Acute myeloid leukemia (AML) is a heterogeneous disease, with individual cases showing variability in clinical presentation, blast-cell morphology, therapeutic response, and long-term prognosis. One of the most common cytogenetic abnormalities described in AML is t(8;21)(q22;q22) found in 10% to 15% of cases.1 As a consequence of the chromosomal translocation, 5 exons of the AML-1 gene are fused to nearly the entire coding sequence of the ETO gene, generating an easily detectable polymerase chain reaction (PCR) product of a constant size (260 bp), corresponding to an in-frame fusion of AML1 exon 5 to ETO exon 2. This novel chimeric gene, AML/ETO, encodes a fusion protein with a primary inhibitory role in the normal hematopoietic differentiation program.
Adult patients with de novo–diagnosed AML enrolled in the Spanish Estudi i Tractament de Leucèmies Agudes i Mielodisplàssies (CETLAM) 99 protocol were tested for the presence of chimeric AML1-ETO and CBFβ-MYH11 mRNA using the BIOMED-1 protocols. Briefly, 1 μg of RNA obtained from fresh leukemic bone marrow was retrotranscribed into cDNA using random hexamers. RNA quality assessment was carried out with 5 μL of cDNA in a 1-step PCR amplification of the normal ABL. cDNA (3 μL) was used for the first-step amplification using specific primers for the AML1-ETO fusion gene transcript, followed by a second-step (nested) amplification. Amplified PCR products from PCR I and II were separated by electrophoresis on a 2% agarose gel.
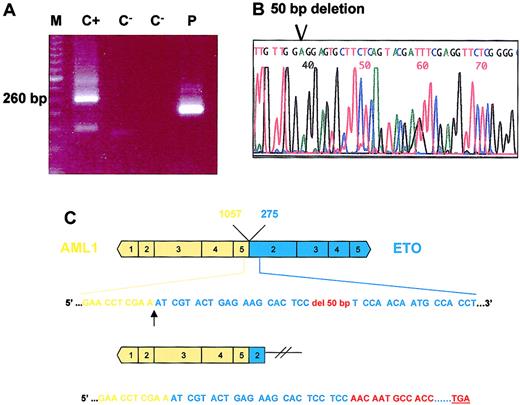
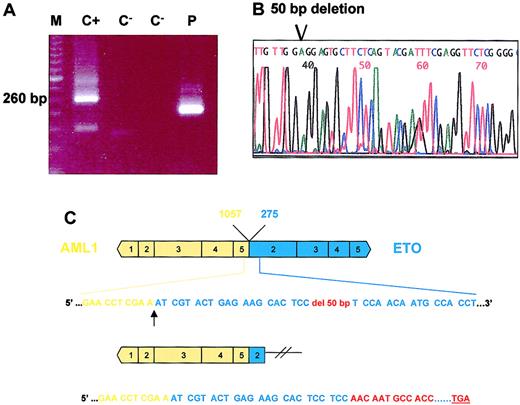
In one patient with a typical M2 leukemia with the t(8;21)(q22;q22), a different-sized PCR amplimer was observed. This faster migrating band was cut from the gel and used for reamplification by PCR. The sequence of this purified product revealed a 50-bp frameshift deletion in exon 2 of ETO. The loss of 50 bp originated a disruption of the reading frame of this transcript resulting in the introduction of a premature stop codon 41 amino acid downstream (Figure 1). The encoded truncated protein would only conserve 31 residues from the ETO coding sequence, and 24 residues would be different from the original transcript as a consequence of the disruption of the reading frame.
Eto deletion in one AML1-ETO leukemia case.
(A) AML1-ETO RT-PCR disclosed an abnormal band that was smaller than the typical specific product. M indicates molecular weight marker; C+, positive control; C−, blank sample; and P, patient. (B) Direct sequencing of the abnormal PCR product revealed a 50-bp ETO deletion. (C) Diagram representation of the 50-bp deletion. Only 7 residues of the ETO sequence are conserved in this abnormal chimeric product.
Eto deletion in one AML1-ETO leukemia case.
(A) AML1-ETO RT-PCR disclosed an abnormal band that was smaller than the typical specific product. M indicates molecular weight marker; C+, positive control; C−, blank sample; and P, patient. (B) Direct sequencing of the abnormal PCR product revealed a 50-bp ETO deletion. (C) Diagram representation of the 50-bp deletion. Only 7 residues of the ETO sequence are conserved in this abnormal chimeric product.
To date, a few alternatively spliced out-of-frame variants consisting of additional small ETO alternative exons have been described.2 3
In the case under discussion, the reverse transcriptase (RT)–PCR showed a single band, which indicates the presence in this patient of a unique transcript.
The expression of this abnormal transcript was investigated through the evolution of the disease by means of real-time quantitative PCR following the recommendations of the European SANCO (Health and Consumer Protection, European Commission) Concerted Action using the ABI PRISM 7700 Sequence Detector (Applied Biosystems, Foster City, CA).4 Based on these findings, fusion transcript values obtained were normalized with respect to the number of ABL transcripts and expressed as fusion-gene copy number per 104 copies of ABL. At the time of diagnosis, the real-time PCR showed 28 000 copy number of [AML1-ETO/abl]× 104, and after therapy the transcript levels rapidly decreased to 1.3 copies. This parallels with the results obtained in typical AML1-ETO fusion cases.
The AML1-ETO fusion protein retains many of the important functional domains of both AML1 and ETO, including the RHD (Runt homology DNA-binding domain) of AML1, and the ETO sequences that mediate homo- and heterodimerization with ETO/MTG family members and interaction with nuclear corepressors. The ability of AML1-ETO to repress transcription is dependent on both the RHD of AML1, and the hydrophobic heptad repeat (HHD) and zinc fingers of ETO.5-7 In the patient described here, the fusion-truncated protein would not have any of the most important ETO domains, and thus it shows that most of the ETO sequence may be dispensable in this AML1-ETO leukemia.
The fact that the patient had a typical M2 leukemia may be explained by the determinant effect on the leukemic phenotype of cooperative and yet unidentified mutations associated with the AML1-ETO rearrangement.8