoptotic 12B1-D1 cells at a 1:1 ratio for 24 hours in the presence of 10 ng/mL GM-CSF and IL-4 for 24 hours. DCs were then collected and treated with mitomycin C and washed as described in “Materials and methods.” A total of 105 splenocytes from C57BL6 mice were added per well and cultured with the indicated ratio of pretreated BALB/c DCs for 4 days. [3H]thymidine was added, and the cells were cultured for an additional 18 hours before the incorporated radioactivity was counted. Representative data from 1 of 3 experiments are shown.
We further examined the effects of apoptotic 12B1-D1 cells on altering the immunostimulatory function of DCs in MLRs. DCs were cultured with nonstressed or stressed apoptotic 12B1-D1 cells for 24 hours and then treated with mitomycin C before they were added as stimulators to allogeneic (H-2b) splenocyte responders. Splenocyte proliferation in the MLR was determined by [3H]thymidine incorporation. Compared with nonstressed apoptotic cells, stressed apoptotic 12B1-D1 cells significantly enhanced the immunostimulatory function of DC (Figure 4B).
Vaccination of mice with stressed apoptotic tumor cells induces secretion of type 1 cytokines and stimulates the generation of specific CTLs
Cell-mediated immunity plays an essential role in combating tumors24 and is characterized by the production of type 1 cytokines, such as IFN-γ, IL-2, and tumor necrosis factor α (TNF-α), and the induction of CTLs. We next explored whether vaccination with stressed versus nonstressed apoptotic tumor cells may have differential abilities to induce type 1 cytokine secretion by T cells and to generate tumor-specific CTLs. We found that splenocytes primed in vivo by vaccination with stressed apoptotic tumor cells responded with increased secretion of IL-2 and IFN-γ upon in vitro restimulation with mitomycin C–treated 12B1-D1 cells (Figure 5A-B). Moreover, proliferation of stressed apoptotic tumor cell–primed splenocytes was significantly higher, as assessed by [3H]thymidine incorporation assays (Figure 5C).
Immunization of mice with stressed apoptotic 12B1-D1 cells induces IFN-γ, IL-2 secretion by splenocytes and T-cell proliferation.
Heat-stressed or nonstressed 12B1-D1 cells (2 × 106) were treated with 40 nM AP20187 for 6 hours and then injected to BALB/c mice subcutaneously. Splenocytes from immunized mice were harvested 5 days later and restimulated with mitomycin C–treated 12B1-D1 cells. (A) CTLL-2 bioassay was used to determine the IL-2 production. (B) IFN-γ secretion was determined by ELISPOT. (C) T-cell proliferation was determined by [3H]thymidine incorporation. Representative data from 1 of 3 experiments are shown.
Immunization of mice with stressed apoptotic 12B1-D1 cells induces IFN-γ, IL-2 secretion by splenocytes and T-cell proliferation.
Heat-stressed or nonstressed 12B1-D1 cells (2 × 106) were treated with 40 nM AP20187 for 6 hours and then injected to BALB/c mice subcutaneously. Splenocytes from immunized mice were harvested 5 days later and restimulated with mitomycin C–treated 12B1-D1 cells. (A) CTLL-2 bioassay was used to determine the IL-2 production. (B) IFN-γ secretion was determined by ELISPOT. (C) T-cell proliferation was determined by [3H]thymidine incorporation. Representative data from 1 of 3 experiments are shown.
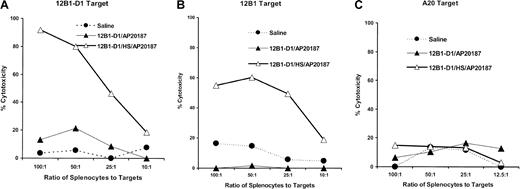
We further examined the CTL activity generated following vaccination with stressed apoptotic tumor cells. Splenocytes from mice immunized with nonstressed or stressed apoptotic 12B1-D1 cells were collected 5 days after immunization and restimulated in vitro with mitomycin C–treated 12B1-D1 cells for 5 another days. These effector cells were tested for cytolytic activity against 12B1-D1, parental 12B1, or A20 targets. We found that vaccination with stressed apoptotic cells resulted in generation of potent CTL activity against 12B1-D1 cells (Figure 6A). In addition, these CTLs were potent and specific enough to lyse the parental 12B1 cells (Figure 6B). No cytolytic activity above background was observed against the A20 B-cell leukemia targets, confirming the specificity of CTLs (Figure6C). In contrast, vaccination with nonstressed apoptotic tumor cells failed to generate CTLs against 12B1-D1 or 12B1.
Immunization of mice with stressed apoptotic 12B1-D1 cells induces tumor-specific CTLs.
BALB/c were immunized with saline heat-stressed or nonstressed 12B1-D1 cells that had been pretreated with 40 nM AP20187 for 6 hours. Splenocytes from immunized mice were harvested 5 days later and restimulated with mitomycin C–treated 12B1-D1 cells for 5 days. Stimulated effector cells were tested for cytolytic activity against (A) 12B1-D1 cells, (B) parental 12B1 cells, or (C) A20 cells, using a 4- to 6-hour cytotoxicity assay. Representative data from 1 of 3 experiments are shown.
Immunization of mice with stressed apoptotic 12B1-D1 cells induces tumor-specific CTLs.
BALB/c were immunized with saline heat-stressed or nonstressed 12B1-D1 cells that had been pretreated with 40 nM AP20187 for 6 hours. Splenocytes from immunized mice were harvested 5 days later and restimulated with mitomycin C–treated 12B1-D1 cells for 5 days. Stimulated effector cells were tested for cytolytic activity against (A) 12B1-D1 cells, (B) parental 12B1 cells, or (C) A20 cells, using a 4- to 6-hour cytotoxicity assay. Representative data from 1 of 3 experiments are shown.
Discussion
DCs are the most potent antigen-presenting cells (APCs) that play a central role in initiating adaptive and innate immune responses.23 Distributed as sentinels throughout the body, DCs are poised to capture antigens, migrate to draining lymphoid organs and, after a process of maturation, select antigen-specific lymphocytes to which they present the processed peptides, thereby inducing immune responses.25 DCs are capable of phagocytosing cells dying by apoptosis.26-28 However, the immunologic outcome following the ingestion of apoptotic cells by DCs remains an issue of debate.9,10 Some reports indicate that the uptake of apoptotic cells may lead to anti-inflammatory signals and possibly tolerance, whereas interaction with necrotic cells will lead to activation of innate and adaptive immune mechanisms.20,29However, some studies have shown that DCs can acquire antigens from apoptotic cells and induce specific CTLs 26,30 in vitro. Others have reported that DCs take up apoptotic tumor cells and transport them to lymph node T-cell areas where they induce a specific immune response leading to tumor rejection in vivo.31 We have previously shown that pulsing of stressed apoptotic 12B1-D1 cells, but not nonstressed ones, onto syngeneic DCs resulted largely in rejection of coinjected viable 12B1-D1 cells.14 Mice rejecting the primary 12B1-D1 inoculum were immune to the same but not to a different leukemia challenge.14 Our current findings indicate that the immune system is capable of distinguishing between stressed and nonstressed cells undergoing programmed cell death. We have demonstrated that both stressed and nonstressed apoptotic cells are efficiently phagocytosed by DCs. This indicates that the difference between the immunologic outcome of stressed and nonstressed apoptotic tumor cells is not due to the differential uptake of apoptotic cells by DCs but rather to the maturation or activation that ensues following contact with the stressed apoptotic bodies.
In addition to antigens that can be acquired, processed, and presented by APCs, secondary signals are needed to activate local APCs, especially DCs, leading to an active immune response against the antigens.1-4 Are DCs differentially activated in response to heat-stressed or nonstressed apoptotic tumor cells? We have demonstrated that DCs exposed to stressed apoptotic cells up-regulated their surface expression of costimulatory molecules and secretion of IL-12, which are both key in determining the type of T-cell responses. IL-12 has been shown to have multiple functions, including modulating TH1 versus TH2 switching, which is critical in antitumor immune responses.24 DCs activated with stressed 12B1-D1 apoptotic tumor cells had improved immunostimulatory function in MLRs. In contrast, nonstressed apoptotic tumor cells displayed limited capacity to induce DC costimulatory molecule expression, IL-12 secretion, and immunostimulatory function. This is consistent with other reports that in the absence of inflammation, infection, and necrosis, apoptotic cells are taken up by immature DCs or macrophages without stimulating autoimmune responses against the self-antigens from apoptotic cells.5,29,32This, as proposed by Steinman et al,33 may represent an important mechanism for establishing peripheral tolerance to self. Therefore, it is possible that tumor cells dying of apoptosis may exploit a similar mechanism to induce tolerance and inhibit the development of tumor-specific immunity. In agreement with our findings are reports demonstrating that engulfment of nonstressed apoptotic cells does not stimulate DC maturation.5,20,29 However, when the dying cells are associated with up-regulation of HSPs14,34,35 or with infectious pathogens,11-13they are capable of stimulating T cells and therefore are highly immunogenic. Using flow cytometry14 and confocal microscopy we demonstrated that stressed apoptotic 12B1-D1 cells up-regulate HSP60 and HSP72 expression on their surface. These membrane-expressed HSPs may act as endogenous adjuvants or danger signals, which stimulate DC maturation, antigen presentation, and, consequently, T-cell priming. Our data confirm that professional APCs can adopt distinct differentiation choices depending upon whether or not cell death is associated with stress, or danger, even if the cells are dying by apoptosis. In fact, we have demonstrated that exogenous HSPs even from normal tissue (which are devoid of tumor antigenic peptides) function as adjuvants, enhancing the immunogenicity of apoptotic tumor cells induced by AP20187 or by mitomycin C.36 Using model systems different from ours, others have documented that HSP expression by viable tumor cells decreases their tumorigenicity.37-39
It is essential for DCs to mature before they can activate naive T cells. Stressed apoptotic 12B1-D1 cells mature DCs, and vaccination with stressed apoptotic tumor cells induces a T-cell–dependent antitumor immunity.14 This is characterized by the production of type 1 cytokines and the generation of tumor-specific cytotoxic T cells. We showed that splenocytes from mice immunized with stressed apoptotic 12B1-D1 cells secreted significantly higher amounts of IL-2 and IFN-γ than spleen cells from mice immunized with nonstressed apoptotic cells. Moreover, we clearly demonstrated that stressed apoptotic 12B1-D1 cells (but not nonstressed ones) induced potent tumor-specific CTLs. Our results are in agreement with our previous report that vaccination with stressed but not nonstressed apoptotic cells significantly retarded the progression of coinjected tumor cells.14 CTLs are particularly important in tumor immunity.40,41 Several reports have shown that APCs can acquire antigens from apoptotic bodies and cross-prime CTLs in vitro.7,13,26 However, evidence that apoptotic tumor cells can prime CTLs in vivo remains limited. In the current study we have demonstrated that stressed apoptotic tumor cells are capable of inducing potent CTLs in vivo. We do not believe that these CTLs are the result of immunization with cellular components released from secondary necrotic tumor cells, because apoptotic cells induced by AP20187 keep their membrane integrity before vaccination as determined by trypan blue exclusions and flow cytometry (data not shown). Moreover, we injected mice with only 5 × 105 AP20187-treated cells, which is unlikely to overwhelm the phagocytic capacity of host APCs.42 Finally, we have previously shown that necrotic 12B1-D1 lysate failed to generate antitumor immunity, retarding the progression of coinjected viable tumor cells.14
In summary, in the present study we provided direct evidence that DCs differentially responded to stressed and nonstressed apoptotic tumor cells. In contrast to nonstressed apoptotic 12B1-D1 leukemia cells, stressed apoptotic tumor cells induced secretion of IL-12, up-regulated the expression of costimulatory molecules on DCs, and augmented their immunostimulatory function. This leads to induction of type 1 cytokines and generation of effective tumor-specific CTLs.
Prepublished online as Blood First Edition Paper, July 25, 2002; DOI 10.1182/blood-2002-05-1389.
Supported in part by the Leukemia and Lymphoma Society of America (E.K.), the Arizona Disease Control Research Commission (E.K.), the Tee Up for Tots Fund (Y.Z.), and the Michael Landon Fund (M.W.G.).
H.F. and Y.Z. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Emmanuel Katsanis, University of Arizona, Department of Pediatrics, 1501 N Campbell Ave, PO Box 245073, Tucson, AZ 85724-5073; e-mail: katsanis@peds.arizona.edu.


![Fig. 4. Effects of stressed apoptotic 12B1-D1 cells on DCs. / (A) Stressed apoptotic 12B1-D1 cells induce IL-12 production by DCs. ELISPOT assays were performed to measure the IL-12 secretion by DCs. A total of 3 × 105 DCs were cocultured with media with heat-stressed or with nonstressed apoptotic 12B1-D1 cells at a 1:1 ratio for 24 hours in the presence of 10 ng/mL GM-CSF and IL-4 for 24 hours. (B) Stressed apoptotic 12B1-D1 cells increase DC capacity to stimulate allogeneic splenocyte proliferation in MLR. DCs were cocultured with heat-stressed or nonstressed apoptotic 12B1-D1 cells at a 1:1 ratio for 24 hours in the presence of 10 ng/mL GM-CSF and IL-4 for 24 hours. DCs were then collected and treated with mitomycin C and washed as described in “Materials and methods.” A total of 105 splenocytes from C57BL6 mice were added per well and cultured with the indicated ratio of pretreated BALB/c DCs for 4 days. [3H]thymidine was added, and the cells were cultured for an additional 18 hours before the incorporated radioactivity was counted. Representative data from 1 of 3 experiments are shown.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/100/12/10.1182_blood-2002-05-1389/5/m_h82323488004.jpeg?Expires=1766512511&Signature=cYGSYvCfybe5QeBYok3YlL-SbBlQ1wHwaF2cwiY0jOvFDgUWcByOuuDE9O6gEW28gtgIVsiXfibFeZsL0K18YL6BeGPGqScFSRr~BvZ-KBPjcxHmthqTq04Fwsk8H-Q7Lay~ddf2dE9By5kgNbVt~Ua9HxgMzAcf6Np4BfCUl6OceL~t8s3YFNU6qPRckQh8ZS1YUQZ78816R~8Y3i4piIhy5zfrpDrLPPaQ2~MyCrv0tPOmYXsGKEUUDhpcT-FPYRYtPZBIaFVENX-Ae0jqRnGiwOLFPwsS4qeRdLaQPM~I3H6s4YCYTb5LYvKxHcChIlsyhgB6ig3wV1xX57ZztQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 5. Immunization of mice with stressed apoptotic 12B1-D1 cells induces IFN-γ, IL-2 secretion by splenocytes and T-cell proliferation. / Heat-stressed or nonstressed 12B1-D1 cells (2 × 106) were treated with 40 nM AP20187 for 6 hours and then injected to BALB/c mice subcutaneously. Splenocytes from immunized mice were harvested 5 days later and restimulated with mitomycin C–treated 12B1-D1 cells. (A) CTLL-2 bioassay was used to determine the IL-2 production. (B) IFN-γ secretion was determined by ELISPOT. (C) T-cell proliferation was determined by [3H]thymidine incorporation. Representative data from 1 of 3 experiments are shown.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/100/12/10.1182_blood-2002-05-1389/5/m_h82323488005.jpeg?Expires=1766512511&Signature=S5ravPJyevhYsOSLDz4dtb2QuBL09pK44pcWG09OYHu-jUpM6o6CapDVay0AAXVmNAT-qmTmoJDOWwAKfcpjhbKtyFC2DagjCmqqoq-ejaYpDP7JlNk6WDY847pXLq7Ohak5-~3fgFcETqcxP5zlRTRNLD854Y8LmKcDY6DXpDbOutjx4soB5uHYQa~PfUwhZLMj6SuoSWgb-~jdgV36QEy4EkvaXP3XN62c6T2Y4oFPmAJgOIFTlrDWL1954JbOKDseHo-m3p2ARMqExrDFVjKPAaf-GT1xv2nIlEtGFnbSg-XKWaTGThGjRhNwtsNZaDRLizfrEhtnTP30Vs~OPw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)




![Fig. 4. Effects of stressed apoptotic 12B1-D1 cells on DCs. / (A) Stressed apoptotic 12B1-D1 cells induce IL-12 production by DCs. ELISPOT assays were performed to measure the IL-12 secretion by DCs. A total of 3 × 105 DCs were cocultured with media with heat-stressed or with nonstressed apoptotic 12B1-D1 cells at a 1:1 ratio for 24 hours in the presence of 10 ng/mL GM-CSF and IL-4 for 24 hours. (B) Stressed apoptotic 12B1-D1 cells increase DC capacity to stimulate allogeneic splenocyte proliferation in MLR. DCs were cocultured with heat-stressed or nonstressed apoptotic 12B1-D1 cells at a 1:1 ratio for 24 hours in the presence of 10 ng/mL GM-CSF and IL-4 for 24 hours. DCs were then collected and treated with mitomycin C and washed as described in “Materials and methods.” A total of 105 splenocytes from C57BL6 mice were added per well and cultured with the indicated ratio of pretreated BALB/c DCs for 4 days. [3H]thymidine was added, and the cells were cultured for an additional 18 hours before the incorporated radioactivity was counted. Representative data from 1 of 3 experiments are shown.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/100/12/10.1182_blood-2002-05-1389/5/m_h82323488004.jpeg?Expires=1766512512&Signature=Z~0HqCNWoJ-POazvA5GFr9IfHj5S9Bq-qLSqHyMigyjq03CDMe0dvxcLRQqs88QPtW4~XsCRiQWM8sYxJWIqQxwK242klBCssk9py1nSFz9tfnZUYpmiGkx6K8uN~3vQhyiwTtUXf2veotTr7D4Ra~oGeWWyCYEFF64JHIXE~2iGTpge61lacgYw2jSN7G8Jy6GuqFMYBnXPvXlJNhPdKHIqecOoRxRO~kbnXQhLBYD3mxN8hS31YOR-Y7n4HPJ0n4pz6OXDeuj7gr0ZKGWwlS4DRnXzA3G5VnR2HtxXza1iKUxjjTnptUE2MTBk91kHpbie1~92c7Wky4spsefJxw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 5. Immunization of mice with stressed apoptotic 12B1-D1 cells induces IFN-γ, IL-2 secretion by splenocytes and T-cell proliferation. / Heat-stressed or nonstressed 12B1-D1 cells (2 × 106) were treated with 40 nM AP20187 for 6 hours and then injected to BALB/c mice subcutaneously. Splenocytes from immunized mice were harvested 5 days later and restimulated with mitomycin C–treated 12B1-D1 cells. (A) CTLL-2 bioassay was used to determine the IL-2 production. (B) IFN-γ secretion was determined by ELISPOT. (C) T-cell proliferation was determined by [3H]thymidine incorporation. Representative data from 1 of 3 experiments are shown.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/100/12/10.1182_blood-2002-05-1389/5/m_h82323488005.jpeg?Expires=1766512512&Signature=vGXtyN-v48rtrAWtwNJ3W1ety62R3DfvmKPpoz3qXOs3SLW1VJrF~ZbMS7K-hI4l7sK5kj0EjzG~emvDnrQRI01uf8BLI7TJDHRV2AP0poi9yTpz3BH7JRyzfltS7oEudqpBx1crAwZG3wCLewwRbHz-wUGoWVKeH25bcogb4QY0d57-SqLIz4fcZa6d4ofqlvT5N56POw10K1n4K8aFeUYmnpP6JzKlxk4VDOUS4WxG6YUgspk2~h0AhM35SuGfCF1x39I664aq2gOHaf9KcrF6oc-DktuOqd8-bpHgKISOq8~hUBSEY4KU0D4XjMZbgma1e6R4x~g12JShznYZNg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)