In a 5-month-old child affected by acute lymphoblastic leukemia (ALL) cytogenetic analysis showed a translocation involving chromosomes 4q13, 11q23, and 17q11. Standard reverse transcriptase–polymerase chain reaction (RT-PCR) analysis of t(4;11) breakpoints did not amplify any knownMLL/AF4 mRNA junction. Therefore, we performed Southern analysis of the MLL locus in DNA from bone marrow cells sampled at diagnosis and found a rearrangement at locus MLLof chromosome 11q23. Panhandle PCR amplification1 of the fragment comprising the der(11) translocation breakpoints yielded a 4.6-kb fragment corresponding to theBamHI-rearranged fragment detected by Southern analysis. The amplified fragment contained sequences corresponding toMLL exon 9 and, after an AluJ0 sequence, a unique nonrepetitive sequence corresponding to the 937 bp of exon 11 of the AF4 gene. These data indicated a hitherto unknown junction between MLL intron 9 and AF4 intron 10 on der(11).
Because the MLL partner fragment included a sequence homologous toAF4 exon 11, we designed a new antisense primer on this exon to amplify the junction sequences of MLL/AF4cDNA, transcribed from the new MLL/AF4 fusion gene. RT-PCR analysis, with the primer couple 5′-TTCCCAAAACCACTCCTAGTGA-3′ (sense-MLL exon 9) and 5′-TCAGAATGCTCCTGACTCGTG-3′ (antisense-AF4 exon 11), yielded a 380-bp (predicted) fragment. Sequence analysis of the amplified product confirmed a hybrid mRNA with an in-frame junction between MLL exon 9 andAF4 exon 11 (Figure 1A).
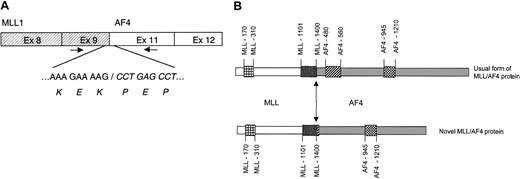
Structural organization of the novel chimericMLL/AF4 transcript and protein.
(A) Analysis of the novel MLL/AF4 transcript. An RT-PCR assay was performed to evaluate whether the novelMLL/AF4 fusion gene generates a chimeric mRNA. cDNA prepared from a bone marrow RNA sample was amplified using the new primers according to sequence analysis of the panhandle product. The amplification reactions consisted of 40 cycles: 94°C, 30 seconds; 65°C, 60 seconds; and 72°C, 60 seconds. (B) Structural organization of the novel chimeric MLL/AF4 protein. The novel chimeric MLL/AF4 protein retains the DNA binding domains but lacks the AF4-derived transactivating domain, which is conserved in the usual type of this fusion protein. The junctions between the 2 protein sequences are aligned and indicated by an arrow. indicatesMLL AT hooks;
indicatesMLL AT hooks; ,MLL repression domain;
,MLL repression domain; ,AF4 transactivation domain; and
,AF4 transactivation domain; and ,AF4 nuclear localization.
,AF4 nuclear localization.
Structural organization of the novel chimericMLL/AF4 transcript and protein.
(A) Analysis of the novel MLL/AF4 transcript. An RT-PCR assay was performed to evaluate whether the novelMLL/AF4 fusion gene generates a chimeric mRNA. cDNA prepared from a bone marrow RNA sample was amplified using the new primers according to sequence analysis of the panhandle product. The amplification reactions consisted of 40 cycles: 94°C, 30 seconds; 65°C, 60 seconds; and 72°C, 60 seconds. (B) Structural organization of the novel chimeric MLL/AF4 protein. The novel chimeric MLL/AF4 protein retains the DNA binding domains but lacks the AF4-derived transactivating domain, which is conserved in the usual type of this fusion protein. The junctions between the 2 protein sequences are aligned and indicated by an arrow. indicatesMLL AT hooks;
indicatesMLL AT hooks; ,MLL repression domain;
,MLL repression domain; ,AF4 transactivation domain; and
,AF4 transactivation domain; and ,AF4 nuclear localization.
,AF4 nuclear localization.
The predicted structure of the new MLL/AF4 chimeric protein lacks the transcription activation MLL motif at residues 2829 to 2883 and the whole AF4 domain spanning residues 480 to 560, which is encoded by exon 10 of the AF4 gene and the first nucleotides of exon 11 (Figure 1B).
The AF4 gene partners the MLL gene in 11q23 translocations in about 50% of childhood and adult ALL cases.2 The MLL gene is fused to at least 25 different gene partners. The sequence and the predicted structure of the proteins encoded by these genes do not appear to have any unifying characteristic that would clarify their role in the leukemogenic process. In addition, in some acute leukemiasMLL may show exon duplications. These issues raised the possibility that an alteration of MLL alone is sufficient to transform hemopoietic precursors and that the fusion partner has no role. However, evidence that partner genes play a role in the leukemogenic process comes from knock-out and knock-in experiments.3 Indeed, an important role for AF4is emerging not only from the epidemiologic restriction ofMLL/AF4 to ALL4 but also from the recent finding that lymphoid development is severely impaired in AF4−/−mice.3
The normal function of the AF4 gene is not known, but a domain with transcriptional activity at nucleotides 480 to 560 and the nuclear localization of the AF4protein5,6 suggests it regulates transcription (ie, theAF4 gene seems to encode a transcription factor whose expression is relevant for committed lymphoid precursors to complete differentiation). This suggests that the MLL/AF4gene arrests the lymphoid differentiation program by altering an early multipotential progenitor cell, either through a gain- or loss-of-function of the MLL/AF4 protein. Therefore the protein might induce aberrant expression of target genes so impairing lymphoid differentiation,7 or alternatively it might lose the capacity to induce the expression of genes important for differentiation.2 The AF4 breakpoints in this novel MLL/AF4 fusion gene lie at least 4 introns further downstream compared with all other MLL/AF4 known translocations. Therefore, the new fusion gene lacks both theAF4-derived and the MLL-derived (nucleotides 2772-3579) transactivating domain5(Figure 1B). The resulting fusion protein is hence able to bind DNA through the MLL-derived AT hook domain, and through the AF4-derived nuclear targeting sequence domains8 but could lose its capacity to activate the expression of these genes. Consequently, it is not inconceivable that loss-of-function, through transcription block, could be the mechanism of leukemogenesis in our ALL patient.
It is not clear whether transcription block could apply to otherMLL/AF4 fusion genes that retain the transactivating domain at residues 480 to 560.
Supported by grants from AIRC (Associazione Italiana per la Ricerca sul Cancro, Milan), Consiglio Nazionale Delce Ricerche (CNR)–Progetto Strategico & Progetto Finalizzato (PF) Biotecnologie (Rome), Biogem (Naples), MIUR (Ministero Dell' Istruzione, Dell' Università e Della Ricerca, Rome), AIL (Associazione Italiana Leucemie, Rome), and Regione Campania (Naples).



 indicatesMLL AT hooks;
indicatesMLL AT hooks; ,MLL repression domain;
,MLL repression domain; ,AF4 transactivation domain; and
,AF4 transactivation domain; and ,AF4 nuclear localization.
,AF4 nuclear localization.