Abstract
Vascular endothelial cadherin (VE-cadherin) is an endothelial-specific, trans-membrane protein that promotes homophilic cell adhesion. Inhibition of VE-cadherin by the blocking monoclonal antibody (mAb) BV13 inhibited angiogenesis and tumor growth in vivo. However, this effect was accompanied by a marked increase in lung and heart permeability. In the present paper, we characterize a different VE-cadherin mAb (BV14) that is able to inhibit angiogenesis without affecting vascular permeability. In vitro studies show that BV14, in contrast to BV13, did not increase paracellular permeability of endothelial monolayers and did not disrupt VE-cadherin clusters at junctions. However, both antibodies could inhibit formation of vascularlike structures in collagen gels and increase migration of endothelial cells into wounded areas. In vivo, BV14 and BV13 were equally active in inhibiting angiogenesis in the mouse cornea and in reducing the growth of hemangioma and C6 glioma. In contrast to BV13, BV14 did not change vascular permeability in all the organs tested and at any dose used. BV14 and BV13 bind to VE-cadherin extracellular repeats EC4 and EC1, respectively. We propose that, in resting vessels, where junctions are stable and well-structured, antibody binding to EC1 but not EC4 disrupts their organization and increases permeability. In contrast, in growing vessels, where endothelial cells are migrating and junctions are weaker, antibody binding to EC4 may be sufficient to disrupt cell-to-cell adhesion and inhibit assembly of new vascular structures.
Introduction
Endothelial cell-to-cell junctions are complex structures formed by different adhesive molecules.1-3Endothelial cells have tight and adherens junctions that present a general organization similar to that described in epithelial cells.4-7 Adherens junctions are ubiquitous along the vascular tree and are formed by transmembrane proteins belonging to the cadherin superfamily.4 Endothelial cells express a cell-specific cadherin called vascular endothelial (VE)–cadherin.1,2 This protein is linked inside the cells to β- and γ-catenin, which, in turn, promote the anchorage to actin cytoskeleton. Although the extracellular domain of VE-cadherin is required for homophilic adhesion and clustering, the intracellular association to catenins and the cytoskeleton are needed for stabilization of the complex and for full control of paracellular permeability.8 In addition, β- and γ-catenins, when released in the cytoplasm, may translocate to the nucleus and modulate cell transcription by interacting with Tcf transcription factors.9 This suggests that the cadherin-catenin complex may take part in the transfer of intracellular signals following homotypic cell-to-cell adhesion.
VE-cadherin plays a morphogenetic role in vascular development. Its expression is required for the normal organization of the vasculature in the embryo,10 and a null mutation in the VE-cadherin gene leads to embryonic lethality within 9.5 days after coitus because of strong alterations in vascular remodeling in yolk sac and embryo proper. These effects are due to marked functional alterations in VE-cadherin null endothelial cells, which lose contact inhibition of growth and show high susceptibility to apoptotic stimuli.10
Taking this into account, we tested whether VE-cadherin mAbs could inhibit angiogenesis in the adult. We found that mAb BV13, which is able to block VE-cadherin adhesive properties, was effective in reducing angiogenesis in different tumor models in the adult.11 These observations suggest that VE-cadherin may be a molecular target to limit angiogenesis in pathological conditions. However, administration of mAb BV13 in vivo markedly increased permeability in the lungs and heart.12 These data discouraged further development of BV13 as a potential therapeutic agent.
Nevertheless, considering the complexity of cadherin homophilic interaction and the multiple binding sites implicated, we tested whether other mAbs, directed to different regions of the protein, could have a more specific effect. The idea behind these studies is that tumor vessels present poorly organized junctions and high permeability in comparison to pre-existing vasculature. VE-cadherin engagement at junctions and the type and number of binding domains may be qualitatively and quantitatively different in tumors, supporting the possibility of developing specific inhibitors of this particular vasculature.
In this paper, we present evidence that a VE-cadherin mAb (BV14) directed to a region closer to the cell membrane was able to inhibit angiogenesis and tumor growth without a significant effect on vascular permeability in other organs. It is therefore possible to develop VE-cadherin blocking agents devoid of undesirable effects on vascular permeability.
Materials and methods
Cells and culture conditions
Endothelial cells from mouse lung (1G11) and H5V mouse endothelioma cell lines were provided by Dr A. Vecchi (Istituto Mario Negri, Milano, Italy).13,14 C6 glioma cells expressing a transfected vascular endothelial growth factor (VEGF) gene under tetracycline control (pTET-VEGF) were provided by Dr E. Keshet (The Hebrew University, Jerusalem, Israel).15 16 All the cell lines were cultured in Dulbecco minimum essential medium (D-MEM) (Life Technologies, Paisley, United Kingdom) supplemented with 10% fetal calf serum (FCS) (Life Technologies).
Endothelial cells from control mice (VE-cadherin+/+) or mice homozygous for a null mutation in the VE-cadherin gene (VE-cadherin−/−) were isolated from 9-day postcoitum embryos as previously described.10,17 To express human wild-type VE-cadherin and the human/murine VE-cadherin chimera, we used retroviral vectors as described.18 The cDNAs were cloned into PINCO plasmid and transfected in amphotropic Phoenix packaging cells. Culture supernatants containing viral particles were used to infect VE-cadherin−/−cells as described previously in detail.18,19 The cells were routinely cultured on 0.1% gelatin-coated (Difco Laboratories, Detroit, MI) flask in D-MEM (Life Technologies) with 20% FCS (HyClone Laboratories, Logan, UT) supplemented with 50 μg/mL endothelial cell growth supplement (ECGS) (Sigma Chemical, St Louis, MO) and 100 μg/mL heparin (Sigma). The endothelial nature of the cultures was confirmed both by Western blot and immunofluorescence microscopy using a set of antibodies directed to endothelial markers as previously described.10,17VE-cadherin−/− cells expressed all the endothelial cell markers tested at amounts comparable to VE-cadherin+/+ cells.20
Antibodies and reagents
Rat mAbs against the extracellular domain of mouse VE-cadherin (BV14 and BV13) were developed as described previously.12Mouse mAbs against the extracellular domain of human VE-cadherin were BV921,22 and Cad 5 (Transduction Laboratories, Lexington, KY). Rat mAb to mouse VEGF-receptor 2 (VEGF-R2) (DC101) and to mouse platelet endothelial cell adhesion molecule (PECAM)–1 (MEC 13.3) were characterized in a previous publication.23-25 All the mAbs were used as purified immunoglobulin G (IgG).
Purified nonimmune rat IgG (Jackson ImmunoResearch Laboratories, West Grove, PA) was used as a control in both in vivo and in vitro experiments.
Rhodamine (TRITC)–conjugated secondary antibody (reactive with either rat or mouse IgG) was purchased from Jackson ImmunoResearch Laboratories. Peroxidase-conjugated goat anti-rat and goat anti-mouse IgG antibodies were used for immunoblotting (Jackson ImmunoResearch Laboratories). Development of peroxidase activity was performed using an enhanced chemiluminescence kit (Amersham-Pharmacia, Uppsala, Sweden).
Immunofluorescence and in vitro permeability
The procedure for immunofluorescence analysis of endothelial cell monolayers was reported previously in detail.26Briefly, cells were seeded on fibronectin-coated glass coverslips and grown to confluence in 1 mL of medium. Fixation was in 3% formaldehyde from paraformaldehyde (PAF) for 15 minutes and was followed by permeabilization with 0.5% TX-100 before staining. After incubation with the primary antibody for 1 hour, cells were labeled with appropriate TRITC-conjugated secondary antibody. Fluorescence was detected with a fluorescence microscope (Leica DMR, Weitzlar, Germany) and images recorded with a Hamamatsu 3CCD camera (Hamamatsu Photonics, Hamamatsu-City, Japan) before processing through Adobe Photoshop for Macintosh.
Paracellular permeability through endothelial cell monolayers was measured as described.21 27 Endothelial cells were cultured to confluency on purified fibronectin-coated (7 μg/mL) Transwell units (0.4 μm pore; Corning Costar, Cambridge, MA) for 4 days. MAbs were added in the upper compartment followed by addition of fluorescein isothiocyanate (FITC)–conjugated dextran (1mg/mL, average molecular mass 40 000, Sigma). After 3 hours, 50 μL samples were taken from the lower compartment to measure fluorescence (492/520 nm, absorption/emission wavelengths).
Capillary tube assay
Three-dimensional cultures of endothelial cells were made as previously described.17 28 Briefly, type I collagen (Collaborative Biomedical Product, Bedford, MA) from rat tail was diluted to a concentration of 1 mg/mL, and the pH was neutralized by adding 1/10 of the volume of 10 × minimum essential medium (MEM) (Life Technologies). Aliquots of 250 μL were added to each of the 24-well culture plates and incubated at 37°C until gelation occurred. Endothelial cells were seeded on the gel at a concentration of 1 × 104 cells/mL in complete medium as described above. MAbs to VE-cadherin or rat IgG (50 μg/mL) were added for 30 minutes. The medium was then aspirated, and overlying collagen gels were prepared including mAbs or nonimmune rat IgG (50 μg/mL). Capillary tube formation was followed by phase contrast microscopy.
In vitro wounding
In vitro wounding for testing cell migration was performed following a previously published procedure.8,21 29Endothelial cells were cultured for 4 to 5 days in 24-well plates on 0.1% gelatin to obtain a tightly confluent monolayer. Culture medium was then aspirated, and the cell monolayer was wounded with a plastic tip along 2 diameters. The total area of wound surface was 27 mm2/well. The wounded cell layer was washed twice with culture medium and incubated with complete medium in the presence of antibodies to VE-cadherin or nonimmune rat IgG (50 μg/mL) for 6 hours. The number of single cells that migrated into the wounded area was counted using a micrograduated scale (Leica) adapted in the ocular of a Leica DMIL inverted microscope under phase contrast (magnification × 100).
Corneal angiogenesis assay
The assays were performed as described,30 using hydron-coated sucralfate pellets containing 50 ng of human recombinant fibroblast growth factor–2 (hrFGF-2) (R&D Systems, Minneapolis, MN) and 1 μg of BV13, BV14, or nonimmune rat IgG (Jackson ImmunoResearch Laboratories). A single pellet was surgically implanted into a corneal micropocket created in both eyes of 6- to 7-week-old BALB/c mice (Charles River Italia, Calco, Italy). On day 6 after implantation, the eyes were photographed and corneal vascularization evaluated in a masked manner by slit-lamp biomicroscopy at × 40 magnification. The area of neovascular response was quantified by measuring maximal vessel length (mm) between the limbic vessel and the pellet, the circumference of neovascularization in clock hours (1 clock hour = 30 degrees of arc), and, finally, calculated as described.31
Tumor transplantation
Male Crl:nu/nu (CD-1)BR (Charles River Italia) mice 6 to 7 weeks old were used for tumor transplantation models.
H5V cells were trypsinized and washed, and a single-cell suspension of 1 × 105 in 0.1 mL of phosphate-buffered saline (PBS) was injected subcutaneously into the right flank of the mice.
Cultured C6 (pTET-VEGF) glioma cells were harvested, washed, and resuspended in PBS at 15 × 106 cells/mL. Two hundred microliters of the cell suspension was then injected into the right flank of the mice.
Twenty-four hours later, mice began receiving an intraperitoneal injection of various doses of mAbs or nonimmune rat IgG every 3 days. Tumors were measured every 3 days with calipers, and tumor volumes were calculated as previously described.11
At 10 days after tumor implantation, C6 glioma tumors were resected, embedded in TissueTEK OCT compound (Miles, Elkhart, IN), snap frozen in liquid nitrogen, and stored at −80°C. Frozen tumors were then sectioned in 10 μm sections, fixed with methanol (10 minutes at −20°C), and stained with anti–PECAM-1 mAb (MEC 13.3) followed by rhodamine-conjugated donkey anti-rat antibody (Jackson ImmunoResearch Laboratories). Fluorescence images of immunostained tumor tissues were analyzed with Leica DMR fluorescence microscope and images recorded with a Hamamatsu 3CCD camera before processing through Adobe Photoshop for Macintosh.
Vascular permeability was measured in both control mice and mice that received C6 glioma transplants at 10 days after implantation by intravenous injection of Evans blue (100 μL/mouse, 1% solution). Fifteen minutes after injection, animals were killed and extravased. Evans blue was extracted from tissues as described.12,32 33 Permeability values obtained in animals treated with VE-cadherin mAbs were expressed as percentage increase in permeability in comparison to animals treated with equal doses of nonimmune rat IgG.
Production and expression of VE-cadherin chimeras
Various truncated VE-cad-Ig chimeric plasmids were constructed by polymerase chain reaction (PCR) technology using a strategy previously described.34 A full-length VE-cad-Ig cDNA was constructed by interrupting VE-cadherin cDNA at the putative membrane insertion site with a EcoRI site and ligated with a cDNA encoding the hinge and Ch2 and Ch3 regions of human IgG1 (MRC). VE-cad-Ig cDNA was subsequently subcloned into theHindIII and XbaI sites of expression vector pcDNA3/Neo (Invitrogen, San Diego, CA). PCR technology was used to produce a series of VE-cadherin constructs differing by the addition of a complete 3′ domain. A common 5′ primer and a series of 3′ primers engineered with HindIII and NotI sites, respectively, were used to generate DNA encoding only the desired segments of VE-cadherin. These were then ligated into pcDNA3/Neo and rejoined with Fc fragment of human IgG (hFc) in a separate step.
The sequence of the 5′ primer was 5-CATAGCAAGCTTATGCAGAGGCTCACA-3′. The boldface represents the HindIII site. The initiator ATG codon is underlined. The sequences of the 3′ primers were: 1. 5′-CATAGCGATATCAAACACAGGCCAATT-3′ 2. 5′-CATAGCGATATCAAAGACGGGGAAGTT-3′ 3. 5′-CATAGCGATATCTCTCTTTTGGCGATG-3′ 4. 5′-CATAGCGATATCTCGCACCAGGGTATTC-3′ 5. 5′-CATAGCGATATCCTGGGCTGCCATTCT-3′.
The boldface marks the EcoRI restriction sites.
Production and assay of truncated VE-cad-Ig chimeras and enzyme-linked immunosorbent assay (ELISA) binding of mAbs to the chimeric proteins were performed as described.34 35 Briefly, plates were coated with truncated VE-cad-Ig chimeras (1 μg/mL) and blocked with 0.1% bovine serum albumin (Sigma). After washing, either BV13 or BV14 (0.5 μg/mL) was added for 1 hour at room temperature, followed by peroxidase-conjugated goat anti–rat IgG (Jackson ImmunoResearch Laboratories).
To construct the human/murine VE-cadherin chimera (h/m VE-cadherin) (Figure 8A), we first amplified, by PCR, the murine cDNA (between nucleotides 674 and 1531) containing the region encoding the EC3-EC4 domains. Using the TA cloning kit (Invitrogen), the PCR fragment was subcloned into the pCR 2.1 vector, sequenced to confirm absence of mutations, and cut with the HindIII and BglII enzymes (the HindIII site was added to the forward oligo used in the PCR amplification). To remove the region encoding the EC3-EC4 domains from the human VE-cadherin cDNA, the human cDNA subcloned into pBluescript vector (Stratagene, La Jolla, CA) was cut with HindIII and BglII. Finally, to form the h/m VE-cadherin chimera, the murine PCR fragment was ligated into the human cDNA. The chimeric construct was then cloned into the PINCO plasmid, and the cells were infected as described above.
The efficiency of cDNA transfer was tested measuring the expression of VE-cadherin chimera by fluorescence-activated cell-sorter scanner (FACS) analysis. Fluorescence flow cytometric analysis was performed by a FACStar Plus apparatus (Becton Dickinson, Mountain View, CA) using FITC-conjugated secondary antibody (reactive with either rat or mouse IgG) (Caltag Laboratories, Burlingame, CA).36
Results
Effect of VE-cadherin mAbs on endothelial permeability in vitro
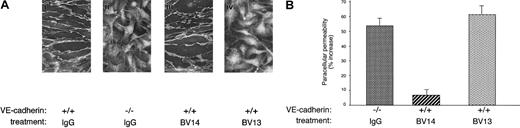
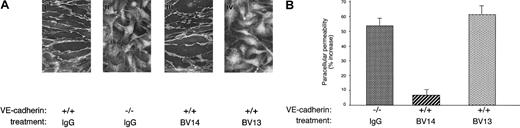
Figure 1A shows that, when added in vitro to an established confluent endothelial cell monolayer, BV14 did not displace VE-cadherin from cell-to-cell contacts. In contrast, BV13 induced redistribution of VE-cadherin from intercellular junctions. Consistently, BV13 but not BV14 induced a significant increase in paracellular permeability of confluent endothelial monolayers (Figure1B). Paracellular permeability in BV13-treated cells was comparable to that observed in endothelial cells where VE-cadherin gene had been inactivated by homologous recombination (Figure 1A-B). The permeability values measured correspond to that of relatively small molecules with a molecular weight comparable to albumin (MW 40 000).
Effect of BV14 and BV13 on VE-cadherin organization at junctions and on permeability of confluent endothelial cells.
(A) VE-cadherin+/+ (i) and VE-cadherin−/− (ii) cells were incubated with BV13 mAb (50 μg/mL) after fixation. Only VE-cadherin–positive cells expressed the antigen at junctions, while VE-cadherin null cells were negative to staining. VE-cadherin+/+ cells were incubated with 50 μg/mL BV14 (iii) or BV13 (iv) for 7 hours before fixation. After this time, cells were fixed, and VE-cadherin staining was evidenced by a rhodhamine-conjugated secondary antibody. (Magnification × 1000.) (B) Permeability across cell monolayers was measured by seeding the cells on Transwell filters and measuring the passage of FITC-dextran from the upper to the lower compartment. VE-cadherin−/− cells presented higher permeability values as compared to VE-cadherin+/+ cells. Addition of BV13 (50 μg/mL) to VE-cadherin–positive cells induced an increase in permeability at 3 hours, similar to that observed in VE-cadherin−/− cells. BV14 at the same concentration was ineffective. Increasing the concentration of the mAbs up to 100 μg/mL did not further increase permeability. Data are expressed as percent increase in comparison to VE-cadherin+/+ cells and are means ± SEM of quadruplicates from a typical experiment out of 4 performed.
Effect of BV14 and BV13 on VE-cadherin organization at junctions and on permeability of confluent endothelial cells.
(A) VE-cadherin+/+ (i) and VE-cadherin−/− (ii) cells were incubated with BV13 mAb (50 μg/mL) after fixation. Only VE-cadherin–positive cells expressed the antigen at junctions, while VE-cadherin null cells were negative to staining. VE-cadherin+/+ cells were incubated with 50 μg/mL BV14 (iii) or BV13 (iv) for 7 hours before fixation. After this time, cells were fixed, and VE-cadherin staining was evidenced by a rhodhamine-conjugated secondary antibody. (Magnification × 1000.) (B) Permeability across cell monolayers was measured by seeding the cells on Transwell filters and measuring the passage of FITC-dextran from the upper to the lower compartment. VE-cadherin−/− cells presented higher permeability values as compared to VE-cadherin+/+ cells. Addition of BV13 (50 μg/mL) to VE-cadherin–positive cells induced an increase in permeability at 3 hours, similar to that observed in VE-cadherin−/− cells. BV14 at the same concentration was ineffective. Increasing the concentration of the mAbs up to 100 μg/mL did not further increase permeability. Data are expressed as percent increase in comparison to VE-cadherin+/+ cells and are means ± SEM of quadruplicates from a typical experiment out of 4 performed.
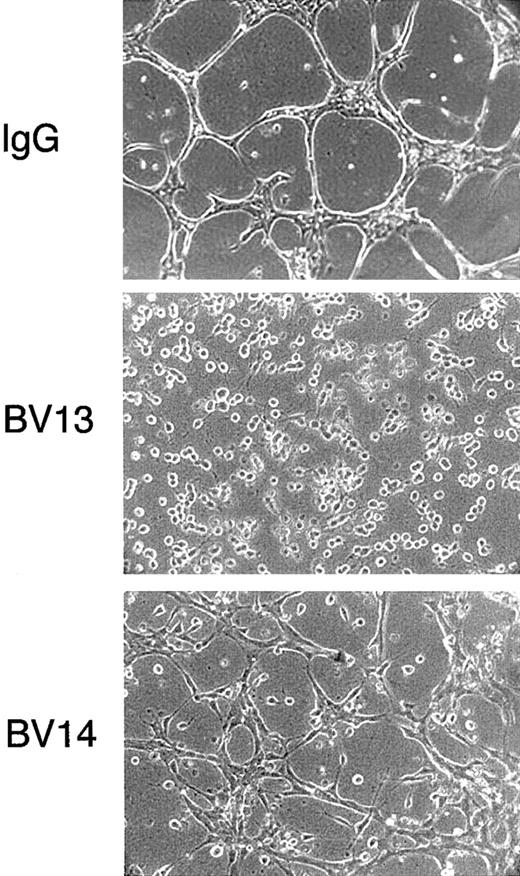
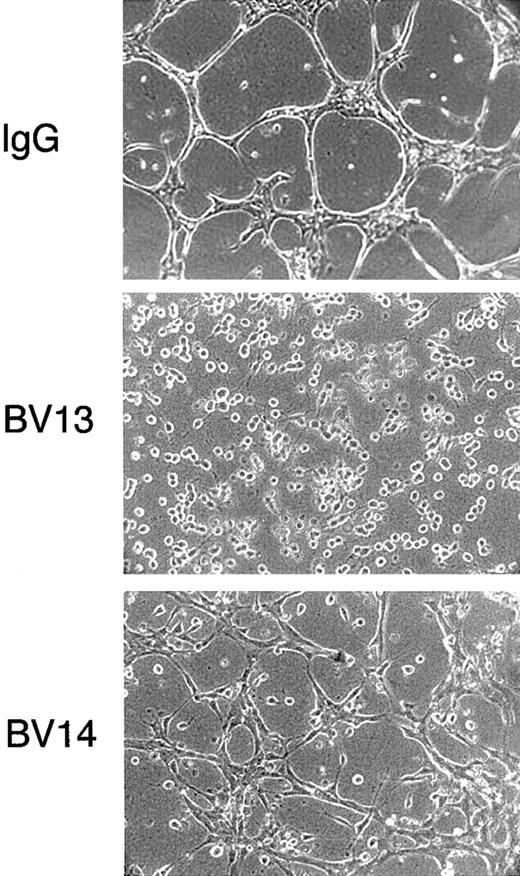
Effect of VE-cadherin mAbs on formation of tubular structures in a 3-dimentional gel
As shown in Figure 2, BV13 blocked the capacity of endothelial cells to organize cords in a gel of collagen. BV14, although less effective, significantly prevented formation of a tubular network and reduced the size and the length of the cords.
Effect of VE-cadherin mAbs on formation of vascularlike structures by endothelial cells.
1G11 cells were cultured in a tridimensional collagen gel. After 48 hours in the presence of nonimmune rat IgG (50 μg/mL), cells reorganized in a network of cordlike structures. In contrast, when exposed to equal concentrations of either mAb BV13 or BV14, the formation of the network was impaired. (Magnification × 100.)
Effect of VE-cadherin mAbs on formation of vascularlike structures by endothelial cells.
1G11 cells were cultured in a tridimensional collagen gel. After 48 hours in the presence of nonimmune rat IgG (50 μg/mL), cells reorganized in a network of cordlike structures. In contrast, when exposed to equal concentrations of either mAb BV13 or BV14, the formation of the network was impaired. (Magnification × 100.)
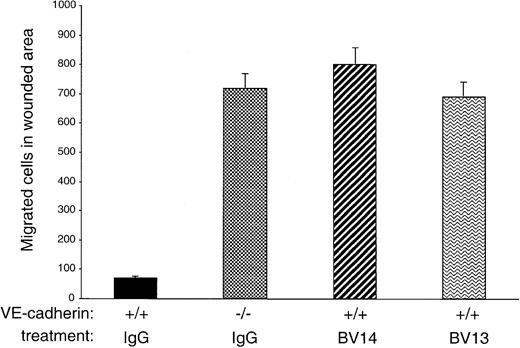
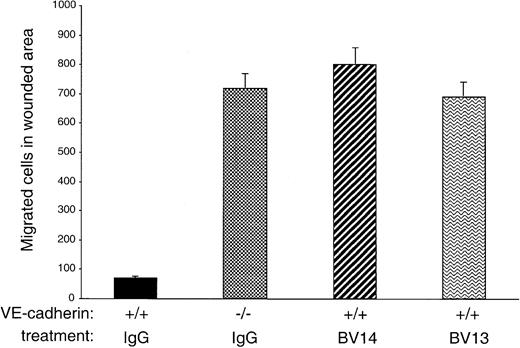
MAb BV14 disrupts homophilic VE-cadherin binding in migrating endothelial cells
The data presented in Figure 2 suggest that, although inactive on confluent endothelial cells, BV14 could exert some disruptive effect when endothelial cells are migrating and forming vascularlike cords. To further test this hypothesis, a standardized wound was produced in confluent endothelial cell monolayers, and the number of migrating endothelial cells was evaluated in the presence or absence of VE-cadherin mAbs. Previous work had shown that VE-cadherin expression in Chinese hamster ovary cells reduced their capacity to migrate into a wounded area.8 21 In agreement with these early observations, VE-cadherin null endothelial cells migrated into a wound much more effectively (up to 7 times) than VE-cadherin+/+ cells (Figure 3). Addition of BV14 and BV13 to VE-cadherin+/+ cells increased the number of migrated cells to a value similar to that of VE-cadherin−/−cells.
Migration of endothelial cells into a wounded area.
A standardized wound of 27 mm2 was produced in monolayers of cultured endothelial cells. The total number of single cells that migrated into the wound area at 6 hours was then counted. VE-cadherin−/− cells migrated more efficiently than VE-cadherin+/+ cells. BV13 or BV14 mAbs (50 μg/mL) were added to VE-cadherin+/+ cells immediately after the wound. Both mAbs increased cell migration to a level comparable to VE-cadherin−/− cells. Data are means ± SEM of quadruplicates from 1 experiment representative of 3 performed.
Migration of endothelial cells into a wounded area.
A standardized wound of 27 mm2 was produced in monolayers of cultured endothelial cells. The total number of single cells that migrated into the wound area at 6 hours was then counted. VE-cadherin−/− cells migrated more efficiently than VE-cadherin+/+ cells. BV13 or BV14 mAbs (50 μg/mL) were added to VE-cadherin+/+ cells immediately after the wound. Both mAbs increased cell migration to a level comparable to VE-cadherin−/− cells. Data are means ± SEM of quadruplicates from 1 experiment representative of 3 performed.
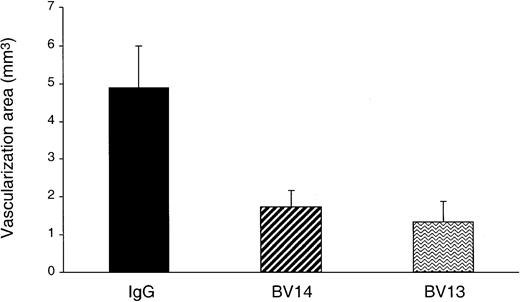
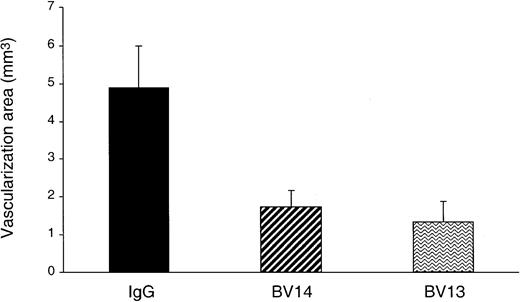
BV14 inhibits angiogenesis in vivo
We then tested whether BV14 could have some effect on angiogenesis in vivo. In the mouse cornea angiogenesis assay (Figure4) BV14 was effective to an extent comparable to BV13.
Inhibition of FGF-2–induced corneal neovascularization by VE-cadherin mAbs.
Hydron pellets containing 50 ng of hrFGF-2 and 1 μg of either one of VE-cadherin mAbs or control IgG were surgically inserted into the mouse corneal stroma adjacent to the temporal limbus. The area of corneal angiogenesis was evaluated as described in the “Materials and methods” section. Eyes implanted with pellets containing BV14 or BV13 mAbs showed a reduction of vascularization of 64% and 72%, respectively, when compared with nonimmune rat IgG–treated group. Data are means ± SEM (n = 6) of a representative experiment out of 3 performed.
Inhibition of FGF-2–induced corneal neovascularization by VE-cadherin mAbs.
Hydron pellets containing 50 ng of hrFGF-2 and 1 μg of either one of VE-cadherin mAbs or control IgG were surgically inserted into the mouse corneal stroma adjacent to the temporal limbus. The area of corneal angiogenesis was evaluated as described in the “Materials and methods” section. Eyes implanted with pellets containing BV14 or BV13 mAbs showed a reduction of vascularization of 64% and 72%, respectively, when compared with nonimmune rat IgG–treated group. Data are means ± SEM (n = 6) of a representative experiment out of 3 performed.
To study whether BV14 could also inhibit tumor vascularization and growth, we selected 2 very aggressive tumor models that are strongly dependent on vascular proliferation for their growth.
We first used a polyoma Middle T immortalized mouse endothelial cell line derived from heart microcirculation (H5V). As also shown in Figure5A, these cells are known to form hemangiomalike tumors when injected subcutaneously in nude mice.14,37,38 These tumors form vascular lacunae largely induced by recruitment of endothelial cells from the host.14 38
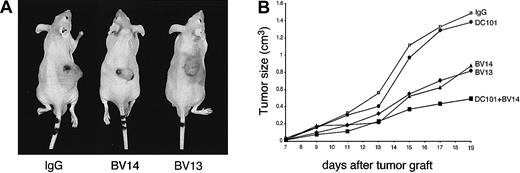
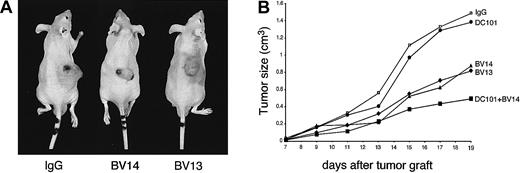
Treatment with VE-cadherin mAbs inhibits growth of H5V hemangiomas.
Hemangiomas were established by injecting 1 × 105H5V cells subcutaneously into the right flank of nu/nu (CD-1)BR mice. The day after injection and every 3 days, mice received equal amounts of BV14 or BV13 (50 μg/mouse), or DC101 (800 μg/mouse), or the combination of BV14 and DC101 (50 μg/mouse and 800 μg/mouse, respectively). Control mice were treated with 50 μg/mouse of nonimmune rat IgG. Tumor size was measured every 3 days. (A) Representative hemangiomas in mice treated with nonimmune IgG, BV13, or BV14 at 19 days after implantation. In animals treated with either BV13 or BV14, the size of tumors was significantly smaller. Note the presence of hematomas around the tumor upon BV13 treatment, indicating local increase in permeability. (B) Hemangioma growth in mice treated with different mAbs. BV13 and BV14 inhibited tumor progression, while DC101 was ineffective. Combination of BV14 with DC101 was more effective. Data are means of 3 separate experiments performed in quintuplicate. SEM never exceeded 20% of the means. Similar results were obtained when the dose of BV14 was increased to 200 μg/mouse.
Treatment with VE-cadherin mAbs inhibits growth of H5V hemangiomas.
Hemangiomas were established by injecting 1 × 105H5V cells subcutaneously into the right flank of nu/nu (CD-1)BR mice. The day after injection and every 3 days, mice received equal amounts of BV14 or BV13 (50 μg/mouse), or DC101 (800 μg/mouse), or the combination of BV14 and DC101 (50 μg/mouse and 800 μg/mouse, respectively). Control mice were treated with 50 μg/mouse of nonimmune rat IgG. Tumor size was measured every 3 days. (A) Representative hemangiomas in mice treated with nonimmune IgG, BV13, or BV14 at 19 days after implantation. In animals treated with either BV13 or BV14, the size of tumors was significantly smaller. Note the presence of hematomas around the tumor upon BV13 treatment, indicating local increase in permeability. (B) Hemangioma growth in mice treated with different mAbs. BV13 and BV14 inhibited tumor progression, while DC101 was ineffective. Combination of BV14 with DC101 was more effective. Data are means of 3 separate experiments performed in quintuplicate. SEM never exceeded 20% of the means. Similar results were obtained when the dose of BV14 was increased to 200 μg/mouse.
As reported in Figure 5A and B, treatment of mice with either BV13 or BV14 markedly inhibited tumor growth. As a comparison, we used mAb DC101 directed to mouse VEGF-R2. This mAb previously has been shown to effectively inhibit neo-vascularization and growth of different experimental tumors.24 39 DC101 was poorly active in this model and only partially increased the inhibitory effect of BV14 when administered in combination.
In addition, about 60% of controls or animals treated with DC101 died within 19 days of tumor implantation, while no mice treated with BV13 or BV14 alone or in combination with DC101 died within the same time frame.
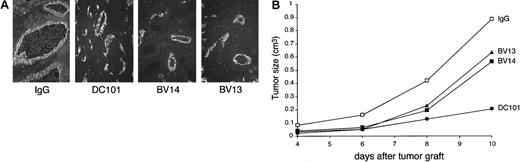
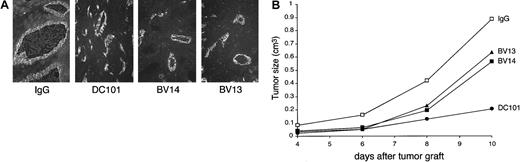
As a second model, we used C6 glioma transfected with VEGF cDNA.15,16 We did not administer tetracycline to the animals in order to get maximal production of VEGF. At early stages of development, this tumor presents highly developed vascular structures (Figure 6A and Benjamin and Keshet15). Inhibition of VEGF-R2 by DC101 blocked tumor growth (Figure 6B), supporting the concept that growth is mostly dependent on VEGF-induced vascular proliferation. Treatment of the animals with BV14 inhibited tumor growth in a way comparable to BV13. At histological analysis, tumor vessels in control animals appeared as enlarged lacunae and irregular vascular structures (Benjamin and Keshet15 and Figure 6A.) MAbs DC101, BV13, and BV14 reduced the number and, even more significantly, the size of tumor vessels (Figure 6A).
VE-cadherin mAbs inhibit growth of C6 glioma tumors.
C6 glioma (3 × 106 cells/mice) was injected subcutaneously into the right flank of nu/nu (CD-1)BR mice. The day after injection and every 3 days, mice received 50 μg/mouse of nonimmune IgG or an equal amount of BV14 or BV13 or DC101 (800 μg/mouse). (A) Immunofluorescence staining of C6 vessels using a rat anti–mouse-PECAM-1 mAb. Vessels present an enlarged lumen and irregular structure in control mice. In the 3 groups of treated mice, the vessels were reduced in number and size. (Magnification ×200.) (B) Time course of C6 tumor growth in animals treated with BV13, BV14, and DC101. All 3 mAbs inhibited C6 growth, but DC101 was the most effective. BV14 and BV13 showed a comparable activity. Data are means of 3 separate experiments performed in quintuplicate. SEM never exceeded 20% of the means. Higher doses of BV14 (up to 200 μg/mouse) showed similar results.
VE-cadherin mAbs inhibit growth of C6 glioma tumors.
C6 glioma (3 × 106 cells/mice) was injected subcutaneously into the right flank of nu/nu (CD-1)BR mice. The day after injection and every 3 days, mice received 50 μg/mouse of nonimmune IgG or an equal amount of BV14 or BV13 or DC101 (800 μg/mouse). (A) Immunofluorescence staining of C6 vessels using a rat anti–mouse-PECAM-1 mAb. Vessels present an enlarged lumen and irregular structure in control mice. In the 3 groups of treated mice, the vessels were reduced in number and size. (Magnification ×200.) (B) Time course of C6 tumor growth in animals treated with BV13, BV14, and DC101. All 3 mAbs inhibited C6 growth, but DC101 was the most effective. BV14 and BV13 showed a comparable activity. Data are means of 3 separate experiments performed in quintuplicate. SEM never exceeded 20% of the means. Higher doses of BV14 (up to 200 μg/mouse) showed similar results.
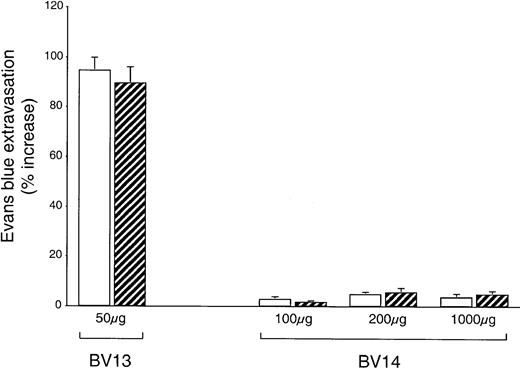
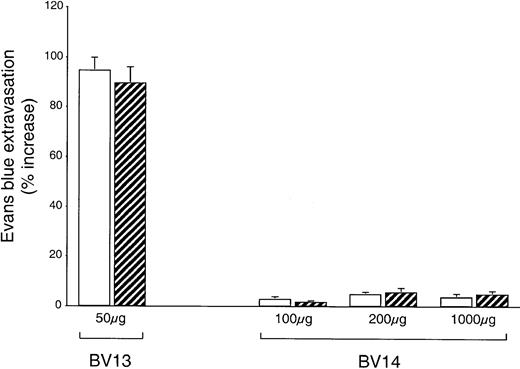
Tumors may produce permeability-increasing substances able to act synergistically with VE-cadherin blocking mAbs. We therefore checked whether lung permeability was altered in animals carrying C6 glioma. As shown in Figure7, while repeated BV13 intraperitoneal treatments significantly increased lung permeability in both control and C6-injected animals, BV14 did not have this effect at any dose in either control or tumor-injected mice. We also were unable to detect an increase in permeability after BV14 treatment in other organs, such as the heart, the kidney, the ears, the spleen, and the brain (not shown). Blue Evans permeability values correspond to that of albumin since the dye rapidly conjugates to this protein in the circulation.32
BV14 does not increase vascular permeability in vivo.
Control nu/nu (CD-1)BR mice (white columns) or mice injected with C6 glioma (striped columns) were treated with nonimmune IgG or BV13 or BV14. Every 3 days mice received the indicated amounts of mAbs. At 10 days after tumor implantation, mice were tested for Evans blue leakage in lungs. While BV13 increased permeability to a significant extent, BV14 was essentially inactive. Data are expressed as percentage increase in Evans blue content in comparison to mice treated with equivalent doses of nonimmune rat IgG. Data are means ± SEM of 3 experiments performed in triplicate.
BV14 does not increase vascular permeability in vivo.
Control nu/nu (CD-1)BR mice (white columns) or mice injected with C6 glioma (striped columns) were treated with nonimmune IgG or BV13 or BV14. Every 3 days mice received the indicated amounts of mAbs. At 10 days after tumor implantation, mice were tested for Evans blue leakage in lungs. While BV13 increased permeability to a significant extent, BV14 was essentially inactive. Data are expressed as percentage increase in Evans blue content in comparison to mice treated with equivalent doses of nonimmune rat IgG. Data are means ± SEM of 3 experiments performed in triplicate.
BV14 and BV13 recognize different regions of VE-cadherin extracellular domain
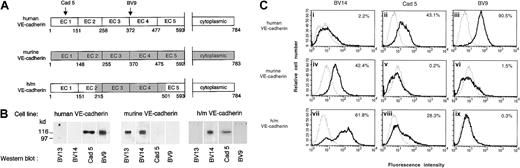
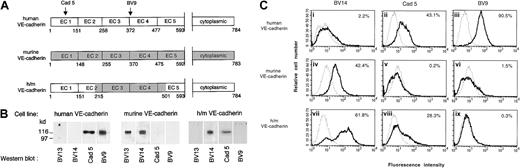
To identify the epitope of BV13 and BV14, recombinant VE-cad-Ig chimeras were produced.34 The fragments spanning different extracellular repeats of the protein EC1 (1-148 AA), EC1-2 (1-255 AA), EC1-3 (1-370 AA), EC1-4 (1-475 AA), and EC1-5 (1-592 AA) were tested for their capacity to bind BV13 and BV14 by ELISA assay.34As reported in Table 1, BV13 was able to effectively bind all the constructs containing the first amino-terminal repeat EC1, while BV14 bound only constructs containing the EC4 repeat. We concluded that while BV13 binds EC1, BV14 binds to a region located in EC4 and closer to the cell membrane.
To further confirm this observation, a human/mouse VE-cadherin chimera was constructed (Figure 8A) and the corresponding cDNA infected in VE-cadherin−/− endothelial cells.
BV14 binds to the membrane proximal region of VE-cadherin.
(A) Schematic representation of VE-cadherin constructs. The extracellular domains EC3 and EC4 in human VE-cadherin were substituted with the murine homologous sequence spanning from AA 215 to AA 501. Recognition binding domains of the mAbs Cad 5 and BV9 on human VE-cadherin are indicated. (B) Western blot analysis of VE-cadherin transfectant cell line extracts using anti–human and anti–mouse VE-cadherin antibodies. As expected, mAb BV13 and BV14 recognized murine VE-cadherin, while mAb Cad 5 and BV9 human VE-cadherin did not. However, only BV14 and Cad 5 could bind to the chimeric construct. (C) Fluorescence flow cytometric analysis of binding of different mAbs to endothelial cells. Saturating concentrations of mAbs were used for staining. Panels indicate fluorescence intensity, and the numbers inside the panels represent the percentage of cells positive to staining with mAbs. MAbs Cad 5 and BV9 bind human VE-cadherin cells (ii) and (iii) and BV14 binds murine VE-cadherin (iv). The analysis was performed at least 3 times for each cell type.
BV14 binds to the membrane proximal region of VE-cadherin.
(A) Schematic representation of VE-cadherin constructs. The extracellular domains EC3 and EC4 in human VE-cadherin were substituted with the murine homologous sequence spanning from AA 215 to AA 501. Recognition binding domains of the mAbs Cad 5 and BV9 on human VE-cadherin are indicated. (B) Western blot analysis of VE-cadherin transfectant cell line extracts using anti–human and anti–mouse VE-cadherin antibodies. As expected, mAb BV13 and BV14 recognized murine VE-cadherin, while mAb Cad 5 and BV9 human VE-cadherin did not. However, only BV14 and Cad 5 could bind to the chimeric construct. (C) Fluorescence flow cytometric analysis of binding of different mAbs to endothelial cells. Saturating concentrations of mAbs were used for staining. Panels indicate fluorescence intensity, and the numbers inside the panels represent the percentage of cells positive to staining with mAbs. MAbs Cad 5 and BV9 bind human VE-cadherin cells (ii) and (iii) and BV14 binds murine VE-cadherin (iv). The analysis was performed at least 3 times for each cell type.
As expected, anti–mouse VE-cadherin antibodies did not recognize human VE-cadherin and, conversely, anti–human VE-cadherin antibodies were unable to bind the mouse homolog. BV14 but not BV13, and Cad 5 but not BV9, could bind the human/mouse chimeric construct in Western blot and flow cytometry (Figure 8B-C). These observations confirm previously published data22and Figure 8A showing that Cad 5 and BV9 bind human VE-cadherin on EC1 and EC3-EC4 domains, respectively. In addition, these results are consistent with the idea that BV14 binds the membrane proximal while BV13 binds the amino-terminal region of murine VE-cadherin.
Discussion
In this paper we present evidence that mAb BV14 directed to VE-cadherin is able to inhibit angiogenesis and reduce tumor growth. As reported previously12 and in the present paper, this mAb does not affect vascular permeability. The lungs, the heart, and other organs did not present a significant increase in permeability even after repeated high doses of the mAb and even in animals carrying well-developed tumors. In vitro experiments support in vivo observations. BV14 did not increase permeability of confluent endothelial monolayers or disrupt VE-cadherin clusters but was able to weaken junctions in migrating endothelial cells and inhibit formation of vascularlike structures.
These results differ from those obtained using another VE-cadherin mAb, BV13, which strongly altered microvascular permeability, leading to strong pathological alterations in treated animals.12These 2 mAbs, therefore, present distinct biologic activities even if they bind to the same protein.
BV14 binds the extracellular domain 4 (EC4), while BV13 recognizes domain 1 (EC1). This difference may explain, in part, their different behavior.
Early studies suggested that cadherin homophilic binding would occur exclusively through the EC1 domain.40 However, more recent publications22 41-44 strongly argue against this possibility and favor a model involving multivalent low-affinity interactions.
Mature junctions between 2 adjacent endothelial cells may require multiple binding domains, while in partially open, weaker junctions, cadherins may interact with a lower number of bonds.
Since EC1 is always required in cadherin homophilic adhesion,43 binding by BV13 would disrupt both mature and weak junctions. In contrast, EC4 may not be necessary to maintain adhesion in stabilized junctions, but its role may be crucial in weak junctions where the number of interacting sites is critical.
Other studies45,46 show that, besides EC1, the membrane proximal region of VE-cadherin extracellular domain is important for adhesion and antibodies directed to it may be strongly inhibitory. We found that mAbs directed to a human VE-cadherin fragment spanning EC3 and EC4 could inhibit homotypic cell adhesion.22 In this last study, EC3-4 antibodies were also able to increase paracellular permeability in vitro in contrast to what was reported here for BV14. This discrepancy may be due to the fact that the work was performed using umbilical vein endothelial cells that, in culture, express poorly organized junctions, easily disorganized. It is also possible, however, that not all the antibodies directed to the membrane proximal region of VE-cadherin have the same disruptive capacity.
An alternative explanation may be that EC4 has a different biologic role than that of promoting homophilic adhesion. A recent publication47 reported that EC4 of N-cadherin mediates epithelial to mesenchimal transition and promotes cell motility. Antibodies directed to this region would inhibit epithelial cell migration. However, in the present paper, BV14 shows opposite effects since it increases endothelial cell detachment from a monolayer and promotes cell movement into the wound.
Whatever the mechanism of action of BV14, these data open the possibility to develop inhibitors of VE-cadherin with a specific activity on angiogenesis and without toxic effects on vascular permeability.
The tumor models we used in this paper are particularly aggressive and strongly dependent on vascular proliferation for growth. The effect of the 2 VE-cadherin mAbs is comparable and was maximal at 50 μg/mouse, which, from previous work,12 appears to be a saturating dose. Interestingly, the 2 types of tumors react to a different extent to VE-cadherin mAbs and to VEGF-R2 mAb DC101. Hemangiomas are essentially insensitive to DC101, while they respond to VE-cadherin mAbs. In contrast and as expected, C6 glioma overexpressing VEGF is more sensitive to DC101 than to VE-cadherin mAbs. Thus, different types of tumors may require a different antiangiogenic strategy. It was found that human hemangiomas produce high amounts of fibroblast growth factor (FGF)48 and that this growth factor, and not VEGF, plays a major role in their growth. This may explain why an inhibitor of VEGF-R2 is quite ineffective, while inhibitors of vascular assembly may be more active.
In conclusion, this study reports that 2 mAbs able to bind to distinct regions of VE-cadherin exert different biologic activities in vitro and in vivo. The possibility to dissociate the effect of VE-cadherin blockers on angiogenesis from that on permeability may be of interest for development of therapeutic tools.
Supported in part by European Community grants (QLG1-CT-1999-01036, QLK3-CT-1999-00020); Consiglio Nazionale delle Ricerche (CNR Grant 97.01299.PF49, CNR 00B9EE_006); Ministero Sanitá (ICS 060.2/RF99.72, RF00.73); Associazione Italiana per la Ricerca sul Cancro; Telethon-Italy (Grant no. E.1254); Agenzia Spaziale Italiana; Associazione Parent Project; L.Z. is a recipient of fellowship of Associazione Italiana per la Ricerca sul Cancro.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Elisabetta Dejana, FIRC Institute of Molecular Oncology via Adamello 16, 20139 Milan, Italy; e-mail:dejana@ifom-firc.it.