Abstract
Secretion of platelet granules is necessary for normal hemostasis. Platelet secretion requires soluble N-ethylmaleimide–sensitive factor attachment protein (SNAP) receptor (SNARE) complex formation between different members of the syntaxin, SNAP-25, and vesicle-associated membrane protein (VAMP) gene families. Using microcapillary reverse-phase high-performance liquid chromatography–nano-electrospray tandem mass spectrometry, we identified VAMP-3 and VAMP-8 as VAMP isoforms coimmunoprecipitated from platelets with syntaxin 4. Immunoblotting experiments confirmed the presence of VAMP-3 and VAMP-8 but not VAMP-1 or VAMP-2 in platelets. To examine the effect of VAMP proteins on platelet secretion, soluble recombinant (r) VAMP-2, rVAMP-3, and rVAMP-8 were incubated with streptolysin O–permeabilized platelets. Secretion of α granules (monitored by flow cytometric measurement of P-selectin) was blocked, and dense-granule secretion (assessed by release of carbon 14–serotonin) was almost completely inhibited by rVAMP-3, whereas rVAMP-8 inhibited secretion of dense granules but not α granules. In contrast, rVAMP-2, which formed SNARE complexes in vitro, had no effect on platelet exocytosis. We conclude that VAMP-3 and VAMP-8 form SNARE complexes with platelet syntaxin 4 and are required for platelet granule secretion.
Introduction
The secretory machinery in platelets has important homologies to the machinery in neurons and other cells (reviewed by Reed et al1). Soluble N-ethylmaleimide–sensitive factor (NSF) attachment protein (SNAP) receptor (SNARE) complexes are formed between vesicle-associated membrane proteins (VAMPs; v-SNAREs) and proteins in the target membranes (SNAP-25 and homologs and syntaxins; t-SNAREs).2 3 Many lines of evidence show that SNARE complexes are crucial for membrane trafficking and fusion events such as secretion and exocytosis.
Platelets contain SNARE proteins4-7 that form SNARE complexes in vitro which support SNAP-dependent NSF–adenosine triphosphatase activity.4 SNAP-dependent NSF is critical for exocytosis of α and dense granules6 as well as lysosomes.8 Platelet membranes contain syntaxin 2 and syntaxin 4, which have been shown to be required for platelet secretion.5,7-9 Platelets contain abundant amounts of SNAP-23 and VAMP proteins, which interact and form ternary SNARE complexes with syntaxin 4.5,7,9 The platelet Sec1 protein (PSP) is identical to human Munc-18c and forms a tight complex with syntaxin 4 that can prevent formation of the SNARE complex.5 Anti-PSP antibodies that dissociate PSP–syntaxin 4 complexes stimulate secretion of all 3 types of granules in permeabilized platelets (A. Houng et al, manuscript submitted), indicating the importance of SNARE complex formation in platelet secretion. PSP5 and syntaxin 410 are phosphorylated in thrombin-activated platelets through a protein kinase C (PKC)–dependent mechanism; phosphorylation of these 2 proteins modulates their interactions and may regulate secretion.
These findings established the existence of a link of the SNARE machinery through PKC signaling to receptor-mediated cell activation in platelets. To understand further the molecular mechanisms that lead to granule secretion, it is important to identify which isoforms of the SNARE protein families play a role in this process. Although SNAP-23, syntaxin 2, and syntaxin 4 have been shown to be important for platelet secretion, the VAMP isoforms required for platelet granule exocytosis are unknown. Polyclonal anti-VAMP antibodies and Botulinum toxin cleavage inhibit secretion in permeabilized platelets, indicating that one or more VAMPs is required for granule secretion.7 In this study, we found that VAMP-3 and VAMP-8 form SNARE complexes in human platelets and mediate secretion.
Study design
Platelet-secretion assays
Human platelet-rich plasma was prepared from freshly drawn blood, and dense granules of platelets were loaded with carbon 14 (14C)–serotonin as described previously.6Platelets were centrifuged and resuspended in a calcium (Ca++) buffer containing 20 mM piperazine diethanesulfonic acid (pH 7.4), 150 mM potassium glutamate, 5 mM glucose, 2.5 mM EDTA, 2.5 mM ethyleneglycoltetraacetic acid, and 0.05% bovine serum albumin (buffer A). The platelet count was adjusted to 8 × 108/mL by using buffer A.
Platelets (20 μL) were mixed with buffer A (25 μL) containing 200 to 400 U/mL streptolysin O (Sigma, St Louis, MO), 0.5 U/mL hirudin, and various concentrations of VAMPs. The samples were incubated at 25°C for 10 minutes and on ice for 30 minutes. Adenosine triphosphate (50 mM) and magnesium diacetate (125 mM) in buffer A (5 μL) were added, and the samples were incubated at 25°C for 10 minutes. Granule secretion was induced by increasing the amount of Ca++ to 10 μM as described previously.11 After 5 minutes of incubation, 3-μL samples were used for measurement of P-selectin expression (described below). The remaining samples were put on ice for 3 minutes and centrifuged (1000g) for 1 minute, and the 14C-serotonin in the supernatant was measured by scintillation counting.
Secretion of α granules was monitored by measuring P-selectin expression with phycoerythrin-conjugated anti-CD62 antibody AC1.2 (Becton Dickinson, Mountain View, CA) and flow cytometry (FACSCalibur; Becton Dickinson) as described previously.12 Total (100%) P-selectin expression was defined as that induced by 1 U/mL thrombin in nonpermeabilized platelets. P-selectin expression on nonstimulated, nonpermeabilized platelets was less than 2%; that on nonstimulated, permeabilized platelets was less than 5%. The recombinant (r) VAMPs induced neither α-granule secretion in nonstimulated (no thrombin and no addition of Ca++), nonpermeabilized platelets nor dense-granule secretion in nonstimulated, permeabilized platelets.
Protein-sequence analysis
Syntaxin 4 was immunoprecipitated from Triton X-100 platelet lysates with use of antisyntaxin 4 antibodies as described previously.10 The samples were analyzed by sodium dodecyl sulfate–polyacrylamide electrophoresis (SDS-PAGE). Coimmunoprecipitated VAMP proteins were identified by their molecular mass and immunoreactivity (immunoblotting with a mixture of anti-VAMP antibodies). VAMP proteins coimmunoprecipitated from a lysate of approximately 1010 platelets could be detected by Coomassie Blue staining. Gel slices containing VAMP proteins, as well as a negative control sample (immunoprecipitation with irrelevant antibodies; no Coomassie Blue–stained protein band in the gel), were excised and analyzed by microcapillary reverse-phase high-performance liquid chromatography (HPLC)–nano-electrospray tandem mass spectrometry (MS) at the Harvard Microchemistry Facility on a quadrupole ion trap mass spectrometer (LCQ Deca; Thermo Finnigan, San Jose, CA).
Preparation of recombinant proteins
The cytosolic domains of human VAMP-2, VAMP-3, and VAMP-8 and rat syntaxin 4 and SNAP-25 were produced as recombinant glutathione-S-transferase (GST)–tagged (Pharmacia, San Diego, CA) proteins in Escherichia coli by using standard protocols.13 The GST tag was cleaved with thrombin. VAMPs were further purified on a column (Mono S; Pharmacia) by using cation exchange fast-protein liquid chromatography as described previously14 and were then concentrated and dialyzed against buffer A.
Antibodies
Monoclonal antisyntaxin 4 antibodies were from Transduction Laboratories (Lexington, KY). Polyclonal antibodies against human SNAP-23 were generated in rabbits. Monoclonal and polyclonal anti-VAMP-1 and VAMP-2 antibodies were obtained from StressGen (Collegeville, PA) and Synaptic Systems (Germany); anti-VAMP-3 and VAMP-8 antibodies were from Abcam (Cambridge, United Kingdom).
Results and discussion
VAMP-3 and VAMP-8 coimmunoprecipitate with syntaxin 4 in platelets
Complex formation between SNARE proteins is required for granule secretion. We sought to identify which VAMP proteins formed SNARE complexes with syntaxin 4, a SNARE protein that is required for platelet secretion. When syntaxin 4 was immunoprecipitated from Triton X-100 platelet lysate with antisyntaxin 4 antibodies, it was found to be in a complex with SNAP-23. We also identified other proteins with molecular sizes consistent with VAMPs and immunoreactive with a mixture of anti-VAMP antibodies. The immunoblotting experiments were not considered definitive for identification of the VAMP isoforms because of the moderate to high sequence identity among the VAMPs (Figure1) and the limited availability of isoform-specific VAMP antibodies. Therefore, SDS-PAGE gel slices containing VAMP proteins were excised and the samples were analyzed by microcapillary reverse-phase HPLC–nano-electrospray tandem MS. The sequences obtained with this method unequivocally identified VAMP-3 and VAMP-8 (Figure 1).
Sequence comparison of human VAMP isoforms.
Platelet VAMPs were isolated by coimmunoprecipitation with antisyntaxin 4 antibodies. The sequences of VAMP-3 and VAMP-8 identified by microcapillary reverse-phase HPLC–nano-electrospray tandem MS are shown in boldface type. Alignments were carried out by using the ClustalW service at the European Bioinformatics Institute (http://www.ebi.ac.uk/clustalw). The 260-amino acid VAMP-7 is not shown in the alignment. Portions of VAMP-4 (N-terminal 25 amino acids, MPPKFKRHLNDDDVTGSVKSERRNL) and VAMP-5 (amino acids 95-116, QSSDSSSAPRTQDAGIASGPGN) also are not shown.
Sequence comparison of human VAMP isoforms.
Platelet VAMPs were isolated by coimmunoprecipitation with antisyntaxin 4 antibodies. The sequences of VAMP-3 and VAMP-8 identified by microcapillary reverse-phase HPLC–nano-electrospray tandem MS are shown in boldface type. Alignments were carried out by using the ClustalW service at the European Bioinformatics Institute (http://www.ebi.ac.uk/clustalw). The 260-amino acid VAMP-7 is not shown in the alignment. Portions of VAMP-4 (N-terminal 25 amino acids, MPPKFKRHLNDDDVTGSVKSERRNL) and VAMP-5 (amino acids 95-116, QSSDSSSAPRTQDAGIASGPGN) also are not shown.
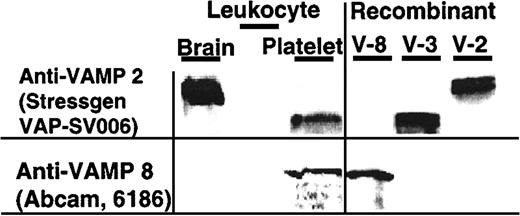
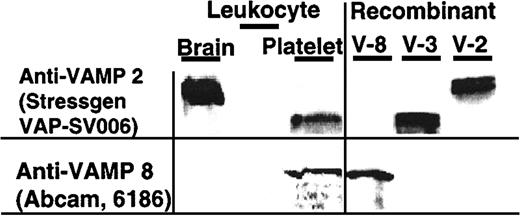
In agreement with these findings, VAMP-3 and VAMP-8 but not VAMP-2 were detected in platelet lysates by immunoblotting (Figure2). The VAMP-3 and VAMP-8 detected were derived from platelets and not the small number of leukocytes contaminating the platelet preparations. No VAMP-3 or VAMP-8 was detected in cell lysates containing twice as many leukocytes as are typically found in our platelet preparations (Figure 2). VAMP-3 and VAMP-8 also coimmunoprecipitated with SNAP-23, another SNARE protein shown to be required for platelet secretion (data not shown).
VAMP-3 and VAMP-8, but not VAMP-2, are VAMP isoforms present in human platelets.
SDS lysate of brain tissue (50 μg; Clontech 7800-1, BD Biosciences, Palo Alto, CA), leukocytes (8 × 103 leukocytes and 1 × 103 platelets), and platelets (4 × 107 platelets containing 4 × 103leukocytes) (left panel) and rVAMP proteins (100 ng each; right panel) were analyzed by SDS-PAGE and immunoblotting. The membranes were probed with the indicated primary anti-VAMP antibodies. There were twice as many leukocytes in the leukocyte sample as in the platelet sample.
VAMP-3 and VAMP-8, but not VAMP-2, are VAMP isoforms present in human platelets.
SDS lysate of brain tissue (50 μg; Clontech 7800-1, BD Biosciences, Palo Alto, CA), leukocytes (8 × 103 leukocytes and 1 × 103 platelets), and platelets (4 × 107 platelets containing 4 × 103leukocytes) (left panel) and rVAMP proteins (100 ng each; right panel) were analyzed by SDS-PAGE and immunoblotting. The membranes were probed with the indicated primary anti-VAMP antibodies. There were twice as many leukocytes in the leukocyte sample as in the platelet sample.
VAMP-3 and VAMP-8 are required for platelet secretion
Our finding that VAMP-3 and VAMP-8 coimmunoprecipitated with syntaxin 4 and SNAP-23 (platelet SNAREs required for secretion) suggested that these VAMP isoforms may play a role in the mechanism of platelet granule secretion. To examine this issue, we studied the effect of various recombinant VAMP proteins on granule secretion in permeabilized platelets. Thus, various concentrations of VAMP-2, VAMP-3, and VAMP-8 with no C-terminal transmembrane domains (rVAMPs) were incubated with streptolysin O–permeabilized platelets. Granule secretion was induced by increasing Ca++.
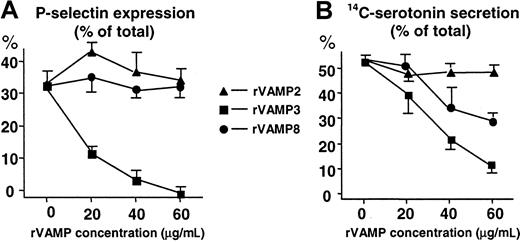
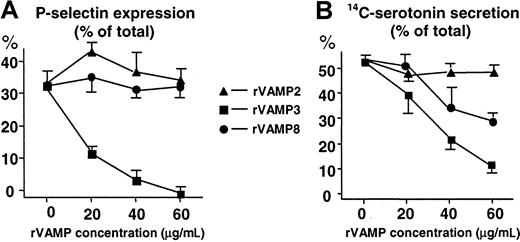
We found that rVAMP-3 was a potent inhibitor of both α-granule secretion (up to 100% inhibition) and dense-granule secretion (80%; Figure 3). The rVAMP-8 inhibited dense-granule secretion (40%) but had no significant effect on α-granule secretion. Complete inhibition of dense-granule secretion could not be obtained with the addition of up to 120 μg/mL rVAMP (data not shown). In contrast to rVAMP-3 and rVAMP-8, rVAMP-2, a VAMP isoform not detected in platelets, had no effect on granule secretion (Figure 3).
The cytoplasmic portions of VAMP 3 and VAMP 8, but not VAMP 2, inhibit granule secretion in permeabilized human platelets.
Streptolysin O–permeabilized platelets were preincubated with various concentrations of soluble rVAMP proteins lacking the C-terminal transmembrane domains. Granule secretion was induced by increasing the amount of Ca++ to 10 μM. (A) Secretion of α granules was monitored by measuring P-selectin expression with flow cytometry. P-selectin expression induced with 1 U/mL thrombin in nonpermeabilized platelets was considered total (100%). (B) Secretion of14C-serotonin from dense granules of platelets. Release of14C-serotonin was measured by scintillation counting. The total 14C-serotonin content (100%) was measured in platelets lysed in 0.4% Triton X-100. All sets of experiments contained samples with no addition of Ca++ for measurement of nonspecific leakage of 14C-serotonin. The amount of nonspecific leakage, typically less than 10% to 15% of the total14C-serotonin, was subtracted from all data points before calculation of the percent secretion. (A,B) The values shown (mean ± SD) are representative results from 3 independent experiments performed in duplicate. In samples with no rVAMP, the α- and dense-granule secretion in permeabilized platelets from different donors represented 30% to 60% of the total inducible by thrombin in nonpermeabilized platelets.
The cytoplasmic portions of VAMP 3 and VAMP 8, but not VAMP 2, inhibit granule secretion in permeabilized human platelets.
Streptolysin O–permeabilized platelets were preincubated with various concentrations of soluble rVAMP proteins lacking the C-terminal transmembrane domains. Granule secretion was induced by increasing the amount of Ca++ to 10 μM. (A) Secretion of α granules was monitored by measuring P-selectin expression with flow cytometry. P-selectin expression induced with 1 U/mL thrombin in nonpermeabilized platelets was considered total (100%). (B) Secretion of14C-serotonin from dense granules of platelets. Release of14C-serotonin was measured by scintillation counting. The total 14C-serotonin content (100%) was measured in platelets lysed in 0.4% Triton X-100. All sets of experiments contained samples with no addition of Ca++ for measurement of nonspecific leakage of 14C-serotonin. The amount of nonspecific leakage, typically less than 10% to 15% of the total14C-serotonin, was subtracted from all data points before calculation of the percent secretion. (A,B) The values shown (mean ± SD) are representative results from 3 independent experiments performed in duplicate. In samples with no rVAMP, the α- and dense-granule secretion in permeabilized platelets from different donors represented 30% to 60% of the total inducible by thrombin in nonpermeabilized platelets.
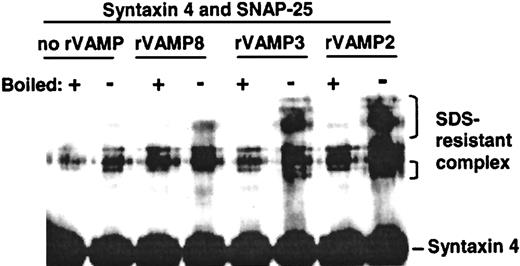
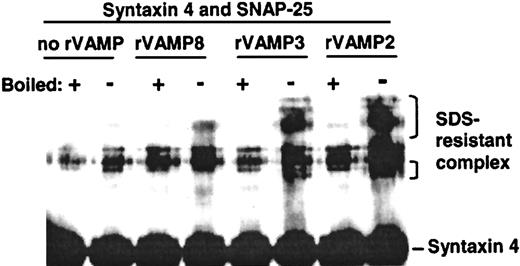
To confirm that the rVAMPs used in these studies were functional, we examined their ability to form SNARE complexes in vitro. Ternary SNARE complexes are resistant to denaturation by 1% SDS if boiling is not done.15 Equimolar amounts of SNAP-25, syntaxin 4, and rVAMP-2, rVAMP-3, or rVAMP-8 were incubated together, and the samples were analyzed by SDS-PAGE and immunoblotting. The incubation time, buffer composition, and temperature mimicked the experimental conditions used for studying the effects of rVAMPs on secretion in permeabilized platelets. All 3 rVAMPs formed ternary complexes under these conditions, confirming the functionality of these proteins (Figure 4). The strongest SNARE complex bands were detected with rVAMP-2 (Figure 4), which had no effect on secretion in platelets.
All 3 recombinant VAMPs formed SDS-resistant ternary SNARE complexes in vitro.
Equimolar amounts of SNAP-25, syntaxin 4, and rVAMP-2, rVAMP-3, or rVAMP-8 were incubated together, and the samples were analyzed by SDS-PAGE and immunoblotting using antisyntaxin 4 primary antibodies. The incubation time, buffer composition, and temperature mimicked the conditions in the experiments studying the effects of rVAMPs on secretion in permeabilized platelets. Note that rVAMP-2, the negative control in the granule-secretion experiments, interacted readily with other SNAREs to form stable SNARE complexes in vitro.
All 3 recombinant VAMPs formed SDS-resistant ternary SNARE complexes in vitro.
Equimolar amounts of SNAP-25, syntaxin 4, and rVAMP-2, rVAMP-3, or rVAMP-8 were incubated together, and the samples were analyzed by SDS-PAGE and immunoblotting using antisyntaxin 4 primary antibodies. The incubation time, buffer composition, and temperature mimicked the conditions in the experiments studying the effects of rVAMPs on secretion in permeabilized platelets. Note that rVAMP-2, the negative control in the granule-secretion experiments, interacted readily with other SNAREs to form stable SNARE complexes in vitro.
Flaumenhaft et al7 found that VAMP-1 and VAMP-2 could not be detected in platelets and that a relatively high concentration of Botulinum toxin was necessary to inhibit platelet secretion. This led them to speculate that platelets may contain a novel VAMP species. Bernstein and Whiteheart16 observed a punctate intracellular staining with anti-VAMP-3 antibodies in human platelets. Our finding that the cytoplasmic domain of VAMP-3 inhibited secretion in permeabilized platelets strongly suggests that VAMP-3 plays an important role in the mechanism of platelet secretion.
VAMP-3 may be the only v-SNARE required for α-granule secretion because rVAMP-3 completely inhibited P-selectin expression whereas rVAMP-2 and rVAMP-8 had no effect. The situation is more complex for dense-granule secretion, in which both rVAMP-3 and rVAMP-8 can inhibit exocytosis. It is conceivable that both VAMP-3 and VAMP-8 are involved in dense-granule secretion; a similar suggestion has been made for syntaxin 2 and syntaxin 4 in platelet lysosomal secretion.8 Alternatively, the involvement of both VAMP-3 and VAMP-8 in dense-granule fusion may be related to the fact that platelet exocytosis proceeds through compound fusion between α and dense granules and the plasma membrane. Flaumenhaft et al17have reported that anti-VAMP-3 (antihuman cellubrevin) antibodies inhibited α-granule secretion in streptolysin O–permeabilized platelets.
We thank Lin Liu and Aeisha Robb for help in cloning VAMPs and producing recombinant proteins.
Supported in part by National Institutes of Health grant HL-64057 (G.L.R.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Guy L. Reed, Cardiovascular Biology Laboratory, Harvard School of Public Health, 665 Huntington Avenue, Boston, MA 02115; e-mail: reed@cvlab.harvard.edu.