Abstract
Acute leukemia with the t(11;17) expressing the PLZF-RARαgene fusion is a rare variant of acute promyelocytic leukemia (APL) that has been associated with poor clinical response to all-trans retinoic acid (ATRA) treatment. However, some recent reports have put into question the absolute refractoriness of this leukemia to ATRA. We describe here a patient withPLZF/RARα APL who was treated at relapse with ATRA and low-dose hydroxyurea. Complete hematologic remission was obtained through differentiation of leukemic blasts, as proven by morphologic, immunophenophenotypic, and genetic studies carried out in sequential bone marrow samples. Moreover, in vitro studies indicated that blast differentiation was potentiated by the addition of the histone deacetylase inhibitor tricostatin A, but not of hydroxyurea, to ATRA. Our findings indicate that the maturation block may be overcome and terminal differentiation obtained in this leukemia subset and support the view that sensitivity/refractoriness of this form to ATRA should be revisited.
Introduction
Acute leukemia with the t(11;17) (q23;q21) translocation is a rare genetic and phenotypic variant of acute promyelocytic leukemia (APL, reviewed in references 1 to 5). Unlike the classic t(15;17) PML/RARα-positive form, t(11;17) APL has been associated with unfavorable prognosis and unresponsiveness to the differentiative action of all-trans retinoic acid (ATRA), both in vitro and in vivo.1-5 At the molecular level, the t(11;17) involves the PLZF (Promyelocytic Leukemia Zinc Finger) gene on chromosome 11, and the RARα (Retinoic Acid Receptor α) gene on chromosome 17, resulting in 2 hybridPLZF-RARα and RARα-PLZF genes. These originate 2 chimeric Plzf-Rarα and Rarα-Plzf proteins that have been shown to produce an APL-like syndrome in transgenic mice.6 Distinct from PML/RARα APL, an ATRA-insensitive site that binds a nuclear co-repressor complex including histone deacetylase (HDAC) activity is located in the Plzf moiety of Plzf-Rarα. This would account for the reported poor response to ATRA of t(11;17) APL. The usage of agents inhibiting HDAC, such as trichostatin A (TSA) in Plzf-Rarα–transfected cell lines and transgenic mice, releases the maturation block induced by the fusion protein, suggesting that combined treatment with HDAC inhibitors and ATRA might prove effective in this leukemia.7-9
Recently, the absolute refractoriness of t(11;17) APL to retinoids has been questioned. In particular, Jansen et al10 reported that, upon combined stimulus with granulocyte colony-stimulating factor (G-CSF), ATRA induced complete remission through blast cell differentiation in a patient with recurrent t(11;17) APL. We describe here an additional case of PLZF/RARα APL that responded to ATRA. Our results indicate that the maturation block can be overcome in this leukemia subset.
Study design
Patient
A 62-year-old man was diagnosed in September 1998 withPLZF-RARα APL. Detailed characterization studies at presentation have been reported (case no. 52 in references 3 and 4). Front-line treatment included 2 cycles of conventional chemotherapy, after which the patient underwent complete remission.3,4Despite clinical and hematologic remission, reverse transcriptase–polymerase chain reaction (RT-PCR) forPLZF-RARα was repeatedly positive in marrow samples collected during follow up. After 15 months in remission, hematologic relapse was documented. Leukemia cells disclosed the same features observed at presentation, including a characteristic morphology, CD56 expression, DNA rearrangement in the RARα gene second intron, and RT-PCR positivity for the PLZF-RARα fusion. As it was seen at diagnosis,4 the reciprocalRARα-PLZF transcript was undetectable by RT-PCR. Because blasts collected at relapse showed functional response to ATRA in vitro (see below) and because of concomitant hyperleucocytosis, reinduction treatment was started with a combination of daily oral ATRA (45 mg/m2/d) and hydroxyurea (HU, 50 mg/kg/d). The latter (HU) was gradually reduced after 10 days of combined therapy and discontinued at day +20. After 40 days of treatment, patient achieved a second remission through leukemia cell differentiation. He then received intermittent cycles of HU and ATRA and remained in second remission, though persistently PCR-positive, for 8 additional months, until March 2001, when second hematologic relapse was documented. At this time, he refused intensive therapy, received palliative treatment, and died with progressive disease in August 2001.
Method
Leukemia blasts from marrow and peripheral blood at diagnosis and during treatment were characterized by conventional light microscopy, cytochemistry, surface marker analysis, Southern blot, and RT-PCR using specific probes and oligonucleotides, respectively, for the PLZF and RARα genes.3,4,11Patient blasts were also used for cell culture studies and assayed for their in vitro response to the HDAC inhibitor TSA, to HU, and to ATRA, as described.12
Results and discussion
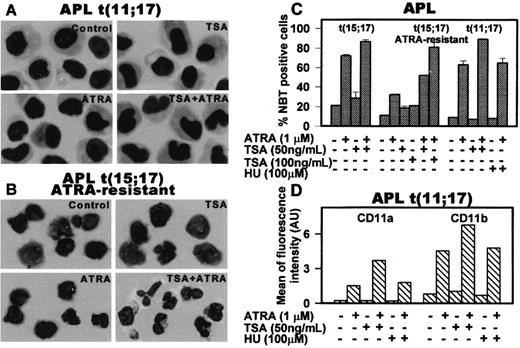
The results of in vitro and in vivo studies are summarized in Figures 1 and 2. In vitro treatment of first-relapse patient blasts with ATRA alone induced minimal morphologic differentiation (Figure1A), whereas it increased the percentage of nitroblue tetrazolium (NBT)–positive cells and the expression of CD11a/CD11b to levels comparable to those obtained in a t(15;17) APL (Figure 1C-D). Furthermore, TSA enhanced the effect of ATRA in inducing NBT and up-regulated myeloid differentiation antigens (Figure 1C-D). In keeping with observations reported in vitro and in vivo by others and by ourselves,7,8,12 13 TSA alone did not significantly induce myeloid differentiation, whereas, in combination with ATRA, it enhanced differentiation of both t(15;17) and t(11;17) APLs and restored ATRA sensitivity in an ATRA-resistant t(15;17) APL (Figure 1B-C).
In vitro effect of ATRA, TSA, and HU in various combinations on t(11;17) APL (patient's blasts), t(15;17) primary APL, and ATRA-resistant t(15;17) APL.
Primary blasts from patient's bone marrow at leukemia relapse, from an ATRA-resistant t(15;17) APL patient and from a t(15;17) APL patient at diagnosis were cultured (1.5 to 2 × 106 cells/mL) in RPMI 1640 medium supplemented with 10% fetal calf serum (FCS) and treated with 1 μM ATRA and/or 100 ng/mL TSA for 5 days as described.12 Differentiation of these blasts was evaluated by morphology in Wright-Giemsa–stained cytospins (panels A and B). Original magnifications, × 40. Differentiation was quantified by the nitroblue tetrazolium (NBT) dye reduction assay (panel C) after treatment of blasts with the indicated agents for 5 days in culture. Results are expressed as the average values of the percentages of positive cells evaluated in at least 10 microscopic fields ± SD. (D) Quantitative fluorescence activated cell sorting (FACS) analysis of the differentiation antigen CD11a and CD11b in APL t(11;17) blasts induced by 4 days of treatment with 1 μM ATRA and/or 50 ng/mL TSA.
In vitro effect of ATRA, TSA, and HU in various combinations on t(11;17) APL (patient's blasts), t(15;17) primary APL, and ATRA-resistant t(15;17) APL.
Primary blasts from patient's bone marrow at leukemia relapse, from an ATRA-resistant t(15;17) APL patient and from a t(15;17) APL patient at diagnosis were cultured (1.5 to 2 × 106 cells/mL) in RPMI 1640 medium supplemented with 10% fetal calf serum (FCS) and treated with 1 μM ATRA and/or 100 ng/mL TSA for 5 days as described.12 Differentiation of these blasts was evaluated by morphology in Wright-Giemsa–stained cytospins (panels A and B). Original magnifications, × 40. Differentiation was quantified by the nitroblue tetrazolium (NBT) dye reduction assay (panel C) after treatment of blasts with the indicated agents for 5 days in culture. Results are expressed as the average values of the percentages of positive cells evaluated in at least 10 microscopic fields ± SD. (D) Quantitative fluorescence activated cell sorting (FACS) analysis of the differentiation antigen CD11a and CD11b in APL t(11;17) blasts induced by 4 days of treatment with 1 μM ATRA and/or 50 ng/mL TSA.
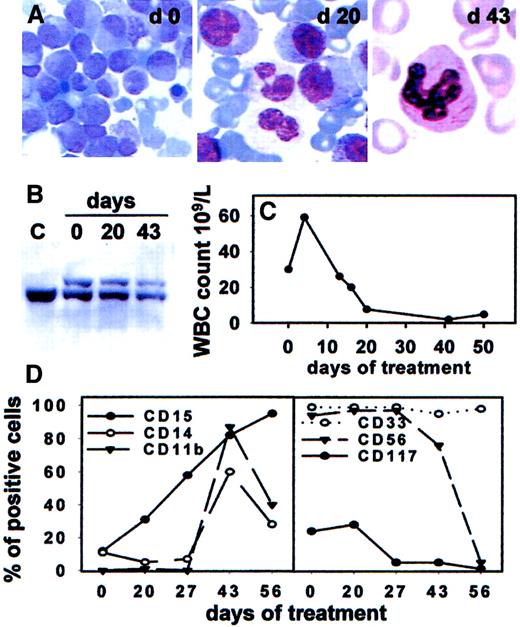
In vivo response of t(11;17)-AML-M3 to combined treatment with ATRA and hydroxyurea.
(A) Wright-Giemsa staining of bone marrow leukemic cells collected at the indicated days of treatment (day 0, 20, 43). A neutrophilic granulocyte displays several Auer rods in the cytosol, indicating its descent from differentiated leukemia blasts. Original magnifications, × 40. (B) Southern blot analysis of the RARα second intron in leukemia blasts pretreatment (day 0), maturing marrow cells collected at day 20, and neutrophils obtained from day 43 bone marrow buffy coat. The germline 19 kilobase (kb) band observed in normal placental DNA (lane C) and in all other lanes is indicated by the bar. An upper band is also visible in marrow DNAs obtained at day 0, 20, and 43, corresponding to the rearranged RARα allele. (C) Time course of peripheral blood leucocytes during treatment. (D) Sequential immunophenotypic study of bone marrow mononuclear cells as determined by FACS analysis. Increase of CD15 staining cells at day 20 was followed by augment of CD11b and CD14-positive elements (day 43) coupled to decrease of CD117 and CD56 staining cells (days 43 and 56).
In vivo response of t(11;17)-AML-M3 to combined treatment with ATRA and hydroxyurea.
(A) Wright-Giemsa staining of bone marrow leukemic cells collected at the indicated days of treatment (day 0, 20, 43). A neutrophilic granulocyte displays several Auer rods in the cytosol, indicating its descent from differentiated leukemia blasts. Original magnifications, × 40. (B) Southern blot analysis of the RARα second intron in leukemia blasts pretreatment (day 0), maturing marrow cells collected at day 20, and neutrophils obtained from day 43 bone marrow buffy coat. The germline 19 kilobase (kb) band observed in normal placental DNA (lane C) and in all other lanes is indicated by the bar. An upper band is also visible in marrow DNAs obtained at day 0, 20, and 43, corresponding to the rearranged RARα allele. (C) Time course of peripheral blood leucocytes during treatment. (D) Sequential immunophenotypic study of bone marrow mononuclear cells as determined by FACS analysis. Increase of CD15 staining cells at day 20 was followed by augment of CD11b and CD14-positive elements (day 43) coupled to decrease of CD117 and CD56 staining cells (days 43 and 56).
Functional response to ATRA in vitro prompted us to treat the relapse with ATRA therapy in vivo. Complete remission was obtained in our patient through leukemia cell differentiation, as shown by the findings of 1) no marrow hypoplasia during treatment, 2) clear morphologic features of cell maturation after 20 days in bone marrow cells, in parallel with increase of CD15 positive cells (Figure2A,D), and 3) polymorphonuclear elements with Auer rods in the blood at remission (Figure 2A). Finally, Southern blot analysis of the RARα locus allowed detection of the same rearranged band in blasts, intermediate maturing elements, and terminally differentiated cells (Figure 2B). As to the possibility that HU administered together with ATRA exerted a synergistic effect on differentiation, we are prone to exclude this hypothesis. In fact, in vitro studies indicated that, in the absence of ATRA, HU did not increase the percentage of NBT positive cells nor CD11a and CD11b expression, while the combination of both agents did not enhance expression of these markers on cultured patient blasts (Figure 1C,D). In addition, when attempted in other ATRA-insensitive AML cases as an approach to induce leukemia differentiation, this combination proved unsuccessful (unpublished observations, May 2001).
Together, our findings suggest that our patient's blasts were sensitive to ATRA. As to the apparent discrepancy with other t(11;17) APLs reported to date,1-4 we remark on 2 distinguishing features of the present case. First, leukemia blasts did not express the reciprocal RARα-PLZF fusion. The latter has been shown to cooperate in t(11;17) APL leukemogenesis and might indeed contribute to confer ATRA-resistance and a more aggressive phenotype in this subset.6 Second, as indicated by the results of surface marker analysis (Figure 2D), a significant in vivo response was observed here after prolonged ATRA treatment.
Because of the exceedingly rare nature of this leukemia, only few cases have been characterized in details to date. Of these, most received conventional chemotherapy, whereas ATRA had not been administered for prolonged periods of time in the few others evaluable,1-4with the only exception being the case who received G-CSF in addition to ATRA, as reported by Jansen et al.10 Finally, semiquantitative RT-PCR analysis of retinoic acid target genes showed significantly increased RARα and RARβexpression following in vivo treatment, further strengthening the view that our case was ATRA sensitive (not shown).
To conclude, our findings support the notion that differentiation therapy is not restricted to PML-RARα APL and suggest that ATRA might have a broader antileukemic efficacy than previously expected.
Prepublished online as Blood First Edition Paper, April 30, 2002; DOI 10.1182/blood-2001-12-0368.
Supported by AIL (Associazione Italiana contro le Leucemie), AIRC (Associazione Italiana per la Ricerca sul Cancro), MURST (Ministero dell' Università e della Ricerca Scientifica e Tecnologica), and Ministero della Salute.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Francesco Lo Coco, Dipartimento di Biotecnologie Cellulari ed Ematologia, Università La Sapienza, Via Benevento 6, 00161 Roma, Italy; e-mail: lococo@bce.med.uniroma1.it.