Abstract
Mutations in the proto-oncogene c-kit, including point mutations, deletions, or duplications in the negative regulatory juxtamembrane (JM) domain or point mutations in the catalytic domain, have been observed in human and canine cancers and often result in constitutive activation of Kit in the absence of ligand binding. To identify a receptor tyrosine kinase (RTK) inhibitor capable of blocking the function of mutant Kit, we evaluated 3 indolinones (SU11652, SU11654, and SU11655) that act as competitive inhibitors of adenosine triphosphate binding to several members of the split kinase family of RTKs, including VEGFR, FGFR, PDGFR, and Kit. Mast cell lines expressing either wild-type (WT) Kit, a point mutation in the JM domain, a tandem duplication in the JM domain, or a point mutation in the catalytic domain were used for these studies. All 3 indolinones inhibited phosphorylation of WT Kit in the presence of stem cell factor at concentrations as low as 0.01 μM. Autophosphorylation of both JM mutants was inhibited at 0.01 to 0.1 μM, resulting in cell cycle arrest within 24 hours, whereas autophosphorylation of the catalytic domain mutant was inhibited at 0.25 to 0.5 μM, resulting in cell death within 24 hours. poly(ADP-ribose) polymerase (PARP) cleavage was noted in all Kit mutant lines after indolinone treatment. In summary, SU11652, SU11654, and SU11655 are effective RTK inhibitors capable of disrupting the function of all forms of mutant Kit. Because the concentrations of drug necessary for receptor inhibition are readily achievable and nontoxic in vivo, these compounds may be useful in the treatment of spontaneous cancers expressing Kit mutations.
Introduction
The proto-oncogene c-kit was first identified as the oncogenic component of the acutely transforming Hardy-Zuckerman 4-feline sarcoma virus.1 It encodes Kit, a type 3 receptor tyrosine kinase (RTK) that binds the ligand stem cell factor (SCF).2 Structurally, Kit is related to the receptors for platelet-derived growth factor, vascular endothelial growth factor, fibroblast growth factor, and FLT-3 ligand (reviewed in Ashman3). All contain an extracellular ligand-binding domain composed of 3 to 7 immunoglobulinlike regions, a transmembrane domain, a negative regulatory juxtamembrane (JM) domain, and 2 kinase domains in which resides a kinase insert. SCF–Kit interactions promote the development of mast cells from hematopoietic progenitors; mice deficient in either SCF or Kit (Sl or W mutant mice) exhibit a near-complete absence of mast cells, anemia, and thrombocytopenia.2,4-6 These mice are also sterile and lack skin pigmentation because of the absence of melanocytes. With regard to mast cells, SCF–Kit interactions are intimately involved in several critical processes. Most important, Kit signaling is required for the differentiation and maturation of mast cells in vivo.2,7-10 Other activities attributable to SCF-Kit binding include chemotaxis and haptotaxis of mast cells and the promotion of cell survival and proliferation.2,7,8,11 12
Several years ago, investigators began to study the potential role of Kit dysfunction in malignant mast cells. Point mutations in c-kit that lead to constitutive activation of Kit in the absence of ligand binding were identified in 3 malignant mast cell lines (human HMC-1, rat RBL, and mouse P815), providing an indication that dysregulation of Kit may promote uncontrolled growth or survival of mast cells.13-15 Following this discovery, similarly activating mutations were identified in the CD34+hematopoietic precursors from patients with mastocytosis and in cells from patients with urticaria pigmentosa, thus providing the first evidence that c-kit may play a role in spontaneous mast cell disease.16,17 Recent studies have consistently demonstrated the presence of activating point mutations in the catalytic domain of c-kit in human patients with aggressive mastocytosis, mast cell leukemia, and hematologic disorders with associated mastocytosis.17-23
In contrast to the disease in humans, mast cell neoplasms are one of the most common malignant tumors of the dog, representing between 7% and 21% of all canine tumors.24,25 Given the link between mast cell disorders and dysfunctional Kit, we investigated whether similar mutations in c-kit were present in canine mast cell tumors (MCTs). Although c-kit derived from the canine MCTs did not contain the previously described activating mutations, 30% to 50% of the tumors examined had novel mutations consisting of tandem duplications in exons 11 and 12 of the gene.26 As is the case with tandem duplications in exons 11 and 12 of Flt-3, these mutations also result in constitutive phosphorylation of Kit in the absence of ligand binding.26-29
In humans and dogs, malignant mast cell disease often suggests an extremely poor prognosis. To date, no reliable effective chemotherapeutic agents have been identified.25,30-34Systemic mast cell disorders in humans have been treated with interferon-α, although the effectiveness of this therapy has been variable.35-43 Recent studies suggest that tumors expressing activating mutations in Kit may be amenable to treatment with Kit inhibitors. This has been particularly relevant for gastrointestinal stromal tumors (GISTs), most of which have deletions of various size in exon 11 of c-kit that lead to autophosphorylation of Kit in the absence of ligand binding.44-48 These tumors respond poorly to conventional chemotherapeutics but exhibit a significant susceptibility (in preliminary trials) to treatment with the tyrosine kinase inhibitor imatinib mesylate (STI571; Gleevec; Novartis Pharmaceuticals, Hanover, NJ).49-51
The purpose of this study was to identify an RTK inhibitor capable of blocking the function of multiple forms of mutant Kit. This is of particular importance because STI571 does not appear to be an effective inhibitor of the catalytic domain Kit mutant (most commonly found in human mastocytosis) and because resistance to STI571 has been noted in treated patients, making the development and application of additional inhibitors necessary.52-57
The earliest small molecule with an indolinone chemical structure to enter clinical testing (SU5416; semaxanib; Sugen Inc, South San Francisco, CA) was targeted to inhibit a single RTK, VEGFR2, based on the hypothesis that broadly targeting multiple RTKs might result in unexpected toxicities. Later, clinical experience with a second indolinone (SU6668) with inhibitory activity against the family of RTKs with split kinase domains—vascular endothelial growth factor receptor-2 (VEGFR2), fibroblast growth factor-receptor (FGFR), and platelet-derived growth factor-receptor (PDGFR), all thought to materially contribute to the process of neovascularization—showed these concerns to be unwarranted.58 The newest series of indolinones, SU11652, SU11654, and SU11655, were also designed to inhibit VEGFR, PDGFR, FGFR, and Kit family members. These indolinone-based compounds all act as competitive inhibitors with respect to adenosine triphosphate (ATP) for binding to the intracellular catalytic domain. For comparison, potency (as measured by 50% inhibitory concentration [IC50] for biochemical and cellular inhibition of phosphorylation of these receptors) is shown in Table 1.59-61
We therefore undertook a series of experiments to determine the potency of these compounds in the functional inhibition of a variety of cell lines expressing mutant Kit using phosphorylation of the receptor, cell viability, and apoptosis as read-outs for activity. Our studies demonstrate that all 3 indolinones are capable of inhibiting autophosphorylation of catalytic domain and JM domain mutant forms of Kit. As such, these compounds may be useful in the treatment of spontaneous cancers expressing Kit mutations.
Materials and methods
Cell culture
The BR canine mastocytoma cell line (with a point mutation in the JM domain Leu575Pro) and the C2 canine mastocytoma cell line (with a 48–base pair (bp) tandem duplication in the JM domain) were generously provided by Dr Warren Gold (Cardiovascular Research Institute, University of California at San Francisco). The P815 mouse mastocytoma cell line (with a point mutation in the catalytic domain Asp814Tyr) was purchased from American Type Culture Collection (Manassas, VA). The C57 mouse mast cell line (expressing wild-type Kit) was generously provided by Dr Stephen Galli (Department of Pathology, Stanford University). All cell lines were maintained in RPMI 1640 supplemented with 10% fetal bovine serum, nonessential amino acids, sodium pyruvate, HEPES, penicillin, streptomycin, andl-glutamine.
Indolinone reagents
The RTK inhibitors SU11652 (5-[(Z)-(5-chloro-2-oxo-1,2-dihydro-3H-indol-3-ylidene)methyl]-N-[2-(diethylamino)ethyl]-2,4-dimethyl-1H-pyrrole-3-carboxamide), SU11654 (5-[(Z)-(5-fluoro-2-oxo-1,2-dihydro-3H-indol-3-ylidene)methyl]-2,4-dimethyl-N-(2-pyrrolidin-1-ylethyl)-1H-pyrrole-3-carboxamide), and SU11655 (5-[(Z)-(5-chloro-2-oxo-1,2-dihydro-3H-indol-3-ylidene)methyl]-2,4-dimethyl-N-(2-pyrrolidin-1-ylethyl)-1H-pyrrole-3-carboxamide) were provided by Sugen. Fresh stock solutions of inhibitor (10 mM) were prepared before each experiment by dissolving approximately 4 mg in 1 mL dimethyl sulfoxide (DMSO). This stock solution was then used for further dilution in DMSO.
Antibodies
For flow cytometry, the Ack45 anti-Kit monoclonal antibody conjugated to phycoerythrin was used at a dilution of 1:200 (BD PharMingen, San Diego, CA). For immunoprecipitation and Western blotting of Kit, the Ab-1 rabbit polyclonal anti-Kit antibody was used (Oncogene Research Products, Boston, MA). A peroxidase-conjugated antiphosphotyrosine antibody (RC20; BD Transduction Laboratories, San Diego, CA) was used to detect phosphorylation of Kit at a concentration of 1:2500. An anti-PARP monoclonal antibody (BD Transduction Laboratories) was used to identify PARP cleavage after indolinone treatment at a concentration of 1:2000. Anti–propidium iodide (PI) 3-kinase and anti–SHP-1 monoclonal antibodies (BD Transduction Laboratories) were used to identify coprecipitation with Kit. Peroxidase-conjugated mouse antirabbit and rat antimouse antibodies (Pierce, Rockford, IL) were used at dilutions of 1:40 000.
Cell viability and proliferation
The BR, C2, and P815 cells were counted and resuspended in 24-well plates at a concentration of 0.5 × 106cells/well (BR and C2) or 0.25 × 106 cells/well (P815) in complete medium. Cells were left untreated, or SU11652, SU11654, or SU11655 was added to a final concentration of 0.01 to 1 μM. Cells were collected after 24, 48, and 72 hours of culture and were counted to determine the number of cells surviving.
Propidium iodide staining and flow cytometry
The BR, C2, and P815 cells were counted and resuspended in 24-well plates at a concentration of 0.5 × 106cells/well (BR and C2) or 0.25 × 106 cells/well (P815) in complete medium. Cells were left untreated, or SU11652, SU11654, or SU11655 was added to a final concentration of 0.01 to 1 μM. Cells were collected after 24, 48, and 72 hours of culture, washed twice with 0.1% glucose–phosphate-buffered saline (PBS), fixed in 1 mL cold 70% ethanol, and stored at 4°C. Before flow cytometric analysis, the cells were centrifuged, and 0.5 mL PI staining solution was added (50 μg/mL PI and 10 μg/mL RNAse in 0.1% glucose–PBS).
Immunoprecipitation and Western blotting
For analysis of PARP cleavage, cell lines were either left untreated or were incubated with SU11652, SU11654, or SU1165 at concentrations of 0.01 to 1 μM. After 6, 24, 48, or 72 hours, the cells were collected, washed once in PBS, and resuspended in lysis buffer consisting of 20 mM Tris-HCl, pH 8.0, 137 mM NaCl, 10% glycerol, 1% IGEPAL CA-630, 10 mM EDTA, 1 μg/mL aprotinin, 1 μg/mL leupeptin, 1 μg/mL pepstatin A, 1 mM phenylmethylsulfonyl fluoride, 1 mM Na orthovanadate, and 10 mM Na fluoride for 1 hour at 4°C. Lysates were then spun, and protein was quantitated. Approximately 40 μg protein per sample was loaded onto a 12% polyacrylamide gel and transferred to polyvinylidene difluoride (PVDF) membrane after electrophoresis, then Western blotting was performed for PARP.
For analysis of Kit autophosphorylation, cell lines were serum starved for 2 hours, then left untreated or incubated with SU11652, SU11654, or SU11655 at concentrations of 0.01 to 1 μM for 2 hours. Cells were collected, washed once in PBS, and resuspended in lysis buffer for 1 hour at 4°C. The cell line expressing wild-type (WT) Kit (C57) was incubated with recombinant mouse SCF (rmSCF) (Sigma, St Louis, MO) at 100 μg/mL for 15 minutes before lysis. The supernatant was precleared with Protein A Sepharose 4 Fast Flow beads (Pierce) for 1 hour at 4°C, then incubated with 2 μg anti-Kit overnight at 4°C. The Protein A beads were added to the supernatant and incubated for 1 hour at 4°C, and the beads were collected and washed before the addition of the loading buffer. Sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) was performed using 7.5% or 10% gels, samples were transferred to PVDF membrane, and the membranes were incubated with either anti–PI 3-kinase, anti–SHP-1, or antiphosphotyrosine antibodies. Membranes were developed using SuperSignal ECL (Pierce), then stripped using a buffer consisting of 2% wt/vol SDS, 62.5 mM Tris HCl, pH 6.8, and 100 mM β-mercaptoethanol for 1 minute at 50°C. Membranes were then reprobed for Kit using the Ab-1 anti-Kit antibody.
Results
Mutation in the juxtamembrane domain or catalytic domain of Kit leads to constitutive phosphorylation and association of PI 3-kinase
Mutations in the kinase domain of Kit arise spontaneously in dogs and humans with mast cell cancer.17-23,26,62 In humans, these mutations occur primarily in the catalytic domain of Kit and have been associated with aggressive mastocytosis.19-23 In dogs, Kit mutations occur almost exclusively in exons 11 and 12 and consist of internal tandem duplications, similar to those reported in the Flt3 gene of human patients with acute myeloid leukemia (AML).26,63-65 Interestingly, gastrointestinal stromal tumors in humans frequently have mutations in Kit, although these consist of deletions in exon 11 rather than duplications.44,45,48 It has previously been demonstrated that mutations in the catalytic domain or JM domain lead to autophosphorylation of Kit in the absence of SCF stimulation.13-15,26,44,62 We were interested to know whether such mutations resulted in the constitutive association of PI 3-kinase because this downstream signaling molecule has been implicated in malignant transformation. In addition, previous studies have indicated that constitutively active forms of Kit lead to an increased rate of SHP-1 degradation and, thus, a markedly decreased level of protein associated with Kit.66 We initially believed that tandem duplications in the JM domain of Kit would lead to conformational changes and prevent SHP-1 binding, thereby resulting in the observed constitutive phosphorylation.
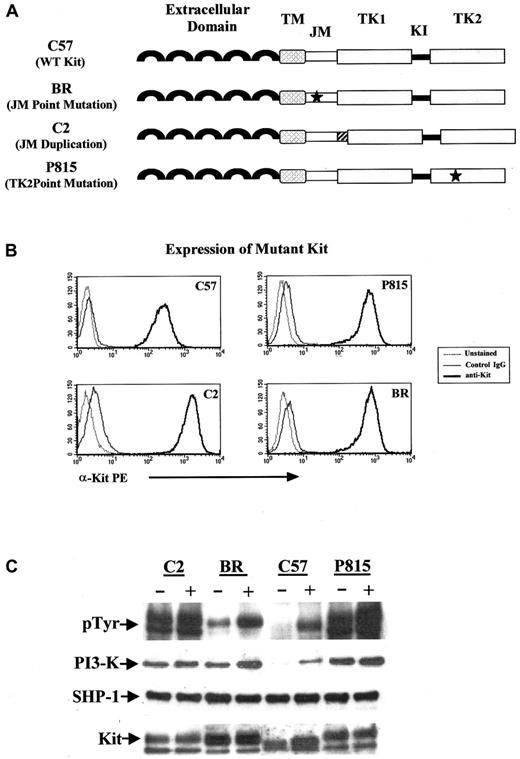
We used 4 malignant mast cell lines expressing various forms of Kit to begin to investigate the effects of catalytic domain and JM domain mutations on cell surface expression of Kit and the association of mutant Kit with PI 3-kinase and SHP-1. The C57 mouse mast cell line expresses WT Kit, the P815 mouse mast cell line has a point mutation in the catalytic domain (Asp816Tyr) and WT Kit, the C2 canine mast cell line has a tandem duplication in exon 11 of the JM domain and does not express WT Kit, and the BR canine mast cell line has a point mutation in exon 11 (Leu575Pro) and WT Kit (Figure1A). We first wanted to know whether the presence of a mutation in either the catalytic domain or the JM domain led to alterations in cell surface expression of Kit. As can be seen in Figure 1B, the expression of Kit was similar among all cell lines, regardless of the presence or absence of a mutation or nature of the mutation.
PI 3-kinase and SHP-1 coprecipitate with mutant Kit.
(A) The C57 mouse mast cell line expressed WT (WT) Kit. C2 and BR lines were derived from spontaneous MCTs; the C2 cell line contains a 48-bp tandem duplication in the exon 11 (the JM domain) of Kit; the BR cell line contains a point mutation (Leu575Pro) in exon 11 of Kit. The P815 line has a point mutation in the catalytic domain (Asp816Tyr) of Kit. TM indicates transmembrane domain; TK1, tyrosine kinase domain 1; KI, kinase insert; and TK2, tyrosine kinase domain 2. (B) The above-described mast cell lines were unstained, stained with a phycoerythrin-labeled isotype-matched control antibody, or the ACK45 anti-KIT monoclonal antibody (PharMingen). Cells were analyzed by flow cytometry on a Becton Dickinson FACScan. Mutation in either the JM domain or the catalytic domain of Kit did not alter cell surface expression of the protein. (C) Cells were cultured in serum-free medium for 2 hours before treatment with SCF (+) at 100 ng/mL and immunoprecipitation with anti-Kit antibody. Western blots were performed for phosphotyrosine, PI 3-kinase, SHP-1, and Kit according to procedures outlined in “Materials and methods.”
PI 3-kinase and SHP-1 coprecipitate with mutant Kit.
(A) The C57 mouse mast cell line expressed WT (WT) Kit. C2 and BR lines were derived from spontaneous MCTs; the C2 cell line contains a 48-bp tandem duplication in the exon 11 (the JM domain) of Kit; the BR cell line contains a point mutation (Leu575Pro) in exon 11 of Kit. The P815 line has a point mutation in the catalytic domain (Asp816Tyr) of Kit. TM indicates transmembrane domain; TK1, tyrosine kinase domain 1; KI, kinase insert; and TK2, tyrosine kinase domain 2. (B) The above-described mast cell lines were unstained, stained with a phycoerythrin-labeled isotype-matched control antibody, or the ACK45 anti-KIT monoclonal antibody (PharMingen). Cells were analyzed by flow cytometry on a Becton Dickinson FACScan. Mutation in either the JM domain or the catalytic domain of Kit did not alter cell surface expression of the protein. (C) Cells were cultured in serum-free medium for 2 hours before treatment with SCF (+) at 100 ng/mL and immunoprecipitation with anti-Kit antibody. Western blots were performed for phosphotyrosine, PI 3-kinase, SHP-1, and Kit according to procedures outlined in “Materials and methods.”
The effect of various Kit mutations on downstream signaling events that regulate cell growth and function is not completely characterized. However, it is now known that the activation of PI 3-kinase by Kit is important for malignant transformation.67 68 As previously reported, Kit autophosphorylation was present in the absence of SCF stimulation in the cell lines with mutations in the catalytic or JM domains. Additionally, we found that PI 3-kinase was constitutively associated with mutant Kit in the presence of mutation in either the JM or the catalytic domain but, as expected, not in the presence of WT Kit (Figure 1C). Interestingly, the point mutation in the JM domain led to slightly less autophosphorylation of Kit than the other 2 mutants and to a lower level of PI 3-kinase association, suggesting that point mutations in the JM domain may be less likely to result in transforming events.
The tyrosine phosphatase SHP-1 is known to bind to Kit in the JM domain and is believed to negatively regulate receptor function. We initially thought that mutations in the JM domain, particularly tandem duplications, might disrupt the conformation of this portion of the receptor, thereby inhibiting SHP-1 association. Interestingly, we found that SHP-1 remained associated with Kit in the presence or absence of mutation in the JM or the catalytic domain. It is therefore likely that the activation of Kit as a consequence of a tandem duplication in the JM domain is secondary to the dimerization of receptor in the absence of ligand binding rather than the loss of tyrosine phosphatase function. Indeed, spontaneous receptor dimerization of Kit has been demonstrated to occur in the presence of deletions and point mutations in the JM domain.69,70 Moreover, a recent study identified critical residues in the Kit intracellular JM domain that exist in a putative α-helical conformation and exert inhibitory effects on the kinase activity of Kit.71 As such, deletions, mutations, or insertions that disrupt this conformation would be expected to result in the constitutive activation of Kit.
Indolinone compounds inhibit the growth of mast cell lines expressing mutant Kit through cell cycle arrest and apoptosis
To identify an RTK inhibitor capable of blocking the function of all forms of mutant Kit, we investigated the effects of 3 indolinones that act as competitive inhibitors with respect to ATP at the intracellular catalytic domain of the receptor.59,60,72-76These indolinones, denoted SU11652, SU11654, and SU11655 (Sugen), inhibit several members of the split kinase family of RTKs including VEGFR, FGFR, PDGFR, and Kit (Figure 2, Table 1). The core structure of these indolinones is predicted to bind to Kit in a fashion similar to that observed in the SU5402/SU6668 cocrystal structure with FGFR1. The functional group off the pyrrole ring extends to the opening of the ATP binding site, which is hydrophilic and exposed to solvent.73 This is in contrast to the crystal structure of an STI571 analog in Abl, in which the inhibitor protrudes to the hydrophobic end of the ATP binding site by pushing up the flexible activation loop.77 To determine whether these compounds were capable of disrupting various forms of mutant Kit, we used the cell lines described above, each of which has a unique mutation in c-kit leading to constitutive phosphorylation in the absence of SCF stimulation.
Structure of indolinones.
Structures of SU11652 (5-[(Z)-(5-chloro-2-oxo-1,2-dihydro-3H-indol-3-ylidene)methyl]-N-[2-(diethylamino)ethyl]-2,4-dimethyl-1H-pyrrole-3-carboxamide), SU11654 (5-[(Z)-(5-fluoro-2-oxo-1,2-dihydro-3H-indol-3-ylidene)methyl]-2,4-dimethyl-N-(2-pyrrolidin-1-ylethyl)-1H-pyrrole-3-carboxamide), and SU11655 (5-[(Z)-(5-chloro-2-oxo-1,2-dihydro-3H-indol-3-ylidene)methyl]-2,4-dimethyl-N-(2-pyrrolidin-1-ylethyl)-1H-pyrrole-3-carboxamide) are shown.
Structure of indolinones.
Structures of SU11652 (5-[(Z)-(5-chloro-2-oxo-1,2-dihydro-3H-indol-3-ylidene)methyl]-N-[2-(diethylamino)ethyl]-2,4-dimethyl-1H-pyrrole-3-carboxamide), SU11654 (5-[(Z)-(5-fluoro-2-oxo-1,2-dihydro-3H-indol-3-ylidene)methyl]-2,4-dimethyl-N-(2-pyrrolidin-1-ylethyl)-1H-pyrrole-3-carboxamide), and SU11655 (5-[(Z)-(5-chloro-2-oxo-1,2-dihydro-3H-indol-3-ylidene)methyl]-2,4-dimethyl-N-(2-pyrrolidin-1-ylethyl)-1H-pyrrole-3-carboxamide) are shown.
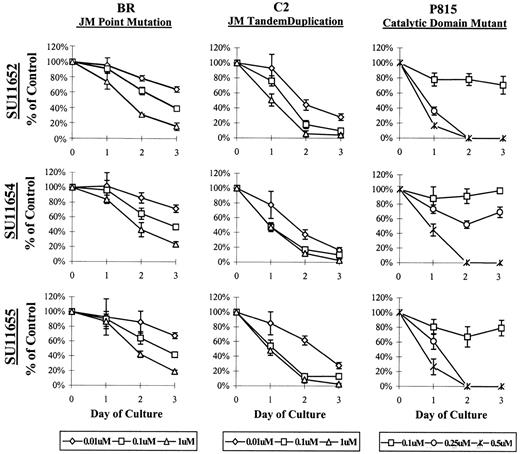
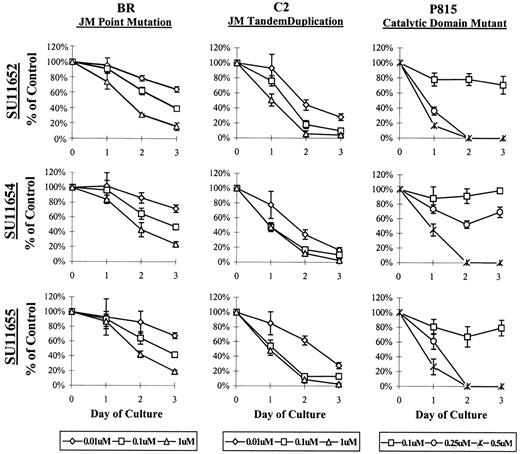
To assess the effects of the indolinones on cell proliferation, BR, C2, or P815 cells were cultured with SU11652, SU11654, or SU11655 at a variety of concentrations, and the number of remaining cells was determined at 24, 48, and 72 hours of culture. Interestingly, the 2 cell lines expressing Kit JM mutations (BR and C2) required lower concentrations of indolinones for the inhibition of cell proliferation than did the cell line expressing a catalytic domain mutation (P815) (Figure 3). Both cell lines demonstrated growth inhibition at concentrations as low as 0.01 μM. However, the effect of indolinone treatment was more profound on the P815 line because no live cells were noted after 24 hours of culture at 0.5 μM (Figure 3). The cell line expressing WT Kit (C57) is a growth factor-independent mast cell line and could not be assessed in this model because additional genomic mutations render it relatively resistant to the effects of these indolinones. As such, the inhibition of cell proliferation was only noted at very high indolinone concentrations (10 μM) (data not shown).
SU11652, SU11654, SU11655 inhibit the growth of mast cell lines expressing mutant Kit.
Cells (5 × 105) (C2, BR, or P815) were cultured in complete medium with no drug or with SU11652, SU11654, or SU11655 at the indicated concentrations. Cells were collected after 24, 48, or 72 hours of culture and were counted to determine the number of remaining cells in each well. No inhibition of cell growth was noted at concentrations lower than 0.01 μM (C2 and BR) or lower than 0.25 μM (P815) (data not shown). Data are presented as percentage of control (untreated) cells. One of 4 representative experiments is shown.
SU11652, SU11654, SU11655 inhibit the growth of mast cell lines expressing mutant Kit.
Cells (5 × 105) (C2, BR, or P815) were cultured in complete medium with no drug or with SU11652, SU11654, or SU11655 at the indicated concentrations. Cells were collected after 24, 48, or 72 hours of culture and were counted to determine the number of remaining cells in each well. No inhibition of cell growth was noted at concentrations lower than 0.01 μM (C2 and BR) or lower than 0.25 μM (P815) (data not shown). Data are presented as percentage of control (untreated) cells. One of 4 representative experiments is shown.
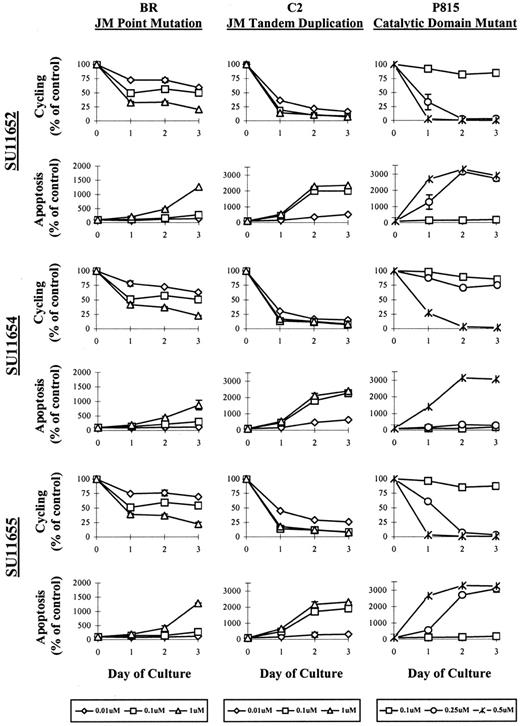
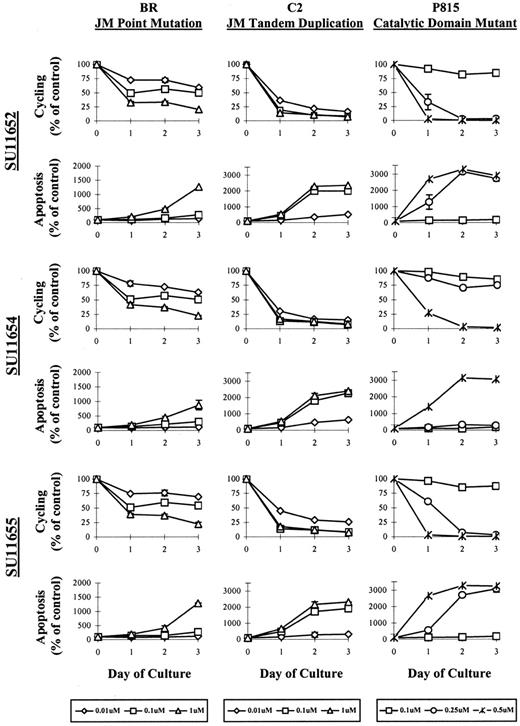
In the cell survival assays detailed above, we observed a decrease in absolute cell numbers and morphologic features consistent with apoptosis. To distinguish between cell cycle arrest and cell death, we evaluated the cells by PI staining after treatment with SU11652, SU11654, or SU11655. Cells were treated with various concentrations of drug for 24, 48, or 72 hours, then collected for flow cytometric analysis. The cell line expressing the catalytic domain mutation (P815) exhibited rapid apoptosis within 24 hours at concentrations between 0.25 and 0.5 μM. Cell cycle arrest was not noted before apoptosis, and apoptosis did not occur at concentrations lower than 0.25 μM (Figure 4). The cell line expressing a JM tandem duplication (C2) exhibited initial cell cycle arrest at concentrations between 0.01 and 0.1 μM after 24 hours of culture. Apoptosis was then noted by 48 to 72 hours of drug treatment, particularly at drug concentrations of 0.1 μM and above. Interestingly, the cell line expressing the JM point mutation (BR) exhibited far less apoptosis after indolinone treatment than the other 2 cell lines expressing Kit mutants, with cell death noted primarily at concentrations of 1 μM. Instead, cell cycle arrest was the principle effect of drug treatment on this cell line. As in the previous experiment, the effects of the 3 indolinones on cell cycling and apoptosis were not evaluated in C57 cells because this growth factor–independent mast cell line expresses additional genomic mutations that render it relatively resistant to the effects of these compounds.
SU11652, SU11654, and SU11 655 induce cell cycle arrest followed by apoptosis in cell lines expressing mutant Kit.
5 × 105 (BR, C2, or P815) were cultured in with no drug or with SU11652, SU11654, or SU11655 at the indicated concentrations. Cells were collected after 24, 48, or 72 hours of culture, permeabilized with 70% EtOH, then incubated with PI. The percentage of cells undergoing cell cycling and apoptosis was assessed by flow cytometry and is represented graphically, presented as the percentage of control (untreated) cells. One of 4 representative experiments is shown.
SU11652, SU11654, and SU11 655 induce cell cycle arrest followed by apoptosis in cell lines expressing mutant Kit.
5 × 105 (BR, C2, or P815) were cultured in with no drug or with SU11652, SU11654, or SU11655 at the indicated concentrations. Cells were collected after 24, 48, or 72 hours of culture, permeabilized with 70% EtOH, then incubated with PI. The percentage of cells undergoing cell cycling and apoptosis was assessed by flow cytometry and is represented graphically, presented as the percentage of control (untreated) cells. One of 4 representative experiments is shown.
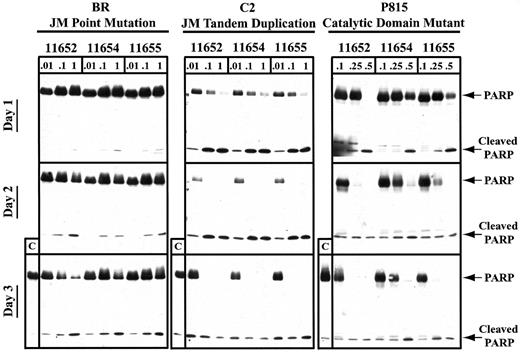
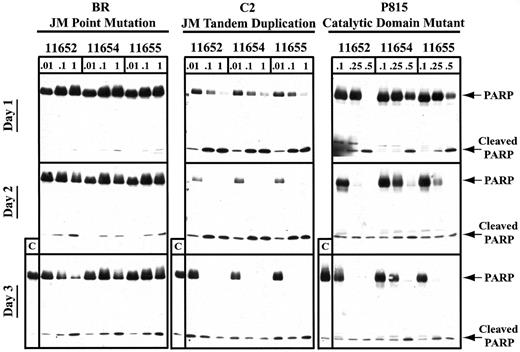
To more specifically evaluate the timing of apoptosis after indolinone treatment, we used PARP cleavage as a sensitive indicator of the apoptotic cascade induced through caspase 3 activation. In agreement with the PI staining results, we found that the catalytic domain mutant underwent complete PARP cleavage within 24 hours of indolinone exposure at 0.5 μM, with some activity observed at 0.25 μM (Figure 5). After 48 hours of exposure to SU11652, SU11654, and SU11655, the catalytic domain mutant exhibited complete PARP cleavage at concentrations of 0.25 μM. In contrast, all 3 indolinones induced PARP cleavage in the JM tandem duplication mutant by 24 hours of culture with 1 μM drug and by 48 hours of culture with 0.01 μM drug. Interestingly, the JM point mutant required at least 72 hours of culture to undergo a similar degree of PARP cleavage. Therefore, although SU11652, SU11654, and SU11655 were capable of inducing cell cycle arrest and apoptosis in cell lines expressing various mutations in Kit, these effects required different concentrations of compound and occurred at different time points after drug exposure. These results support the notion that the 3 Kit mutants result in unique patterns of downstream signaling events and may contribute to malignant transformation through different effects on normal mast cells.
SU11652, SU11654, and SU11655 induce PARP cleavage in cell lines expressing mutant Kit.
Cells (10 × 106) were cultured in complete medium with no drug or with SU11652, SU11654, or SU11655 at the indicated concentrations for 24, 48, or 72 hours. The cells were then collected and lysed, and protein was quantitated. Approximately 40 μg protein/condition was run on a 10% SDS-PAGE gel, and Western blot analysis was performed for PARP.
SU11652, SU11654, and SU11655 induce PARP cleavage in cell lines expressing mutant Kit.
Cells (10 × 106) were cultured in complete medium with no drug or with SU11652, SU11654, or SU11655 at the indicated concentrations for 24, 48, or 72 hours. The cells were then collected and lysed, and protein was quantitated. Approximately 40 μg protein/condition was run on a 10% SDS-PAGE gel, and Western blot analysis was performed for PARP.
Indolinone compounds inhibit autophosphorylation of mutant Kit in a dose-dependent manner
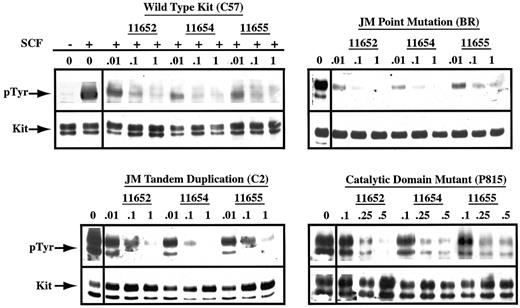
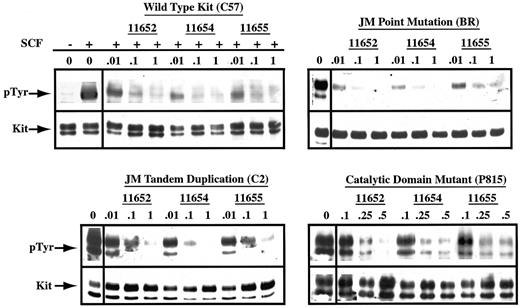
To determine whether the observed cell cycle arrest correlated with the disruption of Kit phosphorylation, we evaluated the effects of indolinone treatment on the phosphorylation status of Kit derived from the previously described cell lines. Inhibition of WT Kit phosphorylation was dose dependent, occurring at concentrations as low as 0.01 μM in the presence of SCF stimulation (Figure6). In agreement with the previous results regarding cell cycle arrest and apoptosis, the catalytic domain mutant required higher concentrations of all 3 indolinones for inhibition, with an IC50 of approximately 0.25 to 0.5 μM. Finally, SU11652, SU11654, and SU11655 inhibited autophosphorylation of both JM mutants with an IC50 of 0.01 to 0.1 μM. Although autophosphorylation of the Kit with the JM point mutation was nearly completely inhibited at 0.01 μM of all 3 indolinones, the cell line expressing this mutant (BR) did not undergo apoptosis at this concentration of drug. This suggests that other mutations are present in the BR line that contribute to growth factor independence and cell survival. Reprobing of all blots for Kit revealed no changes in the expression of Kit protein in cells treated with the indolinones. As such, the dose-dependent decrease in the autophosphorylation of Kit was secondary to the inhibition of kinase activity rather than the down-regulation of protein expression. It is therefore probable that the observed biological effects of SU11652, SU11654, and SU11655 on the malignant mast cell lines are the direct result of the inhibition of Kit signaling.
SU11652, SU11654, and SU11655 inhibit SCF-dependent and SCF-independent phosphorylation of Kit.
Cells (20 × 106 C57) were serum starved for 2 hours, then cultured in complete medium in the absence or presence of SU11652, SU11654, or SU11655 at the indicated concentrations. Cells were collected 2 hours after drug treatment, then incubated with either no rmSCF or rmSCF at 100 ng/mL for 15 minutes at room temperature. 20 × 106 BR, C2, or P815 cells were serum starved for 2 hours, then cultured in complete medium or in SU11652, SU11654, or SU11655 at the indicated concentrations. Cells were collected and lysed, Kit was immunoprecipitated from the lysate, and SDS-PAGE was performed. After transfer to PVDF membrane, the blot was probed for phosphotyrosine, stripped, and reprobed for Kit.
SU11652, SU11654, and SU11655 inhibit SCF-dependent and SCF-independent phosphorylation of Kit.
Cells (20 × 106 C57) were serum starved for 2 hours, then cultured in complete medium in the absence or presence of SU11652, SU11654, or SU11655 at the indicated concentrations. Cells were collected 2 hours after drug treatment, then incubated with either no rmSCF or rmSCF at 100 ng/mL for 15 minutes at room temperature. 20 × 106 BR, C2, or P815 cells were serum starved for 2 hours, then cultured in complete medium or in SU11652, SU11654, or SU11655 at the indicated concentrations. Cells were collected and lysed, Kit was immunoprecipitated from the lysate, and SDS-PAGE was performed. After transfer to PVDF membrane, the blot was probed for phosphotyrosine, stripped, and reprobed for Kit.
Discussion
Mutations in the proto-oncogene c-kit occur in a variety of neoplastic diseases, particularly those associated with mast cells in dogs and humans. These mutations consist of point mutations in the catalytic domain, deletions, point mutations, and tandem duplications in the JM domain, and less commonly, point mutations in the extracellular domain. Most of these mutations lead to constitutive phosphorylation of Kit in the absence of ligand binding. Interestingly, neoplastic diseases expressing activating mutations in Kit (mast cell tumors and GISTs) are relatively resistant to standard chemotherapeutic regimens. As such, novel approaches to the treatment of these diseases are critical for improved therapeutic outcome.
The recent success of the RTK inhibitor, STI571, in the treatment of chronic myelogenous leukemia has demonstrated that identification of particular aberrant signaling pathways in neoplastic cells and subsequent targeting of these pathways with specific inhibitors (so-called targeted therapy) may yield substantial anticancer benefits. Ideally, an effective RTK inhibitor would exhibit good oral bioavailability, a large volume of distribution, an elimination half-life that permits daily dosing, and the ability to block the function of a restricted set of proteins. We chose to investigate the potential usefulness of 3 multitargeted indolinones in the inhibition of dysfunctional Kit signaling because these compounds have a number of such desirable features.
We used 4 mast cell lines expressing either WT Kit or a variety of Kit mutants to investigate the ability of 3 indolinones (denoted SU11652, SU11654, and SU11655) to disrupt Kit function. These compounds were effective in blocking cell proliferation in all cell lines expressing mutant Kit (Figure 3). For lines expressing a JM Kit mutation, treatment with all 3 indolinones initially led to cell cycle arrest, followed by apoptosis after 24 to 72 hours of drug exposure (Figures 4, 5). The cell line with the catalytic domain mutant exhibited significant apoptosis after 24 hours of treatment, with no cells surviving at higher concentrations of each drug.
The concentrations of SU11652, SU11654, and SU11655 necessary for inducing cell cycle arrest and apoptosis correlated directly with those required for the inhibition of Kit phosphorylation. Our results demonstrate that these indolinones are effective at inhibiting the function of WT Kit; the IC50 was 0.01 to 0.1 μM, translating to a target plasma concentration of 4 to 40 ng/mL (Figure6). Both JM mutants were also inhibited by SU11652, SU11654, and SU11655 with a similar IC50 (Figure 6). Interestingly, the catalytic domain mutant required a higher concentration of drug for maximal inhibition, with an IC50 of 0.25 to 0.5 μM (Figure 6). This is in agreement with a prior study examining an earlier indolinone (SU6577), in which higher concentrations of drug were necessary to block the catalytic domain mutant when compared with a JM domain mutant.78 Differences in levels of protein are unlikely to be responsible for this discrepancy because cell surface expression of Kit is comparable among the various mutants (Figure 1). Given that activating mutations in the catalytic domain of Kit induce significant PI 3-kinase activity and are potent inducers of malignant transformation, it is possible that this form of mutation may result in greater constitutive activation of Kit when compared with the JM mutations.
In conclusion, our data demonstrate that, in contrast to other tyrosine kinase inhibitors, the multitargeted indolinones SU11652, SU11654, and SU11655 are potent inhibitors of WT and mutant forms of Kit at concentrations of drug that are readily achievable in vivo (Table2).52,53,79,80 Although we have focused our investigations on the application of these compounds to malignant mast cell disease, aberrant Kit function (through mutation, inappropriate expression, and generation of autocrine loops of growth factor stimulation) has been documented in several other neoplastic disorders including GISTs, leukemias, small cell lung carcinoma, genitourinary tumors, and glioblastoma.81-85 It is possible that these indolinones will have broad applicability in the treatment of a wide variety of cancers.
We thank Dr Alison L. Hannah for helpful discussions and critical review of the manuscript and Dr Chris Liang for structural information relevant to the indolinones.
Prepublished online as Blood First Edition Paper, April 17, 2002; DOI 10.1182/blood-2001-12-0350.
Supported by grant 0038F from the Center for Companion Animal Health at the School of Veterinary Medicine, University of California at Davis.
Several of the authors (N.S., D.B.M., G.M., J.M.C.) are employed by a company (Sugen, Inc) whose potential product was studied in the present work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Cheryl A. London, Department of Surgical and Radiological Sciences, School of Veterinary Medicine, 2112 Tupper Hall, One Shields Ave, University of California at Davis, Davis, CA 95616; e-mail: calondon@ucdavis.edu.

![Fig. 2. Structure of indolinones. / Structures of SU11652 (5-[(Z)-(5-chloro-2-oxo-1,2-dihydro-3H-indol-3-ylidene)methyl]-N-[2-(diethylamino)ethyl]-2,4-dimethyl-1H-pyrrole-3-carboxamide), SU11654 (5-[(Z)-(5-fluoro-2-oxo-1,2-dihydro-3H-indol-3-ylidene)methyl]-2,4-dimethyl-N-(2-pyrrolidin-1-ylethyl)-1H-pyrrole-3-carboxamide), and SU11655 (5-[(Z)-(5-chloro-2-oxo-1,2-dihydro-3H-indol-3-ylidene)methyl]-2,4-dimethyl-N-(2-pyrrolidin-1-ylethyl)-1H-pyrrole-3-carboxamide) are shown.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/100/2/10.1182_blood-2001-12-0350/6/m_h81422854002.jpeg?Expires=1763498575&Signature=FpjLdbR4six2vlF5V4PdMrHOlKHXo6VbCBeX8T1fnAjcbSqxV9eo2g2Ehuf~heGJWcjSbugoe6W5tVc7WBTZCU~F-R9kHLChzNt19IkLxhmbNkHbOxFsEzSmIe-0qSSAELg2xnpD0nCJQGEkXQ61VNmTttB8hzCM2BX9CK4036qkN0B-kX5aNTlIvX2WbfOi-bYPApFdMi0IGojo7ZMrwqMwGVsb6mGg-99j4AO91CmVvFoHEdYG03a6sEMPadapaK7TvI5pnG037EiXjvkxAc2vr4SI~rPAHq3HqVn1Gr7B9S1qi0jxUpM~CunyWtSk5X6ayxlRn44c49KqVA4rJw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Fig. 2. Structure of indolinones. / Structures of SU11652 (5-[(Z)-(5-chloro-2-oxo-1,2-dihydro-3H-indol-3-ylidene)methyl]-N-[2-(diethylamino)ethyl]-2,4-dimethyl-1H-pyrrole-3-carboxamide), SU11654 (5-[(Z)-(5-fluoro-2-oxo-1,2-dihydro-3H-indol-3-ylidene)methyl]-2,4-dimethyl-N-(2-pyrrolidin-1-ylethyl)-1H-pyrrole-3-carboxamide), and SU11655 (5-[(Z)-(5-chloro-2-oxo-1,2-dihydro-3H-indol-3-ylidene)methyl]-2,4-dimethyl-N-(2-pyrrolidin-1-ylethyl)-1H-pyrrole-3-carboxamide) are shown.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/100/2/10.1182_blood-2001-12-0350/6/m_h81422854002.jpeg?Expires=1763498576&Signature=H1eL0BxF0vln4OfuBHG4mcGSPZikfwSfhKtsWOdRIxPncVh2wSi5ocrJHvjF0k5s3FIW2ubEBYrkzledsA3B2DGsnDo97IlPi67YyiZBJEa4N5lrMCEMrFhaKKKk4P61X~90weyeuphLTMsGKpfSxbNi4YvdQiUMyLB9AmMhJcQoP1CwmVEfz0qASqzJvYsdVcMwlB~SSz1J10jXgt~hmytiE3p7Tk1rKP1EiF-pr28D2ct1KfPjRdvMpWvZCQvZsccInabXsyKW9H4KOzW2WIUQT9~~6AljaA2HLzkAcvKc6TtlIu8SN8um0ssOCB0TLfxYVVICNOh60xUAGgmtJw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)