Abstract
Long-term survivors of acquired aplastic anemia (AA) have an increased risk of developing myelodysplastic syndrome (MDS) and acute myeloid leukemia (AML) after immunosuppressive therapy (IST). It is uncertain whether the increased survival time simply discloses the natural history of AA as a premalignant disease or whether secondary disease is related to the therapy itself. Between November 1992 and September 1997, 113 AA children with normal cytogenetics at diagnosis were treated with IST using antithymocyte globulin, cyclosporin, and danazol with or without granulocyte colony-stimulating factor (G-CSF). We assessed risk factors for developing MDS/AML by Cox proportional hazards models. Twelve of 113 patients developed MDS between 9 and 81 months following the time of diagnosis, giving a cumulative incidence of 13.7 ± 3.9%. The following cytogenetic abnormalities were observed at the time of diagnosis of MDS: monosomy 7 (6 patients), monosomy7/trisomy21 (1 patient), trisomy 11 (1 patient), del (11) (9?:14) (1 patient), add (9q) (1 patient), add 7 (q 32) (1 patient), and trisomy 9 (1 patient). The number of days of G-CSF therapy and nonresponse to therapy at 6 months were statistically significant risk factors by multivariate analysis. The present study suggests a close relationship between long-term use of G-CSF and secondary MDS in nonresponders to IST.
Introduction
Long-surviving patients with aplastic anemia (AA) who have been treated with immunosuppressive therapy (IST) have an increased risk of developing myelodysplastic syndrome (MDS) or acute myeloid leukemia (AML).1 At present, it is uncertain whether the increased survival time displays the natural history of AA as a premalignant disease or whether the secondary disease is related to the therapy itself.
We first reported cases of MDS/AML in children with AA following treatment with granulocyte colony-stimulating factor (G-CSF) and had never observed this phenomenon before the introduction of G-CSF therapy.2 In our previous nationwide retrospective study, development of MDS/AML was observed in 11 of 50 children with AA who were treated with the combination of cyclosporin and G-CSF. Monosomy 7 was detected in most patients at the time of diagnosis of MDS/AML.3 Malignant transformation with monosomy 7 was also observed in children with severe congenital neutropenia (SCN) after treatment with G-CSF.4 Because hematopoietic growth factors can stimulate leukemic clones, long-term administration may facilitate malignant transformation in patients with nonmalignant diseases.5 Those findings prompted us to conduct a prospective multicenter study to examine the incidence and risk factors for malignant transformation in children with AA.
Patients, materials, and methods
Patients
Since 1992, the Japan Childhood Aplastic Anemia Study Group has conducted a prospective multicenter trial of immunosuppressive agents and G-CSF for children with acquired aplastic anemia. One hundred thirty-four children were entered into the study. Among these, 119 children were newly diagnosed (within 180 days) and had not received antithymocyte globulin (ATG). The details of this cohort study were previously reported.6 Eligibility criteria for the present report were: age < 18 years, moderate to very severe AA, an adequate number of mitoses for cytogenetic studies, the absence of a clonal cytogenetic abnormality at the time of entry to the study, and at least one subsequent cytogenetic analysis at follow-up. Disease was considered severe if at least 2 of the following were noted: neutrophil count < 0.5 × 109/L, platelet count < 20 × 109/L, or reticulocyte count < 20 × 109/L with a hypocellular bone marrow.7 AA was considered very severe if the above criteria for severe disease were fulfilled and the neutrophil count was < 0.2 × 109/L. Moderate disease was defined by at least 2 of the following hematologic values: neutrophil count < 1.0 × 109/L, platelet count < 50 × 109/L, or reticulocyte count < 60 × 109/L with a hypocellular bone marrow. Patients were excluded if they had congenital AA. Informed written consent was obtained from all patients or their parents, and the study was approved by the ethics committee of each participating hospital.
Study design
All patients with very severe AA received ATG, cyclosporin, danazol, and G-CSF. Other patients were treated with either ATG, cyclosporin, danazol, and G-CSF or ATG, cyclosporin, and danazol. Horse ATG (IMTIX, Lyon, France) was administered at a dose of 15 mg/kg/d from day 1 to day 5. For prevention of serum sickness, methylprednisolone 2 mg/kg/d was given for 7 days, then the dose was halved every 7 days until discontinuation on day 30. Cyclosporin (6 mg/kg/d orally) and danazol (5 mg/kg/d orally) were started on day 1 and continued at least until day 180. The dose of cyclosporin was adjusted to achieve a whole blood through level of 100 to 200 ng/mL. Blood levels of cyclosporin were measured by radioimmunoassay with monoclonal antibody. Both agents were continued at therapeutic doses until blood counts reached a plateau, and then the doses were reduced gradually to prevent relapse of aplasia. G-CSF (Filgrastim, Kirin-Sankyo, Tokyo, Japan) was administered intravenously or subcutaneously at a dose of 400 μg/m2/d from day 1 to day 90. After the neutrophil count reached more than 5 × 109/L, G-CSF was administered 3 times a week. In nonresponders, G-CSF was continued until neutrophil count increased to > 5 × 109/L even after day 90.
Bone marrow aspiration for morphology and cytogenetic studies was performed at the time of entry to the study and at 12-month intervals thereafter. The diagnosis of MDS was established based on characteristic morphologic abnormalities in the bone marrow with the presence of cytogenetic abnormalities and peripheral cytopenia. Patients with MDS or AML were subclassified according to the French-American-British classification.8 All participating hospitals completed case report forms that included the results of cytogenetic studies and dosing records for each immunosuppressive agent and G-CSF.
Statistics
Follow-up continued until death, stem cell transplantation, or April 1, 2001, whichever came first. The Kaplan-Meier method was used to estimate the probability of evolution to MDS/AML.9 The Cox proportional hazards model was used to assess the risk factors for evolution to MDS/AML in both univariate and multivariate analyses. The following demographic, hematologic, and dosing parameters were analyzed for their relation to the development of MDS/AML: age; sex; severity of disease; response to therapy at 6 months; treatment duration of cyclosporin, danazol, and G-CSF; history of previous therapies; history of G-CSF therapy before registration; and use of a second ATG course. In patients who previously received treatments for AA, treatment duration of cyclosporin, danazol, and G-CSF and use of ATG before entering this study were included for analyses. The estimated magnitude of the relative risk (RR) is shown along with its 95% confidence interval (CI). The statistical package SAS was used for all analyses.
Results
From November 1992 to September 1997, 134 patients were entered in the study. At the time of entry to the study, an inadequate number of mitoses for cytogenetic studies were available in 18 patients. A clonal cytogenetic abnormality was detected in 3 patients. A total of 113 patients fulfilled the eligibility criteria, and their data were available for analysis (Table 1). Among them, 98 patients were newly diagnosed. Stem cell transplantation was attempted in 27 patients in whom the initial IST failed or who had relapse of aplasia after an initial response. Their data were censored at the time of transplantation. Fifteen nonresponders received a second course of ATG therapy. Of the 113 evaluable patients, 15 died. Causes of death were MDS (9), BMT-related toxicities (4), intracranial hemorrhage (1), and car accident (1). Median follow-up period for surviving patients is 64 months, ranging from 45 months to 107 months.
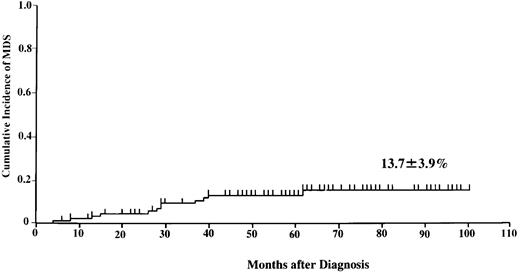
Of the 113 patients, 12 developed MDS between 9 and 81 months (median, 37 months) following the time of diagnosis of AA (Table2). Cytogenetic analysis of bone marrow at the time of diagnosis of MDS revealed monosomy 7 (6 patients), monosomy 7/trisomy 21 (1 patient), trisomy 11 (1 patient), del 11 (9?:14) (1 patient), add (9q) (1 patient), add 7 (q32) (1 patient), and trisomy 9 (1 patient). The prognosis of these patients was very poor. Only 2 patients who underwent bone marrow transplantation and one recently evolved patient are still alive. Figure 1 shows the cumulative incidence of MDS at 8 years (13.7 ± 3.9%). We did not observe any patient with MDS/AML among the 21 patients who were excluded from this analysis by eligibility criteria.
Probability of developing myelodysplastic syndrome in 113 children with aplastic anemia treated with immunosuppressive therapy with or without granulocyte colony-stimulating factor.
Probability of developing myelodysplastic syndrome in 113 children with aplastic anemia treated with immunosuppressive therapy with or without granulocyte colony-stimulating factor.
In addition to the 12 patients with MDS, 4 other patients developed cytogenetic clonal changes in bone marrow cells without the distinctive morphologic features of MDS (Table3). Trisomy 8 (3 patients) and del 13 (1 patient) were noted in these patients. The overall cumulative incidence of clonal cytogenetic abnormalities was 21.9 ± 5.8% at 8 years. One patient received a bone marrow transplant from an HLA-matched sibling. Abnormal clones still remain in 3 patients who did not receive bone marrow transplants. All 4 patients are alive without transformation to malignant disease.
In the analyses of risk factors for developing MDS, 113 patients were eligible for a total of 12 events. Results of a univariate analysis are summarized in Table 4. The following parameters were statistically significant risk factors: the number of days of G-CSF therapy; nonresponse to therapy at 6 months; history of previous therapies; and history of G-CSF therapy before registration. RR for patients who received G-CSF longer than 120 days was 4.4 times higher than that for patients who received G-CSF for < 120 days (P = .02). RR increased in proportion to the duration of G-CSF therapy. For example, RR was 8.7 times higher for patients who received G-CSF for longer than 180 days, compared to patients who received G-CSF for < 180 days (P = .0004). By multivariate analysis, 2 factors, the number of days of G-CSF therapy and nonresponse to therapy at 6 months, remained statistically significant (Table 5). We also analyzed risk factors for developing MDS among 98 patients who were newly diagnosed by multivariate analysis. The number of days of G-CSF therapy was a statistically significant risk factor (RR: 1.003 [1.001-1.005],P = .01). The following number of patients developed MDS in each subgroup: 8 patients in the G-CSF (+) and response (−) group; 3 patients in the G-CSF (+) and response (+) group; and 1 patient in G-CSF (−) and response (−) group.
Discussion
Our prospective study examined the incidence and risk factors of MDS/AML after IST in patients with AA. In the present study, 12 of 113 patients (10.6%) developed MDS/AML at a median follow-up of 56 months. Recently, 2 study groups reported on the incidence of MDS/AML after combination therapy with ATG, cyclosporin, and G-CSF. In a study reported by the German-Austrian Pediatric AA Working Group, 4 of 86 patients (4.7%) developed MDS/AML.10 The frequency of MDS/AML in 100 patients was 6.0% in data from the Gruppo Italiano Trapianti di Midollo Osseo (GITMO)/the European Group for Blood and Marrow Transplantation (EBMT) pilot study.11 Restricted to 29 children with normal cytogenetics at diagnosis, in the GITMO/EBMT study, 2 children (6.9%) developed MDS with monosomy 7. This incidence is not different from the 5% to 15% values in previous reports in which G-CSF was not included in treatment.1 However, the median follow-up periods for survivors were 24 months, 47 months, and 56 months in these 3 reports, much shorter intervals compared to previous studies. Longer follow-up is necessary to determine whether there is a difference in the incidence of malignant conversion between the recent studies that included intensive immunosuppression and hematopoietic growth factors versus previous studies with less intensive immunosuppression alone. The recent study from the GITMO addressed this issue by comparing the incidence of secondary malignancies among 2 patient groups.12 One group consisted of 57 patients who received IST between 1978 and 1991 (non–G-CSF group), and the other consisted of 87 patients who received IST and G-CSF between 1991 and 1996 (G-CSF group). The risk of developing secondary malignancies at 5 years was 9% for the G-CSF group, and 7% for the non–G-CSF group (P = .99). GITMO concluded that large doses of G-CSF administered over a long period of time in conjunction with immunosuppressive agents do not increase the risk of developing MDS/AML in patients with AA. However, their conclusion was derived from a retrospective analysis, which compared 2 cohorts with different patient characteristics and duration of follow-up. Prospective studies are indispensable to clarify this issue.
Contrary to the conclusion of the Italian study, our data show that long-term use of G-CSF is related to development of MDS/AML. Until now, only the study from the EBMT group had identified risk factors for malignant transformation in patients with AA by multivariate analysis.13 Their risk factors were as follows: addition of androgens to the IST (RR: 0.28); older age (RR: 1.03); treatment in 1982 or later, as compared with 1981 or earlier (RR: 3.01); splenectomy (RR: 3.65); and treatment with multiple courses of IST (RR: 2.26). However, this retrospective analysis did not include information on dosing of drugs. In the EBMT group's study population, only a few patients received cyclosporin and none received hematopoietic growth factors. As cyclosporin and hematopoietic growth factors are now widely used in the treatment of AA, further studies are warranted to examine the incidence and risk factors for secondary malignancies in patients with AA treated with this combination of agents.
Reports of malignant transformation in patients with AA after G-CSF therapy come mainly from Japan. In our previous report,3development of MDS/AML with monosomy 7 was observed in children treated with the combination of cyclosporin and G-CSF. None of the 41 patients who received either cyclosporin or G-CSF, but not the combination, developed MDS/AML. MDS with monosomy 7 was also observed in 4 of 18 adult patients, all of whom had been treated with G-CSF and cyclosporin for more than 1 year.14 Identification of the risk factors for malignant transformations of AA is very important, and the present study is the first prospective study to address this issue. What is the role of G-CSF in the evolution of AA into MDS/AML? It seems unlikely that G-CSF causes DNA damage or is directly leukemogenic. Prior to the availability of G-CSF, it was well recognized that malignant transformation occurred in a proportion of patients with AA.15 In the current study, one patient who was not given G-CSF developed MDS with monosomy 7 at 9 months after the diagnosis of AA. It is likely that abnormal clones might have existed in this patient at the time of diagnosis. To determine this possibility, we examined the bone marrow for the presence of monosomy 7 clones using fluorescent in situ hybridization (FISH) at the time of diagnosis and 3 months after the start of IST. However, we could not detect any abnormal clones in this patient.
Genetic instability is suggested by the fact that cytogenetic abnormalities are found frequently in patients with AA.16,17 To clarify chromosomal instability, we previously performed FISH analysis for monosomy 7 in colony-forming cells from patients with AA who later developed MDS with monosomy 7.18 Colonies derived from bone marrow mononuclear cells (BMMNCs) of these patients consisted of a mixture of cells of normal karyotype and monosomy 7, which suggested that colony-forming cells initially had a normal karyotype but subsequently lost chromosome 7 during colony formation. This finding suggests the existence of genetically unstable clones in patients with AA whose condition later evolves into MDS.
These genetically unstable clones may decrease following complete response to IST but remain in patients who failed to respond to IST. In the present study, 9 of the 12 patients with MDS/AML were nonresponders to IST. Evolution into MDS/AML was not observed in any of the 30 complete responders. In a report from the GITMO/EBMT Group, the probability of developing abnormal cytogenetic clones was 40% (2 of 5) in nonresponders, 6% (1 of 19) in partial responders, and 10% (3 of 30) in complete responders.11
In an in vitro suspension culture study using BMMNCs from MDS patients with a mosaic of normal and monosomy 7 karyotypes, only pharmacologic doses of G-CSF significantly increased aberrant clones with monosomy 7.19 No increase in monosomy 7 clones over baseline was noted on exposure to other hematopoietic growth factors, such as granulocyte macrophage colony-stimulating factor or interleukin-3. When cells from patients with trisomy 8 or 5q− were cultured with G-CSF, there was no increase in the proportion of cells with these specific cytogenetic abnormalities. Thus, monosomy 7 may provide a particular growth advantage to the clones in the presence of G-CSF.
These in vitro findings may explain why monosomy 7 is so frequently detected in affected patients in previous reports from Japan. This high incidence of monosomy 7 has not been observed in studies from Europe and North America.1 Because effective ATG products were not available in the early 1990s, the response rate to IST was much lower in Japanese studies to those in European and North American studies.20 Most nonresponders received G-CSF and cyclosporin for a prolonged duration.21 Trisomy 8 is one of the cytogenetic abnormalities frequently observed in patients with AA. Contrary to patients with monosomy 7, this abnormality is rarely observed in patients with a malignant transformation.16Because the risk of progression to MDS/AML may vary among patients with different cytogenetic abnormalities, it may be particularly important that G-CSF facilitates the growth of aberrant clones with monosomy 7.
We previously reported the limited value of G-CSF with respect to hematologic response, incidence of documented infections, and overall survival rate in patients with moderate to severe AA.6Combined with the findings in the present study, it is clear that long-term administration of G-CSF should not be used for patients with AA.
The following centers and persons participated in the Japan Childhood Aplastic Anemia Study Group: Japanese Red Cross Nagoya First Hospital (T. Matsuyama, K. Kato); Kyoto Prefectural University of Medicine (S. Hibi); Kobe University School of Medicine (Y. Kosaka); Hyogo College of Medicine (M. Yamamoto); Ibaragi Children's Hospital (M. Tsuchida); Nihon University (H. Mugishima); Yamanashi Medical University (K. Sugita); Tokai University (H. Yabe); Toho University School of Medicine (A. Ohara, I. Tsukimoto); Shizuoka Children's Hospital (J. Mimaya); Shinshu University School of Medicine (K. Koike); Fukushima Medical University (A. Kikuta); Chiba Children's Hospital (Y. Okimoto); Kiyose Children's Hospital (T. Kaneko); Tokyo Medical and Dental University (Y. Ohkawa); Osaka City General Hospital (M. Sako); Nagoya University (S. Kojima, K. Horibe); Jichi Medical School (T. Yamauchi); Mie University (E. Azuma); Sapporo Medical University (T. Kudo); Kagoshima University (K. Kawakami); Shiga University of Medical Science (S. Ohta); The Jikei University School of Medicine (K. Fujisawa); Toyohashi City Hospital (Y. Nishimura); Okayama University (M. Oda); Hokkaido University (R. Kobayashi); Hiroshima University (K. Ueda); Fukuoka University (K. Nibu); Sapporo National Hospital (Y. Hatae); Nara Medical University (Y.-D. Park); Nagoya National Hospital (J. Yoshida); Akita University School of Medicine (A. Watanabe); Kyushu University (S. Ohga); Yamagata University (Y. Shimizu); Kinki University School of Medicine (H. Miyata); Osaka University Faculty of Medicine (J. Hara); Showa University School of Medicine (K. Isoyama); Tohoku University (M. Imaizumi); Kyoto First Red Cross Hospital (H. Ikuta); Maebashi Red Cross Hospital (M. Shimoda); Kyoto University (Y. Akiyama); Osaka Medical College (M. Miyake); Okazaki City Hospital (N. Nagai); Ohtsu Red Cross Hospital (T. Takimoto); Fukui Red Cross Hospital (S. Hayashi); Kyoto Second Red Cross Hospital (S. Matsuo); Kurume University (H. Eguchi); Yokohama City University (K. Ikuta); and Dokkyo University School of Medicine (K. Sugita).
Supported by a grant from the Intractable Hemopoietic Disease Research from the Ministry of Health and Welfare of Japan.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Seiji Kojima, Department of Developmental Pediatrics, Nagoya University School of Medicine, 65 Tsurumai-cho, Showa-ku, Nagoya 466-8550, Japan; e-mail:kojimas@med.nagoya-u.ac.jp.